Acclimation of Microbial Consortia to Ammonia and Salt in Methane Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Anaerobic Digestion and Acclimation Culture
2.2. Preparation of DNA Samples and 16S rRNA Gene Amplicon-Based Metagenomic Analysis
2.3. Analysis
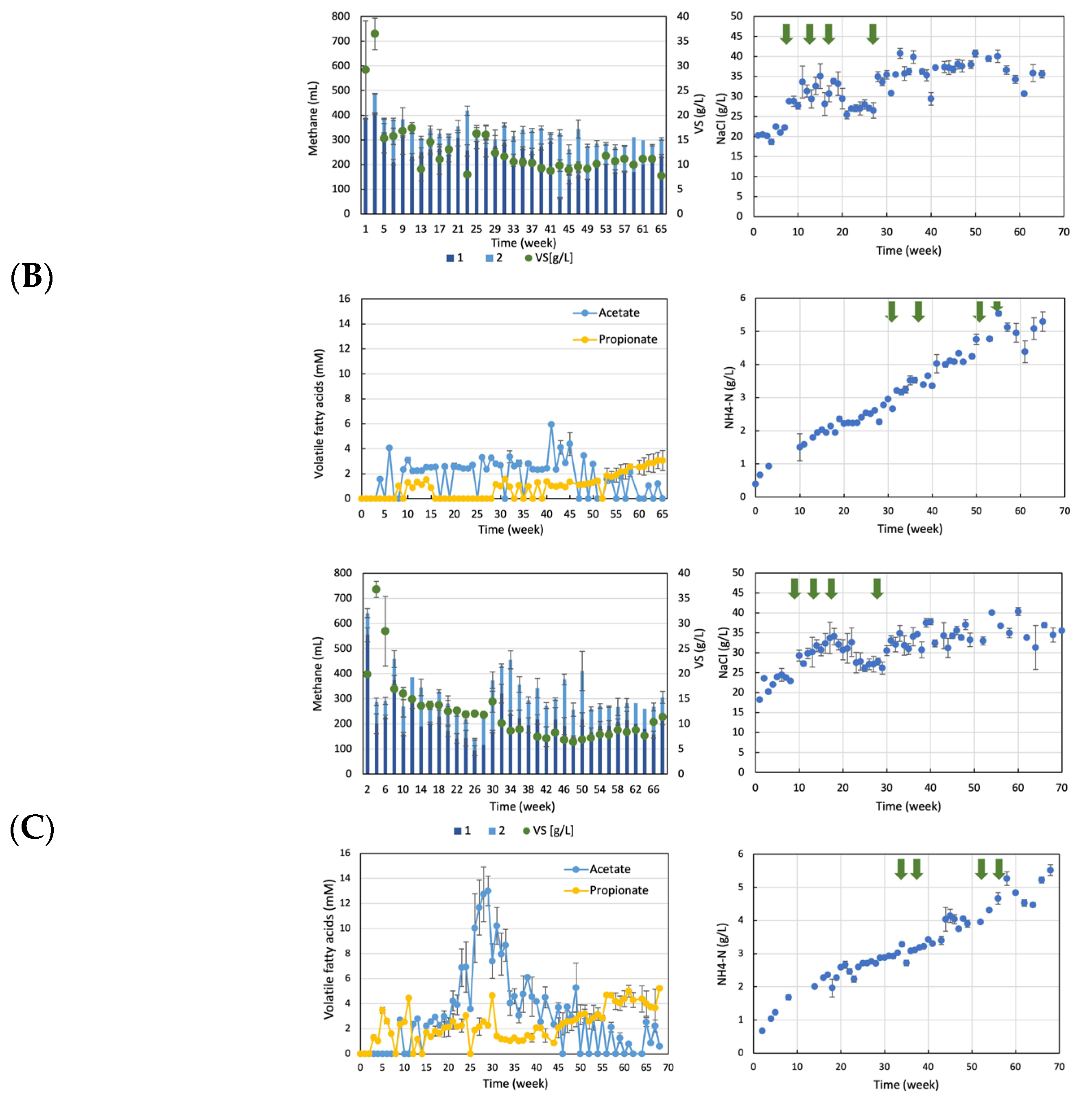
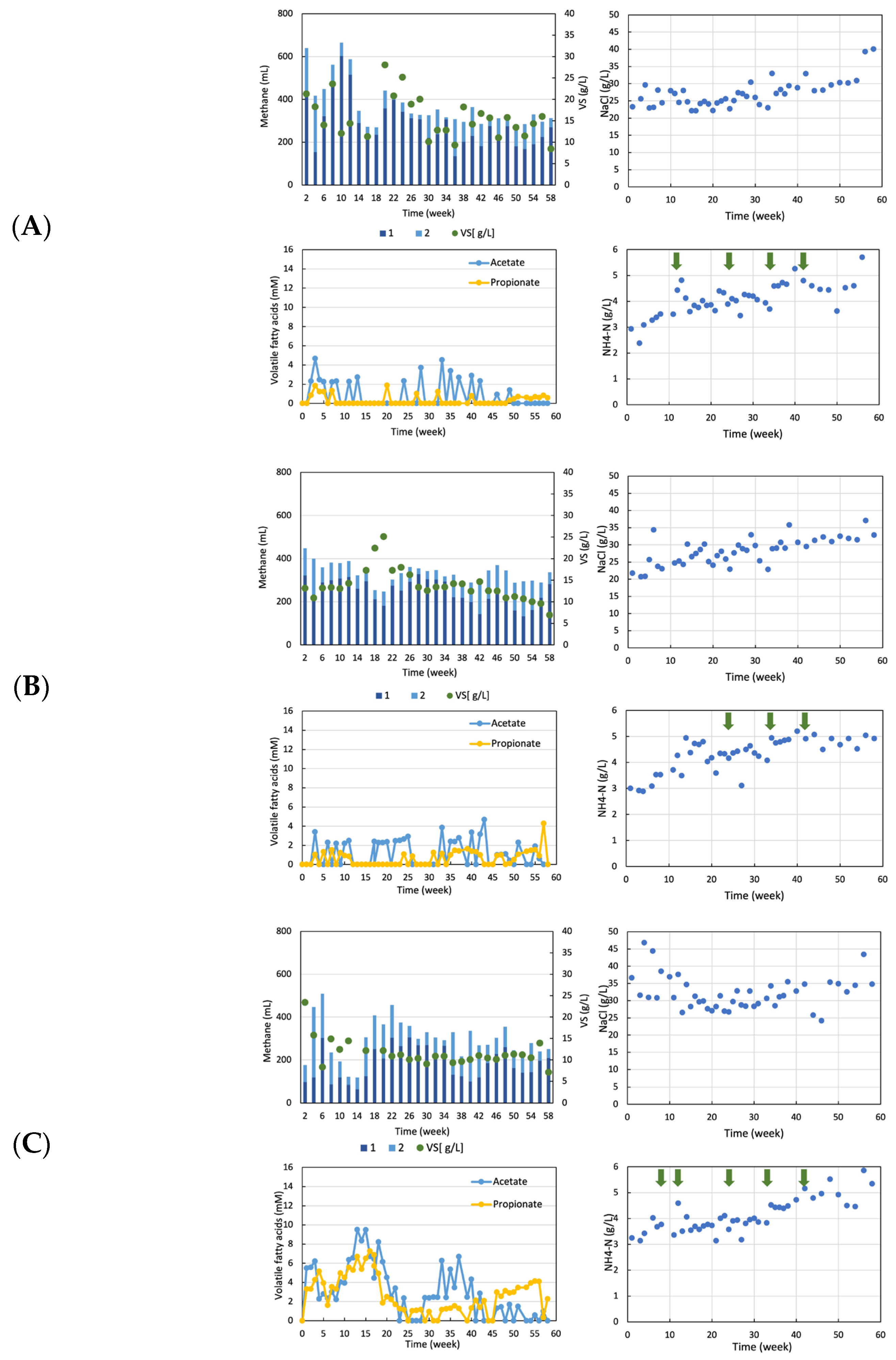
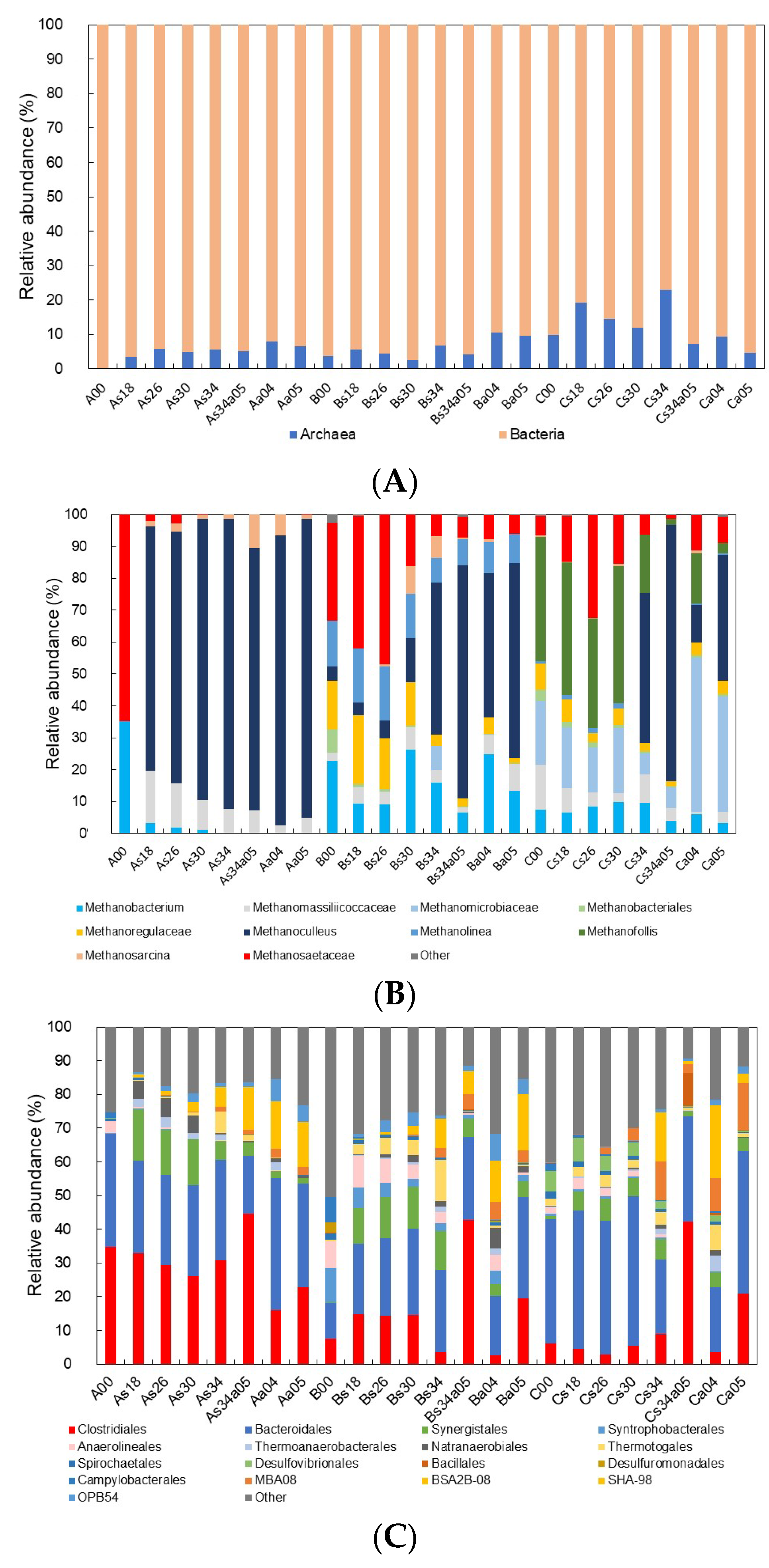
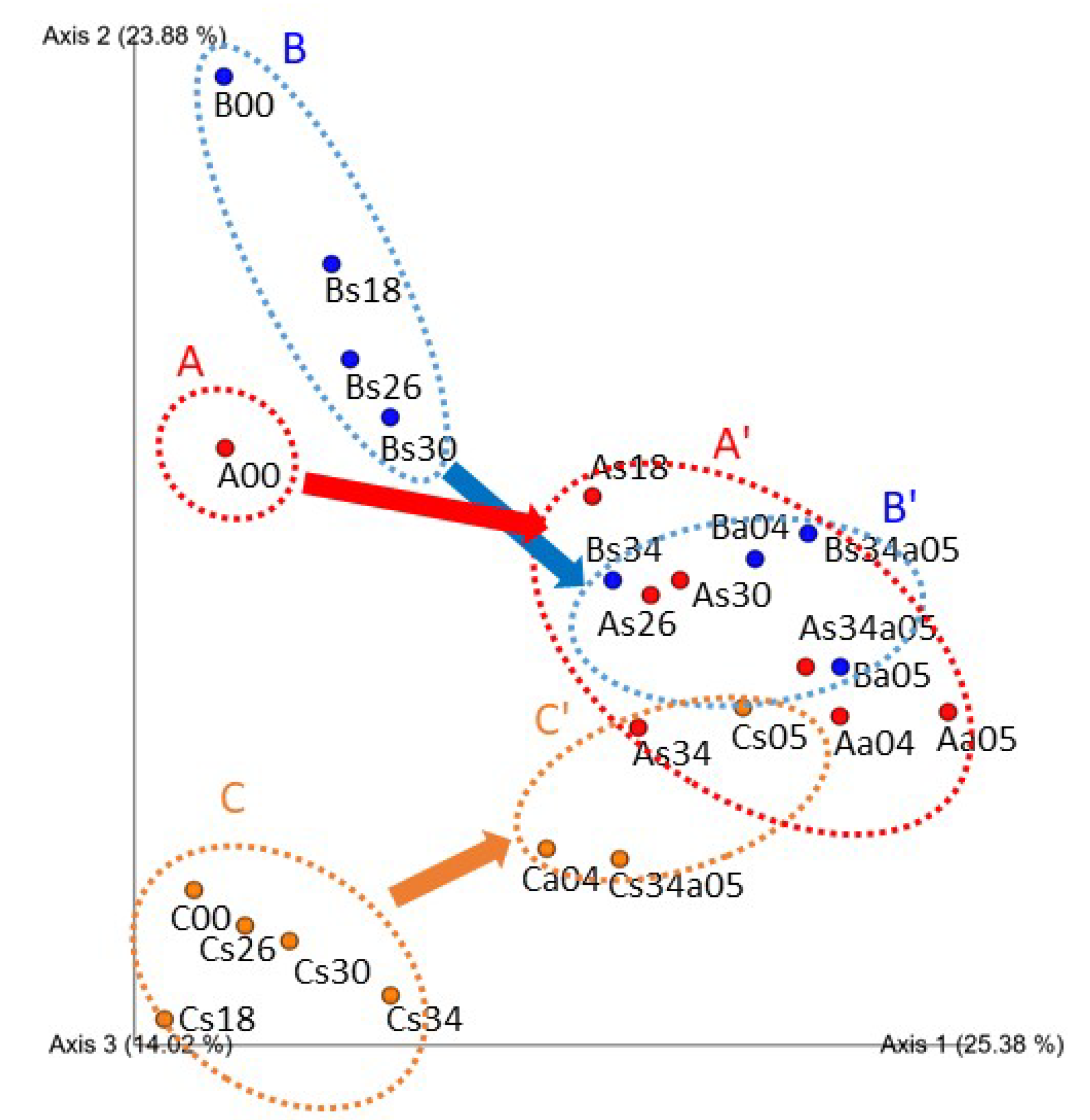
3. Results and Discussion
3.1. Anaerobic Digestion of Sludge from Wastewater Treatment Plants in Food Factories with Ammonia or Salt
3.2. Acclimation Culture for High Concentrations of Ammonia and Salt
3.3. Structure of Bacterial Communities Tolerant to High Concentrations of Ammonia and Salt
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, B.; Chang, J.; Min, Y.; Ge, Y.; Zhu, Q.; Galloway, J.N.; Peng, C. The role of industrial nitrogen in the global nitrogen biogeochemical cycle. Sci. Rep. 2013, 3, 2579. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Sustainability. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F.; Drüke, M.; Fetzer, I.; Bala, G.; von Bloh, W.; et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, R.R.; Nakagawa, K.; Hasegawa, S.; Matsuoka, A.; Kumagai, K.; Yoshioka, T.; Matsuyama, H. Ammoniacal nitrogen concentration by osmotically assisted reverse osmosis. J. Membr. Sci. 2023, 665, 121122. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Nakagawa, K.; Kumagai, K.; Hasegawa, S.; Matsuoka, A.; Li, Z.; Mai, Z.; Yoshioka, T.; Hori, T.; Matsuyama, H. Hybrid osmotically assisted reverse osmosis and reverse osmosis (OARO-RO) process for minimal liquid discharge of high strength nitrogenous wastewater and enrichment of ammoniacal nitrogen. Water Res. 2023, 246, 120716. [Google Scholar] [CrossRef] [PubMed]
- Nitrogen Circular Technologies. Theme 2-1. R&D on Microbial Conversion of Nitrogen Compounds to Ammonia. (English version). 2021. Available online: https://www.n-cycle.jp/en/suiso-en/henkan-en/ (accessed on 27 September 2023).
- Tang, Y.Q.; Shigematsu, T.; Morimura, S.; Kida, K. Dynamics of the microbial community during continuous methane fermentation in continuously stirred tank reactors. J. Biosci. Bioeng. 2015, 119, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Abouelenien, F.; Fujiwara, W.; Namba, Y.; Kosseva, M.; Nishio, N.; Nakashimada, Y. Improved methane fermentation of chicken manure via ammonia removal by biogas recycle. Bioresour. Technol. 2010, 101, 6368–6373. [Google Scholar] [CrossRef]
- Amha, Y.M.; Anwar, M.Z.; Brower, A.; Jacobsen, C.S.; Stadler, L.B.; Webster, T.M.; Smith, A.L. Inhibition of anaerobic digestion processes: Applications of molecular tools. Bioresour. Technol. 2018, 247, 999–1014. [Google Scholar] [CrossRef]
- Tan, L.; Cheng, Q.S.; Sun, Z.Y.; Tang, Y.Q.; Kida, K. Effects of ammonium and/or sulfide on methane production from acetate or propionate using biochemical methane potential tests. J. Biosci. Bioeng. 2019, 127, 345–352. [Google Scholar] [CrossRef]
- Sanjaya, E.H.; Cheng, H.; Li, Y.Y. Mesophilic methane fermentation performance and ammonia inhibition of fish processing wastewater treatment using a self-agitated anaerobic baffled reactor. Bioresour. Technol. 2020, 313, 123644. [Google Scholar] [CrossRef]
- Ye, M.; Zhu, A.; Sun, B.; Qin, Y.; Li, Y.Y. Methanogenic treatment of dairy product wastewater by thermophilic anaerobic membrane bioreactor: Ammonia inhibition and microbial community. Bioresour. Technol. 2022, 357, 127349. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, L.; Li, C.; Angelidaki, I.; Lv, N.; Ning, J.; Cai, G.; Zhu, G. Deep insights into the network of acetate metabolism in anaerobic digestion: Focusing on syntrophic acetate oxidation and homoacetogenesis. Water Res. 2021, 190, 116774. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Dolfing, J.; Schnürer, A. Growth characteristics and thermodynamics of syntrophic acetate oxidizers. Environ. Sci. Technol. 2019, 53, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Kita, A.; Okamura, Y.; Aki, T.; Matsumura, Y.; Tajima, T.; Kato, J.; Nakashimada, Y. Improved methanization and microbial diversity during batch mode cultivation with repetition of substrate addition using defined organic matter and marine sediment inoculum at seawater salinity. Bioresour. Technol. 2017, 245, 833–840. [Google Scholar] [CrossRef]
- Miura, T.; Kita, A.; Okamura, Y.; Aki, T.; Matsumura, Y.; Tajima, T.; Kato, J.; Nakashimada, Y. Effect of salinity on methanogenic propionate degradation by acclimated marine sediment-derived culture. Appl. Biochem. Biotechnol. 2015, 177, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Kita, A.; Okamura, Y.; Aki, T.; Matsumura, Y.; Tajima, T.; Kato, J.; Nakashimada, Y. Improved methane production from brown algae under high salinity by fed-batch acclimation. Bioresour. Technol. 2015, 187, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Duc, L.V.; Nagao, S.; Mojarrad, M.; Miyagawa, Y.; Li, Z.Y.; Inoue, D.; Tajima, T.; Ike, M. Bioaugmentation with marine sediment-derived microbial consortia in mesophilic anaerobic digestion for enhancing methane production under ammonium or salinity stress. Bioresour. Technol. 2023, 376, 128853. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.P.; Loetscher, L.H.; Sharvelle, S.E.; De Long, S.K. Microbial community acclimation enhances waste hydrolysis rates under elevated ammonia and salinity conditions. Bioresour. Technol. 2013, 146, 15–22. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Angelidaki, I. Different cultivation methods to acclimatise ammonia-tolerant methanogenic consortia. Bioresour. Technol. 2017, 232, 1–9. [Google Scholar] [CrossRef]
- Van Lier, J.B.; van der Zee, F.P.; Frijters, C.T.M.J.; Ersahin, M.E. Celebrating 40 years anaerobic sludge bed reactors for industrial wastewater treatment. Rev. Environ. Sci. Biotechnol. 2015, 14, 681–702. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Xu, X.; Zhu, L. Response of methanogenic granules enhanced by magnetite to ammonia stress. Water Res. 2022, 212, 118123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yuan, W.; Dong, Q.; Wu, D.; Yang, P.; Peng, Y.; Li, L.; Peng, X. Integrated multi-omics analyses reveal the key microbial phylotypes affecting anaerobic digestion performance under ammonia stress. Water Res. 2022, 213, 118152. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Treu, L.; Konstantopoulos, K.; Fotidis, I.A.; Angelidaki, I. 16S rRNA gene sequencing and radioisotopic analysis reveal the composition of ammonia acclimatized methanogenic consortia. Bioresour. Technol. 2019, 272, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, P.; Hao, L.; Lü, F.; Shao, L.; Zhang, H. Diverse acetate-oxidizing syntrophs contributing to biogas production from food waste in full-scale anaerobic digesters in China. Renew. Energy 2022, 193, 240–250. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wegner, C.E.; Bei, Q.; Liu, P.; Liesack, W. Metatranscriptomics reveals a differential temperature effect on the structural and functional organization of the anaerobic food web in rice field soil. Microbiome 2018, 6, 169. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Q.; Lu, Y. Inhibitory effects of ammonia on syntrophic propionate oxidation in anaerobic digester sludge. Water Res. 2018, 146, 275–287. [Google Scholar] [CrossRef]
- Wang, H.Z.; Yan, Y.C.; Gou, M.; Yi, Y.; Xia, Z.Y.; Nobu, M.K.; Narihiro, T.; Tang, Y.Q. Response of propionate-degrading methanogenic microbial communities to inhibitory conditions. Appl. Biochem. Biotechnol. 2019, 189, 233–248. [Google Scholar] [CrossRef]
- Cao, L.; Cox, C.D.; He, Q. Patterns of syntrophic interactions in methanogenic conversion of propionate. Appl. Microbiol. Biotechnol. 2021, 105, 8937–8949. [Google Scholar] [CrossRef]
- Dao, H.T.; Kuroda, K.; Nakahara, N.; Danshita, T.; Hatamoto, M.; Yamaguchi, T. 16S rRNA gene-based comprehensive analysis of microbial community compositions in a full-scale leachate treatment system. J. Biosci. Bioeng. 2016, 122, 708–715. [Google Scholar] [CrossRef]
- Hao, L.; Fan, L.; Chapleur, O.; Guenne, A.; Bize, A.; Bureau, C.; Lü, F.; He, P.; Bouchez, T.; Mazéas, L. Gradual development of ammonia-induced syntrophic acetate-oxidizing activities under mesophilic and thermophilic conditions quantitatively tracked using multiple isotopic approaches. Water Res. 2021, 204, 117586. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Ma, J.; Yu, Z.; Wang, Y.; Tang, J. Magnetite accelerates syntrophic acetate oxidation in methanogenic systems with high ammonia concentrations. Microb. Biotechnol. 2018, 11, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Li, M.T.; Rao, L.; Wang, L.; Gou, M.; Sun, Z.Y.; Xia, Z.Y.; Song, W.F.; Tang, Y.Q. Bioaugmentation with syntrophic volatile fatty acids-oxidizing consortia to alleviate the ammonia inhibition in continuously anaerobic digestion of municipal sludge. Chemosphere 2022, 288, 132389. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Mancini, E.; Treu, L.; Angelidaki, I.; Fotidis, I.A. Bioaugmentation strategy for overcoming ammonia inhibition during biomethanation of a protein-rich substrate. Chemosphere 2019, 231, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Xu, X.; Sun, Y.; Xing, T. Bioaugmentation with a propionate-degrading methanogenic culture to improve methane production from chicken manure. Bioresour. Technol. 2022, 346, 126607. [Google Scholar] [CrossRef]
- Yan, M.; Treu, L.; Campanaro, S.; Tian, H.; Zhu, X.; Khoshnevisan, B.; Tsapekos, P.; Angelidaki, I.; Fotidis, I.A. Effect of ammonia on anaerobic digestion of municipal solid waste: Inhibitory performance, bioaugmentation and microbiome functional reconstruction. Chem. Eng. J. 2020, 401, 126159. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajima, T.; Kawaguchi, S.; Matsutani, T.; Hida, A.; Kato, J. Acclimation of Microbial Consortia to Ammonia and Salt in Methane Fermentation. Fermentation 2024, 10, 98. https://doi.org/10.3390/fermentation10020098
Tajima T, Kawaguchi S, Matsutani T, Hida A, Kato J. Acclimation of Microbial Consortia to Ammonia and Salt in Methane Fermentation. Fermentation. 2024; 10(2):98. https://doi.org/10.3390/fermentation10020098
Chicago/Turabian StyleTajima, Takahisa, Shiina Kawaguchi, Tomoka Matsutani, Akiko Hida, and Junichi Kato. 2024. "Acclimation of Microbial Consortia to Ammonia and Salt in Methane Fermentation" Fermentation 10, no. 2: 98. https://doi.org/10.3390/fermentation10020098
APA StyleTajima, T., Kawaguchi, S., Matsutani, T., Hida, A., & Kato, J. (2024). Acclimation of Microbial Consortia to Ammonia and Salt in Methane Fermentation. Fermentation, 10(2), 98. https://doi.org/10.3390/fermentation10020098





