Mechanisms of Health Improvement by Lactiplantibacillus plantarum Based on Animal and Human Trials: A Review
Abstract
:1. Introduction
2. Physiological Characteristics of L. plantarum
3. Market Prospect of L. plantarum
4. L. plantarum with Intestinal Flora
4.1. Improvement in Intestinal Flora
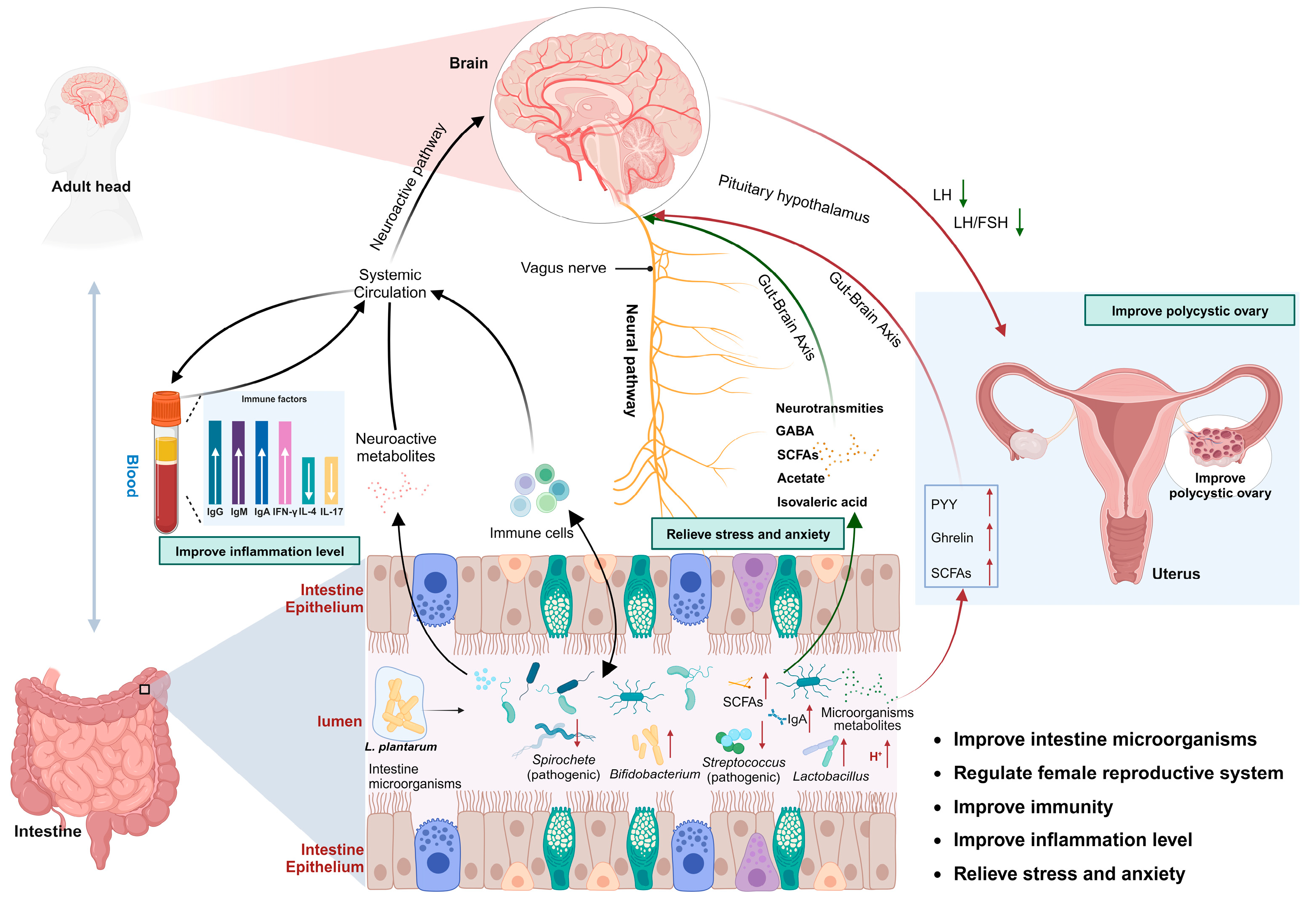
4.2. Intestinal Flora Mediates Female Reproductive Micro-Ecological System
5. L. plantarum with Human Metabolism
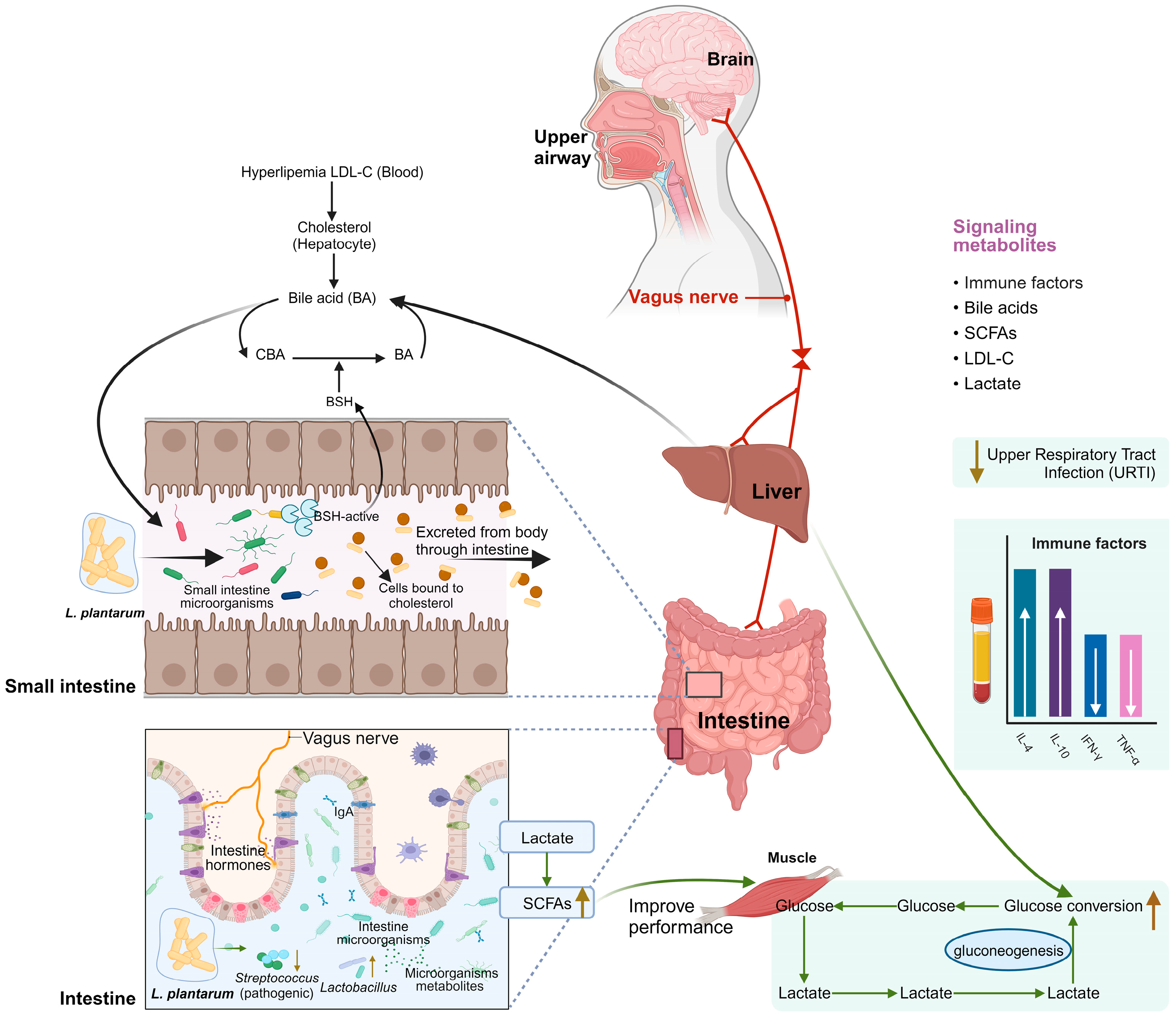
5.1. Reducing Cholesterol
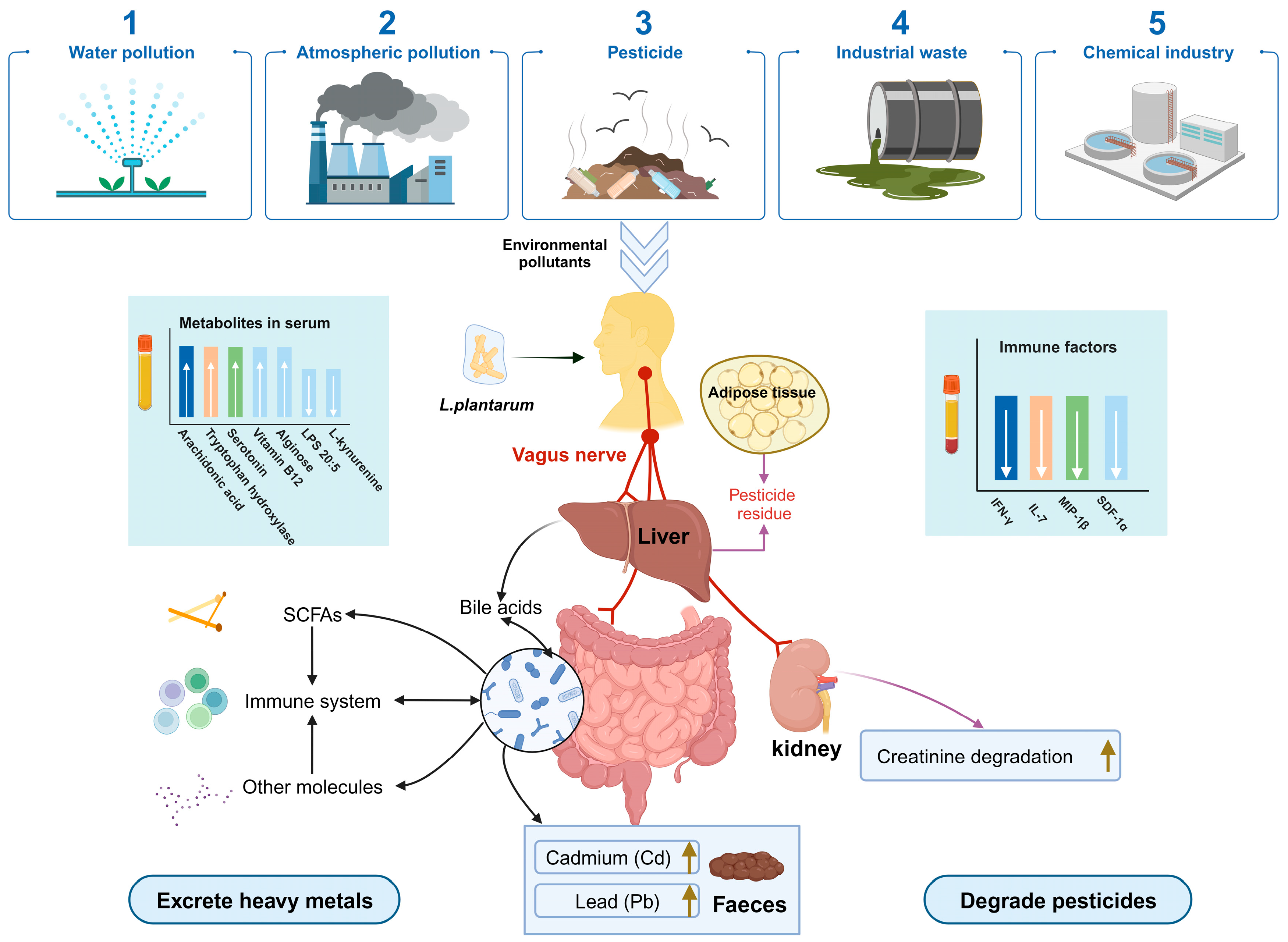
5.2. Promoting Heavy Metal Excretion
5.3. Degradation of the Pesticides
5.4. Improving Body Movement Ability
6. L. plantarum with Immunity
6.1. Regulation of Immunity
6.2. Effect on Absorption of the Trace Elements
7. Effect of L. plantarum on Nervous System
8. L. plantarum with Oral and Skin
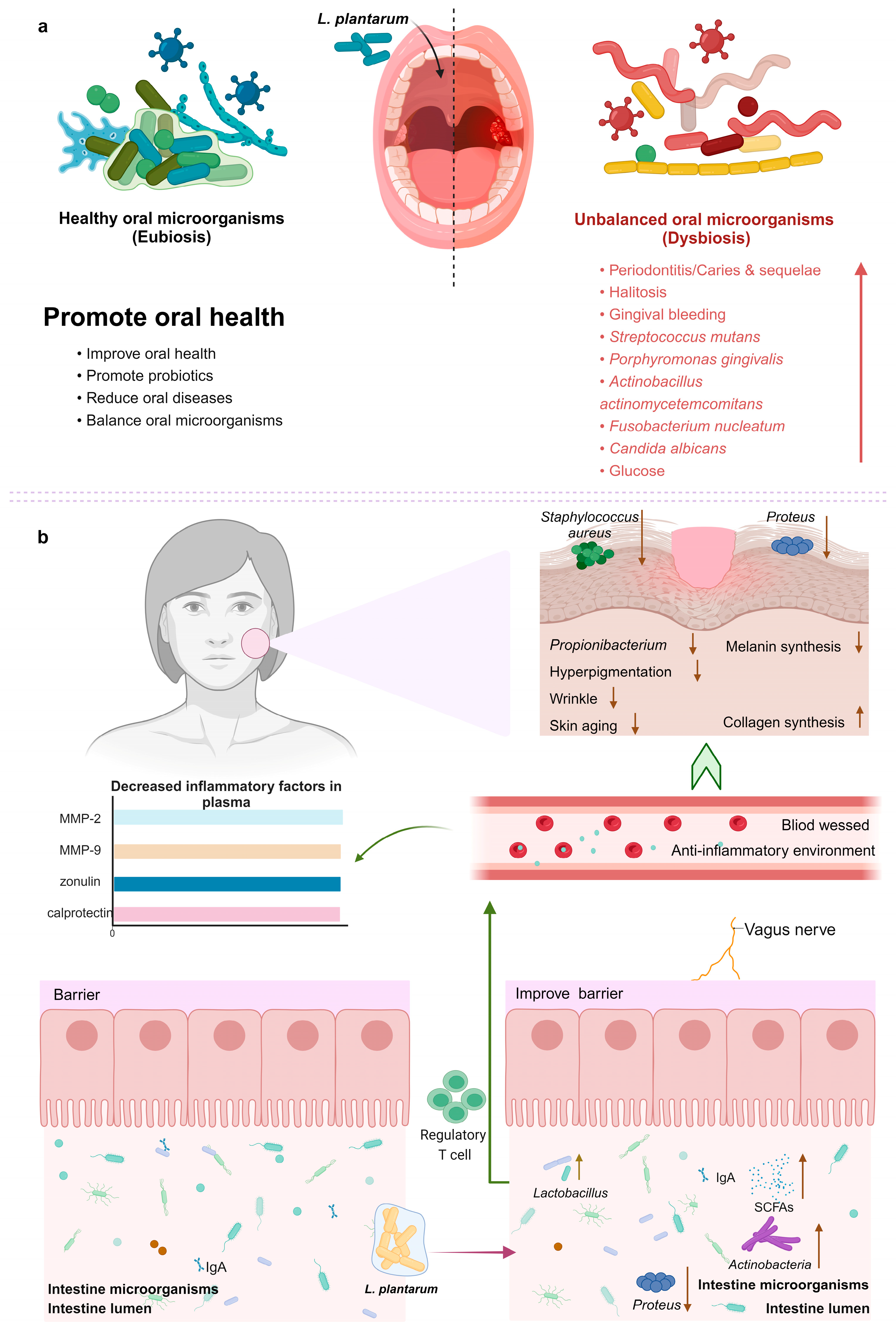
8.1. Promoting Oral Health
8.2. Improving Skin Flora
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J.; Tzeneva, V.A.; Castioni, A.; Wels, M.; Phan, H.T.K.; Rademaker, J.L.W. Phenotypic and genomic diversity of Lactobacillus plantarum strains isolated from various environmental niches. Environ. Microbiol. 2010, 12, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Arasu, M.V.; Jung, M.W.; Ilavenil, S.; Jane, M.; Kim, D.H.; Lee, K.D.; Park, H.S.; Hur, T.Y.; Choi, G.J.; Lim, Y.C. Isolation and characterization of antifungal compound from Lactobacillus plantarum KCC-10 from forage silage with potential beneficial properties. J. Appl. Microbiol. 2013, 115, 1172–1185. [Google Scholar] [CrossRef]
- Martino, M.E.; Bayjanov, J.R.; Caffrey, B.E. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ. Microbiol. 2016, 18, 4974–4989. [Google Scholar] [CrossRef]
- Parente, E.; Ciocia, F.; Ricciardi, A.; Zotta, T.; Felis, G.E.; Torriani, S. Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: A multivariate screening study. Int. J. Food Microbiol. 2010, 144, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Neethu, J.; Craig, B.; Malik, H. Comparison of Microbiological and Probiotic Characteristics of Lactobacilli Isolates from Dairy Food Products and Animal Rumen Contents. Microorganisms 2015, 3, 198–212. [Google Scholar]
- Agaliya, P.J. Screening of Lactobacillus plantarum isolated from fermented idli batter for probiotic properties. Afr. J. Biotechnol. 2012, 11, 12856–12864. [Google Scholar]
- Hsiu, C.M.; Feng, H.S.; Hua, C.J.; Fang, L.M.; Chen, W.S. Antibacterial activity Lactobacillus plantarum isolated from fermented vegetables and investigation of the plantaricin genes. Afr. J. Microbiol. Res. 2016, 10, 796–803. [Google Scholar] [CrossRef]
- Kong, Y.N.; Zhang, W.Y.; Bai, M.; Wu, Y.; Zhao, Y.R.; Zhang, H.P. Stability of the probiotic Lactobacillus plantarum P-8 after long-term continuous subculturing for 1000 generations. China Dairy Ind. 2013, 41, 15–18. [Google Scholar]
- Khemariya, P.; Singh, S.; Jaiswal, N. Isolation and identification of Lactobacillus plantarum from vegetable samples. Food Biotechnol. 2016, 30, 49–62. [Google Scholar] [CrossRef]
- Milioni, C.; Martinez, B.; Degl’Innocenti, S.; Turchi, B.; Fischetti, R. A novel bacteriocin produced by Lactobacillus plantarum LpU4 as a valuable candidate for biopreservation in artisanal raw milk cheese. Dairy Sci. Technol. 2015, 95, 479–494. [Google Scholar] [CrossRef]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2016, 247, 48–54. [Google Scholar] [CrossRef]
- Schillinger, U.; Lückes, F.K. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 1989, 55, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Petra, H.; Rolf, G.; Johanna, B.; Ulrich, S. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73 (Suppl. S2), 365S–373S. [Google Scholar] [CrossRef]
- Won, T.J.; Kim, B.; Lee, Y.; Bang, J.S.; Oh, E.S.; Yoo, J.S.; Hyung, K.E.; Yoon, J.; Hwang, S.; Park, E.S.; et al. Therapeutic potential of Lactobacillus plantarum CJLP133 for house-dust mite-induced dermatitis in NC/Nga mice. Cell Immunol. 2012, 277, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.P.; Oh, H.N.; Choi, C.Y.; Ahn, H.; Yun, H.S.; Chung, Y.M.; Kim, B.; Lee, S.J.; Chun, T. Oral administration of Lactobacillus plantarum CJLP133 and CJLP243 alleviates birch pollen-induced allergic rhinitis in mice. J. Appl. Microbiol. 2018, 124, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kubota, N.; Kakiyama, S.; Miyazaki, K.; Sato, K.; Harima-Mizusawa, N. Effect of Lactobacillus plantarum YIT 0132 on Japanese cedar pollinosis and regulatory T cells in adults. Allergy 2020, 75, 453–456. [Google Scholar] [CrossRef]
- Maeda, N.; Nakamura, R.; Hirose, Y.; Murosaki, S.; Yamamoto, Y.; Kase, T.; Yoshikai, Y. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009, 9, 1122–1125. [Google Scholar] [CrossRef]
- Kim, K.; Lee, G.; Thanh, H.D.; Kim, J.H.; Konkit, M.; Yoon, S.; Park, M.; Yang, S.; Park, E.; Kim, W. Exopolysaccharide from Lactobacillus plantarum LRCC5310 offers protection against rotavirus-induced diarrhea and regulates inflammatory response. J. Dairy Sci. 2018, 101, 5702–5712. [Google Scholar] [CrossRef]
- Shin, D.Y.; Yi, D.Y.; Jo, S.; Lee, Y.M.; Kim, J.H.; Kim, W.; Park, M.R.; Yoon, S.M.; Kim, Y.; Yang, S.; et al. Effect of a new Lactobacillus plantarum product, LRCC5310, on clinical symptoms and virus reduction in children with rotaviral enteritis. Medicine 2020, 99, e22192. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Xiong, T.; Nie, S.P.; Xie, M.Y. Polysaccharide from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. Carbohydr. Polym. 2018, 201, 624–633. [Google Scholar] [CrossRef]
- Xie, J.; Yu, Q.; Nie, S.; Fan, S.; Xiong, T.; Xie, M. Effects of Lactobacillus plantarum NCU116 on Intestine Mucosal Immunity in Immunosuppressed Mice. J. Agric. Food Chem. 2015, 63, 10914–10920. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ding, Q.; Nie, S.P.; Zhang, Y.S.; Xiong, T.; Xie, M.Y. Carrot juice fermented with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. J. Agric. Food Chem. 2014, 62, 11884–11891. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sukumaran, V.; Oviya, M. Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol. 2013, 34, 660–666. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.; Zakaria, N. Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: A randomized, double-blind, placebo-controlled study. J. Dairy Sci. 2019, 102, 4783–4797. [Google Scholar] [CrossRef]
- Wang, T.; Teng, K.; Liu, Y.; Shi, W.; Zhong, J. Lactobacillus plantarum PFM 105 Promotes Intestinal Development Through Modulation of Gut Microbiota in Weaning Piglets. Front. Microbiol. 2019, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Goossens, D.; Jonkers, D.; Stobberingh, E.; Stockbrügger, R. The effect of the probiotic L. plantarum 299v on the faecal and mucosal bacterial flora of patients with inactive ulcerative colitis. Eur. J. Gastroenterol. Hepatol. 2005, 17, A62. [Google Scholar]
- Foo, H.L.; Loh, T.C.; Lai, P.W.; Lim, Y.Z.; Rusul, G. Effects of Adding Lactobacillus plantarum I-UL4 Metabolites in Drinking Water of Rats. Pak. J. Nutr. 2003, 2, 283–288. [Google Scholar]
- Liu, W.; Li, C.; Li, B.; Shang, Q.; Han, Z.; Zhang, Y.; Liu, X.; Fan, H.; Zhang, J.; Chen, Y.; et al. Lactiplantibacillus plantarum P9 improved gut microbial metabolites and alleviated inflammatory response in pesticide exposure cohorts. iScience 2022, 25, 104472. [Google Scholar] [CrossRef]
- Zhai, Q.; Liu, Y.; Wang, C.; Qu, D.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Lactobacillus plantarum CCFM8661 modulates bile acid enterohepatic circulation and increases lead excretion in mice. Food Funct. 2019, 10, 1455–1464. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Xiao, Y.; Liu, Q.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Relief of Cadmium-Induced Intestinal Motility Disorder in Mice by Lactobacillus plantarum CCFM8610. Front. Immunol. 2020, 11, 619574. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q. Lactobacillus plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering 2021, 7, 10. [Google Scholar] [CrossRef]
- Hu, T.; Song, J.; Zeng, W.; Li, J.; Suo, H. Lactobacillus plantarum LP33 attenuates Pb-induced hepatic injury in rats by reducing oxidative stress and inflammation and promoting Pb excretion. Food Chem. Toxicol. 2020, 143, 111533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-M.; Wei, L.; Chiu, Y.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Wang, M.-F.; Huang, C.-C. Lactobacillus plantarum TWK10 Supplementation Improves Exercise Performance and Increases Muscle Mass in Mice. Nutrients 2016, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Huang, W.C.; Lin, J.S.; Mon-Chien, L.; Ker-Sin, N.G. Use of Lactobacillus plantarum TWK10 in Preparation of Composition for Resistance to Post-Exercise Inflammation or for Reducing Body Fat. European Patent EP3896151A1, 20 October 2021. [Google Scholar]
- Liu, Y.Y.; Zeng, S.Y.; Leu, Y.L.; Tsai, T.Y. Antihypertensive Effect of a Combination of Uracil and Glycerol Derived from Lactobacillus plantarum Strain TWK10-Fermented Soy Milk. J. Agric. Food Chem. 2015, 63, 7333. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Tsai, Y.C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liong, M.T.; Chung, Y.E.; Huang, H.Y.; Peng, W.S.; Cheng, Y.F.; Lin, Y.S.; Wu, Y.Y.; Tsai, Y.C. Effects of Lactobacillus plantarum PS128 on Children with Autism Spectrum Disorder in Taiwan: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 820. [Google Scholar] [CrossRef]
- Chen, H.M.; Kuo, P.H.; Hsu, C.Y. Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial. Nutrients 2021, 13, 3731. [Google Scholar] [CrossRef]
- Cui, S.; Guo, W.; Chen, C.; Tang, X.; Zhao, J.; Mao, B.; Zhang, H. Metagenomic Analysis of the Effects of Lactiplantibacillus plantarum and Fructooligosaccharides (FOS) on the Fecal Microbiota Structure in Mice. Foods 2022, 11, 1187. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Z.; Jiang, S.; Bai, X.; Ma, C.; Peng, Q.; Chen, K.; Chang, H.; Fang, T.; Zhang, H. Probiotic Bifidobacterium lactis V9 Regulates the Secretion of Sex Hormones in Polycystic Ovary Syndrome Patients through the Gut-Brain Axis. mSystems 2019, 4, e00017–e00019. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.E.; Kim, M.S.; Shim, K.W. Effects of Lactobacillus plantarum Q180 on Postprandial Lipid Levels and Intestinal Environment: A Double-Blind, Randomized, Placebo-Controlled, Parallel Trial. Nutrients 2020, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, K.P.; Choi, E.; Yim, J.H.; Choi, C.; Yun, H.S.; Ahn, H.Y.; Oh, J.Y.; Cho, Y. Effects of Lactobacillus plantarum CJLP55 on Clinical Improvement, Skin Condition and Urine Bacterial Extracellular Vesicles in Patients with Acne Vulgaris: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 1368. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, B.; Xu, X.; Mao, B.; Tang, X.; Zhao, J.; Zhang, H.; Cui, S.; Chen, W. Lactiplantibacillus plantarum CCFM8724 Reduces the Amounts of Oral Pathogens and Alters the Oral Microbiota in Children with Dental Caries: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Nutr. Assoc. 2023, 42, 361–370. [Google Scholar] [CrossRef]
- Tsai, W.H.; Chou, C.H.; Chiang, Y.J.; Lin, C.G.; Lee, C.H. Regulatory effects of Lactobacillus plantarum-GMNL6 on human skin health by improving skin microbiome. Int. J. Med. Sci. 2021, 18, 1114–1120. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Guo, Z.; Kwok, L.; Ma, C.; Zhang, W.; Lv, Q.; Huang, W.; Zhang, H. Effect of oral consumption of probiotic Lactobacillus planatarum P-8 on fecal microbiota, SIgA, SCFAs, and TBAs of adults of different ages. Nutrition 2014, 30, 776–783.e771. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, Y.; Tian, Y.; Chen, Y.Y.; Guan, W.Y.; Piao, C.H.; Wang, Y.H. Lactobacillus plantarum LP104 ameliorates hyperlipidemia induced by AMPK pathways in C57BL/6N mice fed high-fat diet. J. Funct. Foods 2020, 64, 103665. [Google Scholar] [CrossRef]
- Lew, L.C.; Hor, Y.Y.; Yusoff, N.A.A.; Choi, S.B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Nam, B.; Kim, S.A.; Park, S.D.; Kim, H.J.; Kim, J.S.; Bae, C.H.; Kim, J.Y.; Nam, W.; Lee, J.L.; Sim, J.H. Regulatory effects of Lactobacillus plantarum HY7714 on skin health by improving intestinal condition. PLoS ONE 2020, 15, e0231268. [Google Scholar] [CrossRef]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Kassayová, M.; Bobrov, N.; Strojn, L.; Kisková, T.; Bomba, A. Preventive effects of probiotic bacteria Lactobacillus plantarum and dietary fiber in chemically-induced mammary carcinogenesis. Anticancer Res. 2014, 34, 4969. [Google Scholar] [PubMed]
- Khan, I.; Wei, J.; Li, A.; Liu, Z.; Yang, P.; Jing, Y.; Chen, X.; Zhao, T.; Bai, Y.; Zha, L. Lactobacillus plantarum strains attenuated DSS-induced colitis in mice by modulating the gut microbiota and immune response. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2022, 25, 587–603. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.D.; Jabri, B.; Bendelac, A. Diverse developmental pathways of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2018, 18, 514–525. [Google Scholar] [CrossRef]
- Jung, K.; Kim, A.; Lee, J.H.; Cho, D.; Seo, J.; Jung, E.S.; Kang, H.J.; Roh, J.; Kim, W. Effect of Oral Intake of Lactiplantibacillus plantarum APsulloc 331261 (GTB1(TM)) on Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 2015. [Google Scholar] [CrossRef] [PubMed]
- Nordstrm, E.A.; Teixeira, C.; Montelius, C.; Jeppsson, B.; Larsson, N. Lactiplantibacillus plantarum 299v (LP299V): Three decades of research. Benef. Microbes 2021, 12, 441–465. [Google Scholar] [CrossRef]
- Olek, A.; Woynarowski, M.; Ahrén, I.L.; Socha, P.; Larsson, N. Efficacy and Safety of Lactobacillus plantarum DSM 9843 (LP299V) in the Prevention of Antibiotic-Associated Gastrointestinal Symptoms in Children—Randomized, Double-Blind, Placebo-Controlled Study. J. Pediatr. 2017, 186, 82–86. [Google Scholar] [CrossRef]
- Aguilera, M.; Ontiveros, Y.G.; Rivas, A. Endobolome, a New Concept for Determining the Influence of Microbiota Disrupting Chemicals (MDC) in Relation to Specific Endocrine Pathogenesis. Front. Microbiol. 2020, 11, 578007. [Google Scholar] [CrossRef]
- López-Moreno, A.; Aguilera, M. Probiotics Dietary Supplementation for Modulating Endocrine and Fertility Microbiota Dysbiosis. Int. J. Appl. Mech. 2020, 12, 757. [Google Scholar] [CrossRef]
- He, Y.; Mei, L.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Lactiplantibacillus plantarum CCFM1019 attenuate polycystic ovary syndrome through butyrate dependent gut–brain mechanism. Food Funct. 2022, 13, 1380–1392. [Google Scholar] [CrossRef]
- Vladareanu, R.; Mihu, D.; Mitran, M.; Mehedintu, C.; Vladareanu, S. New evidence on oral L. plantarum P17630 product in women with history of recurrent vulvovaginal candidiasis (RVVC): A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 262. [Google Scholar]
- Liang, T.; Wu, L.; Xi, Y. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 61, 1670–1688. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.C.; Choi, S.B.; Khoo, B.Y.; Sreenivasan, S.; Ong, K.L.; Liong, M.T. Lactobacillus plantarum DR7 Reduces Cholesterol via Phosphorylation of AMPK That Down-regulated the mRNA Expression of HMG-CoA Reductase. Food Sci. Anim. Resour. 2018, 38, 350–361. [Google Scholar]
- Keleszade, E.; Kolida, S.; Costabile, A. The cholesterol lowering efficacy of Lactobacillus plantarum ECGC 13110402 in hypercholesterolemic adults: A double-blind, randomized, placebo controlled, pilot human intervention study. J. Funct. Foods 2022, 89, 104939. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Lajo, T.; Carrión, J.M.; Cuñé, J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br. J. Nutr. 2013, 109, 1866–1872. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhang, Y.; Li, H.; Liu, Y.; Wang, S.; Dong, X.; Su, F.; Yao, G.; Sun, T.; Zhang, H. In vitro screen of Lactobacillus plantarum as probiotic bacteria and their fermented characteristics in soymilk. Ann. Microbiol. 2012, 62, 1311–1320. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, W.; Shan, M.; Lu, Z.; Lu, Y. Yogurt-derived Lactobacillus plantarum Q16 alleviated high-fat diet-induced non-alcoholic fatty liver disease in mice. Food Sci. Hum. Health 2022, 5, 11. [Google Scholar] [CrossRef]
- Kucukgul, A.; Erdogan, S.; Gonenci, R.; Ozan, G. Beneficial effects of nontoxic ozone on H2O2-induced stress and inflammation. Biochem. Cell Biol. 2016, 94, 577–583. [Google Scholar] [CrossRef]
- Burnase, N.; Jaiswal, S.; Barapatre, A. Metal Toxicity in Humans Associated with Their Occupational Exposures Due to Mining. In Medical Geology in Mining: Health Hazards Due to Metal Toxicity; Springer International Publishing: Cham, Switzerland, 2022; pp. 127–186. [Google Scholar]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef]
- Dwyana, Z.; Haedar, N.; Priosambodo, D.; Alam, M.R. The use of probiotic Lactobacillus plantarum and Lactobacillus bifermentans as antidote of mercury metal (Hg) to the mice Rattus norvegicus. Proc. IOP Publ. 2019, 1341, 022009. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.; Chen, W. Protective Effects of Lactobacillus plantarum CCFM8610 against Acute Cadmium Toxicity in Mice. Appl. Environ. Microbiol. 2013, 79, 1508–1515. [Google Scholar] [CrossRef]
- Li, Y.; Liu, A.; Chen, L.; Xiang, Y.; Huang, D.; Huang, W.; Chen, Z.; Fan, H.; Meng, X. Lactobacillus plantarum WSJ-06 alleviates neurobehavioral injury induced by lead in mice through the gut microbiota. Food Chem. Toxicol. 2022, 167, 113308. [Google Scholar] [CrossRef]
- Gu, A.; Zhang, C.; Liu, Q.; Wang, Q.; Jiang, Z. Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ. Pollut. 2017, 226, 268–276. [Google Scholar]
- Harishankar, M.K.; Sasikala, C.; Ramya, M. Efficiency of the intestinal bacteria in the degradation of the toxic pesticide, chlorpyrifos. 3 Biotech 2013, 3, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, Y.; Mi, Z.; Huo, R.; Zhou, T.; Hai, H.; Kwok, L.; Sun, Z.; Chen, Y.; Zhang, H. Screening for Lactobacillus plantarum Strains That Possess Organophosphorus Pesticide-Degrading Activity and Metabolomic Analysis of Phorate Degradation. Front. Microbiol. 2018, 9, 2048. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Moustafa, E.M.; Gewaily, M.S. Ameliorative effects of Lactobacillus plantarum L-137 on Nile tilapia (Oreochromis niloticus) exposed to deltamethrin toxicity in rearing water. Aquat. Toxicol. 2020, 219, 105377. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A. Microbiota and muscle highway—Two way traffic. Nat. Rev. Endocrinol. 2020, 16, 71–72. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; Macdonald, T.; Kostic, A.D. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, B.R.; Hao, Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2022, 8, CD006895. [Google Scholar]
- Jäger, R.; Mohr, A.E.; Carpenter, K.C.; Kerksick, C.M.; Purpura, M.; Moussa, A.; Townsend, J.R.; Lamprecht, M.; West, N.P.; Black, K.; et al. International Society of Sports Nutrition Position Stand: Probiotics. J. Int. Soc. Sports Nutr. 2019, 16, 62. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Li, H.S.; Kan, N.W.; Chen, Y.M. Effect of Lactobacillus plantarum TWK10 on Improving Endurance Performance in Humans. Chin. J. Physiol. 2018, 61, 163. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lee, M.-C.; Lee, C.-C.; Ng, K.-S.; Hsu, Y.-J.; Tsai, T.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C. Effect of Lactobacillus plantarum TWK10 on Exercise Physiological Adaptation, Performance, and Body Composition in Healthy Humans. Nutrients 2019, 11, 2836. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Hsu, Y.J.; Ho, H.H.; Kuo, Y.W.; Lin, W.Y.; Tsai, S.Y.; Chen, W.L.; Lin, C.L.; Huang, C.C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci. Rep. 2016, 6, 34561. [Google Scholar] [CrossRef] [PubMed]
- Prakoeswa, C.R.S.; Herwanto, N.; Prameswari, R.; Astari, L.; Sawitri, S.; Hidayati, A.N.; Indramaya, D.M.; Kusumowidagdo, E.R.; Surono, I.S. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef. Microbes 2017, 8, 833–840. [Google Scholar] [CrossRef]
- Xing, J.H.; Shi, C.W.; Sun, M.J.; Gu, W.; Zhang, R.R.; Chen, H.L.; Li, Y.; Wang, D.; Li, J.; Niu, T.M.; et al. Lactiplantibacillus plantarum 0111 Protects Against Influenza Virus by Modulating Intestinal Microbial-Mediated Immune Responses. Front. Microbiol. 2022, 13, 820484. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Scheers, N.; Rossander-Hulthen, L.; Torsdottir, I.; Sandberg, A.S. Increased iron bioavailability from lactic-fermented vegetables is likely an effect of promoting the formation of ferric iron (Fe3+). Eur. J. Nutr. 2016, 55, 373–382. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Hulthén, L. Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females-Double isotope sequential single-blind studies in menstruating women. PLoS ONE 2017, 12, e0189141. [Google Scholar] [CrossRef]
- Axling, U.; Önning, G.; Combs, M.A.; Bogale, A.; Högström, M.; Svensson, M. The Effect of Lactobacillus plantarum 299v on Iron Status and Physical Performance in Female Iron-Deficient Athletes: A Randomized Controlled Trial. Nutrients 2020, 12, 1279. [Google Scholar] [CrossRef] [PubMed]
- Shamsipour, S.; Sharifi, G.; Taghian, F. Impact of interval training with probiotic (L. plantarum/Bifidobacterium bifidum) on passive avoidance test, ChAT and BDNF in the hippocampus of rats with Alzheimer’s disease. Neurosci. Lett. 2021, 756, 135949. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.Y.; Sun, Z.; Liong, M.T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, C.; Zhou, X.; Zhang, L.; Gu, L.; Liu, Z.; Ma, J.; Hou, J.; Jiang, Z. Lactobacillus plantarum Combined with Galactooligosaccharides Supplement: A Neuroprotective Regimen Against Neurodegeneration and Memory Impairment by Regulating Short-Chain Fatty Acids and the c-Jun N-Terminal Kinase Signaling Pathway in Mice. J. Agric. Food Chem. 2022, 70, 8619–8630. [Google Scholar] [CrossRef] [PubMed]
- Pietri, F.K.; Rossouw, P.E.; Javed, F.; Michelogiannakis, D. Role of Probiotics in Oral Health Maintenance Among Patients Undergoing Fixed Orthodontic Therapy: A Systematic Review of Randomized Controlled Clinical Trials. Probiotics Antimicrob. Proteins 2020, 12, 1349–1359. [Google Scholar] [CrossRef]
- Pudgar, P.; Povšič, K.; Čuk, K.; Seme, K.; Petelin, M.; Gašperšič, R. Probiotic strains of Lactobacillus brevis and Lactobacillus plantarum as adjunct to non-surgical periodontal therapy: 3-month results of a randomized controlled clinical trial. Clin. Oral. Investig. 2021, 25, 1411–1422. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244. [Google Scholar] [CrossRef]
- Charles, B.; Clayman, M.D. Physiology of the Gastrointestinal Tract. JAMA 1981, 246, 2863. [Google Scholar]
- Jo, C.S.; Myung, C.H.; Yoon, Y.C.; Ahn, B.H.; Min, J.W.; Seo, W.S.; Lee, D.H.; Kang, H.C.; Heo, Y.H.; Choi, H.; et al. The Effect of Lactobacillus plantarum Extracellular Vesicles from Korean Women in Their 20s on Skin Aging. Curr. Issues Mol. Biol. 2022, 44, 526–540. [Google Scholar] [CrossRef]
- Yoshitake, R.; Nakai, H.; Ebina, M.; Kawasaki, K.; Murosaki, S.; Hirose, Y. Beneficial Effect of Heat-Killed Lactiplantibacillus plantarum L-137 on Skin Functions in Healthy Participants: A Randomized, Placebo-Controlled, Double-Blind Study. Front. Med. 2022, 9, 912280. [Google Scholar] [CrossRef] [PubMed]
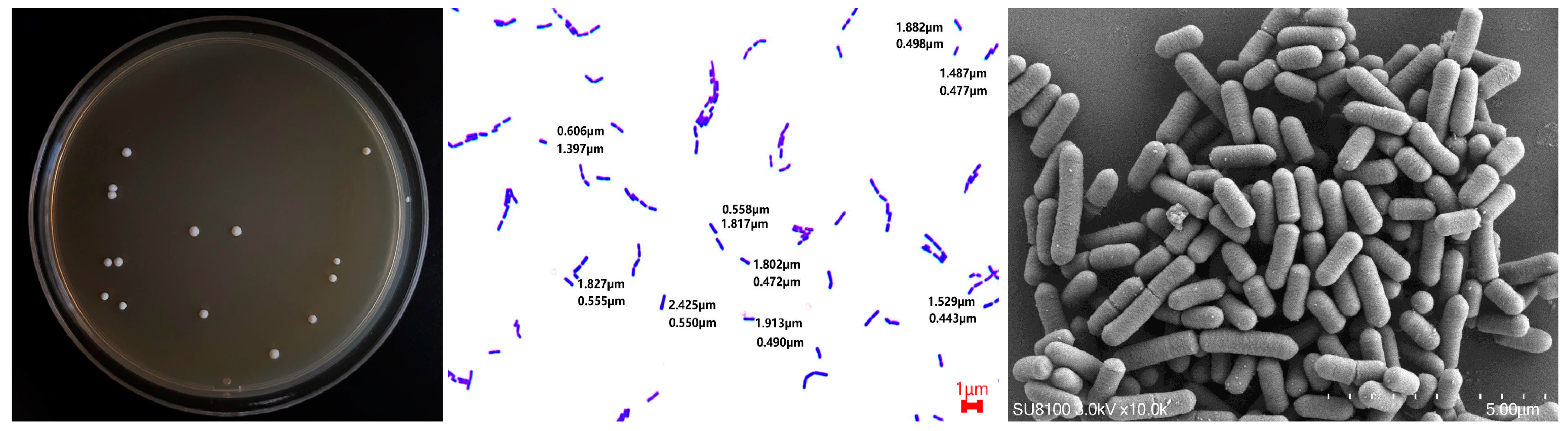
| Strain | Source | Physiological Function | Experimental Object | Reference |
|---|---|---|---|---|
| L. plantarum CJLP133 | Kimchi | Antiallergy, regulating Th1/Th2 balance | Animal (mice) trials | [16] |
| L. plantarum CJLP243 | [17] | |||
| L. plantarum YIT0132 | Fermented food | Human trials | [18] | |
| L. plantarum L-137 | A traditional fermented food produced in the Philippines | Antiallergy, regulating Th1/Th2 balance; degraded pesticide | Animal (mice) trials | [19] |
| L. plantarum LRCC5310 | Kimchi | Produced EPS, resists virus, and relieves intestinal problems such as diarrhea caused by rotavirus | Animal (mice) trials and human trials | [20,21] |
| L. plantarum NCU116 | Pickled vegetables | Ameliorated Type 2 diabetes; reduce cholesterol | Animal (mice) (rat) trials | [22,23,24] |
| L. plantarum VSG3 | The gut of healthy rohu (Labeo rohita) | Enhanced immunity and anti-streptococcal infection ability | Animal (fish) trials | [25] |
| L. plantarum DR7 | Bovine milk | Alleviated the symptoms of upper respiratory tract infection; regulates immunity | Human trials | [26] |
| L. plantarum PFM105 | The rectum of a healthy sow | Improved the intestinal flora, increased the number of beneficial bacteria in the intestine, and reduced the number of conditional pathogenic bacteria; attenuated resistance genes and antibiotic residues | Animal (weaning piglet) trials | [27] |
| L. plantarum 299v | Dehydrated fermented milk | Improved intestinal flora and female reproductive system; enhanced the memory | Human trials | [28] |
| L. plantarum I-UL4 | Malaysian Tempeh | Reduced cholesterol and increases the number of intestinal beneficial bacteria | Animal (post weaning rats) trials | [29] |
| L. plantarum P9 | Traditional fermented food | Improved the degradation rate of pesticides and the stability of intestinal flora | Animal (mice) trials | [30] |
| L. plantarum CCFM8661 | Kimchi | Modulated bile acid enterohepatic circulation and promotes heavy metal excretion | Animal (mice) trials | [31] |
| L. plantarum CCFM8610 | Kimchi | Alleviated irritable bowel syndrome and prevents gut microbiota dysbiosis | Animal (mice) trials | [32,33] |
| L. plantarum LP33 | Yogurt, Xinjiang, China | Promoted heavy metal excretion, regulates oxidative stress, and alleviates heavy metal toxicity in the tissues | Animal (rat) trials | [34] |
| L. plantarum TWK10 | Taiwan pickled vegetables | Improved exercise performance and increased muscle mass | Animal (mice) (rat) trials and human trials | [35,36,37] |
| L. plantarum PS128 | Traditional fermented food–Fu-Tsai | Improved the depressive symptoms and sleep quality of insomniacs and in allay autism spectrum disorder (ASD) | Human trials | [38,39,40] |
| L. plantarum ST-III | Homemade pickles in China | Improved social behavior in a male mouse model of ASD and contribute to more balanced intestinal homeostasis. | Animal (mice) trials | [41] |
| L. plantarum P17630 | Healthy vagina | Treated or alleviated letrozole-induced polycystic ovarian syndrome | Human trials | [42] |
| L. plantarum Q180 | The feces of adults | Reduced cholesterol | Human trials | [43] |
| L. plantarum CJLP55 | Kimchi | Improved the count and grading of acne lesions, increase hydration | Human trials | [44] |
| L. plantarum CCFM8724 | Fermented milk wine | Reduced the number of oral pathogens and altered the oral microbiota in children with dental caries. | Human trials | [45] |
| L. plantarum GMNL6 | - | Reduced the erythema and melanin, improve skin condition | Human trials | [46] |
| L. plantarum P8 | Natural fermented yoghurt | Improve intestinal flora, reduce cholesterol, regulate immunity and the nervous system | Animal (mice) (rat) trials and human trials | [26,47,48,49] |
| L. plantarum HY7714 | The breast milk of healthy women | Improved skin health and regulate permeability | Human trials | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Li, J.; Wang, J.; Chen, Y. Mechanisms of Health Improvement by Lactiplantibacillus plantarum Based on Animal and Human Trials: A Review. Fermentation 2024, 10, 73. https://doi.org/10.3390/fermentation10020073
Hao Y, Li J, Wang J, Chen Y. Mechanisms of Health Improvement by Lactiplantibacillus plantarum Based on Animal and Human Trials: A Review. Fermentation. 2024; 10(2):73. https://doi.org/10.3390/fermentation10020073
Chicago/Turabian StyleHao, Yu, Jianli Li, Jicheng Wang, and Yongfu Chen. 2024. "Mechanisms of Health Improvement by Lactiplantibacillus plantarum Based on Animal and Human Trials: A Review" Fermentation 10, no. 2: 73. https://doi.org/10.3390/fermentation10020073
APA StyleHao, Y., Li, J., Wang, J., & Chen, Y. (2024). Mechanisms of Health Improvement by Lactiplantibacillus plantarum Based on Animal and Human Trials: A Review. Fermentation, 10(2), 73. https://doi.org/10.3390/fermentation10020073






