Application of Fermentation as a Strategy for the Transformation and Valorization of Vegetable Matrices
Abstract
1. Introduction
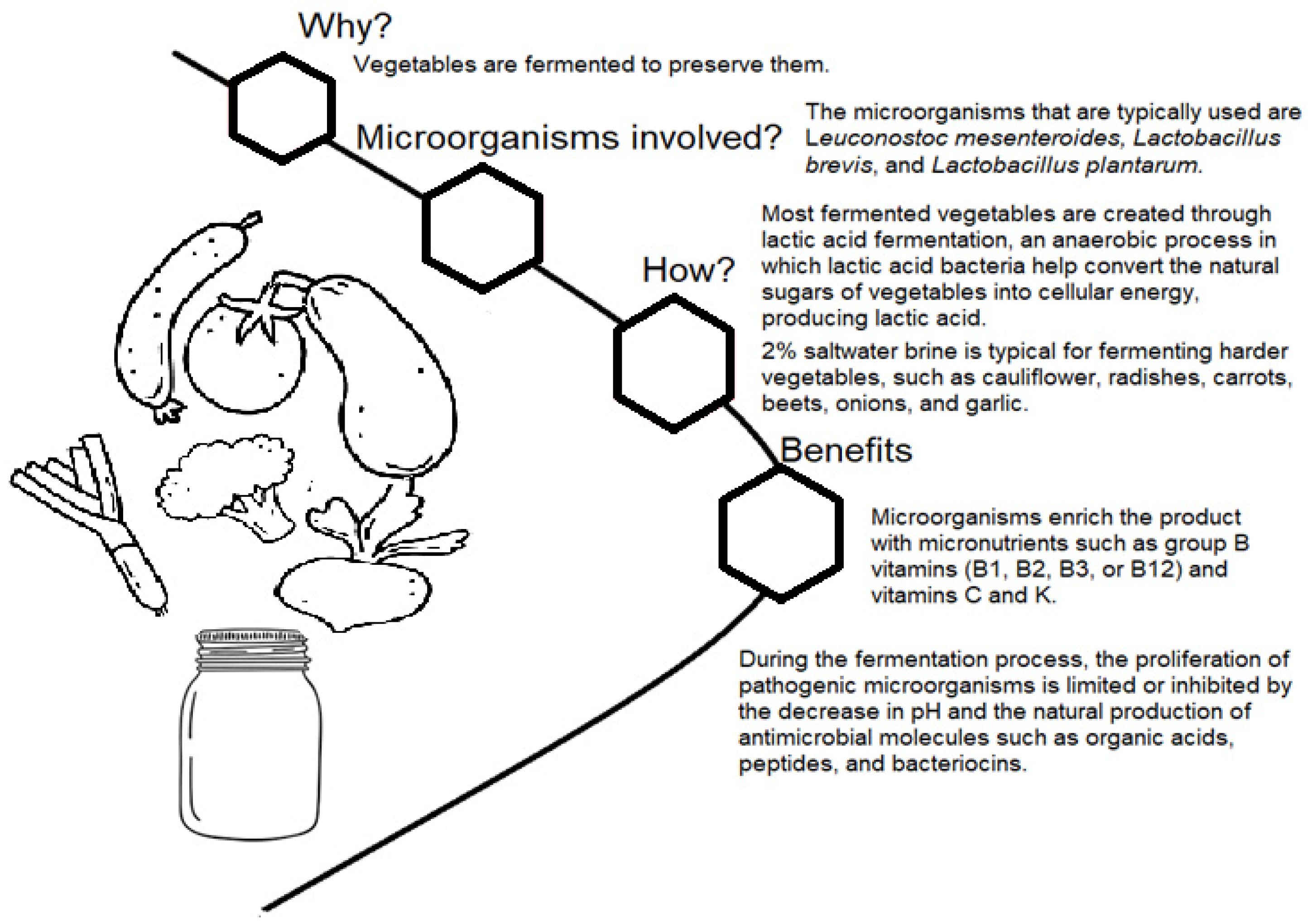
2. Fermentation of Vegetables to Increase Shelf Life
2.1. Vegetable Fermentation
2.2. Fermentation of Vegetables as an Alternative Preservation and Added Value
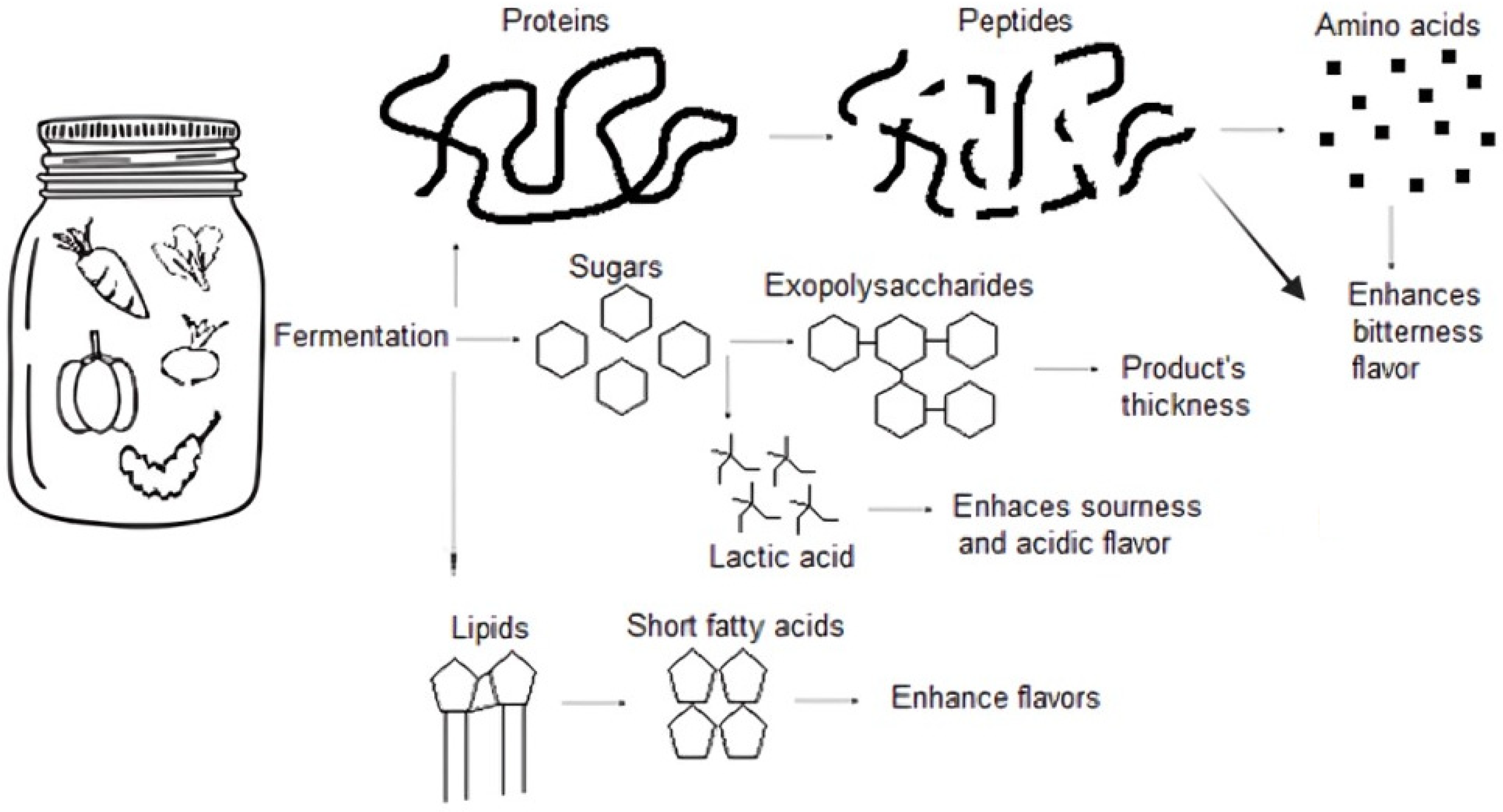
2.3. The Sensory Effect of Fermentation on Plant-Based Products
3. Fermentation of Vegetables to Increase Functional Properties
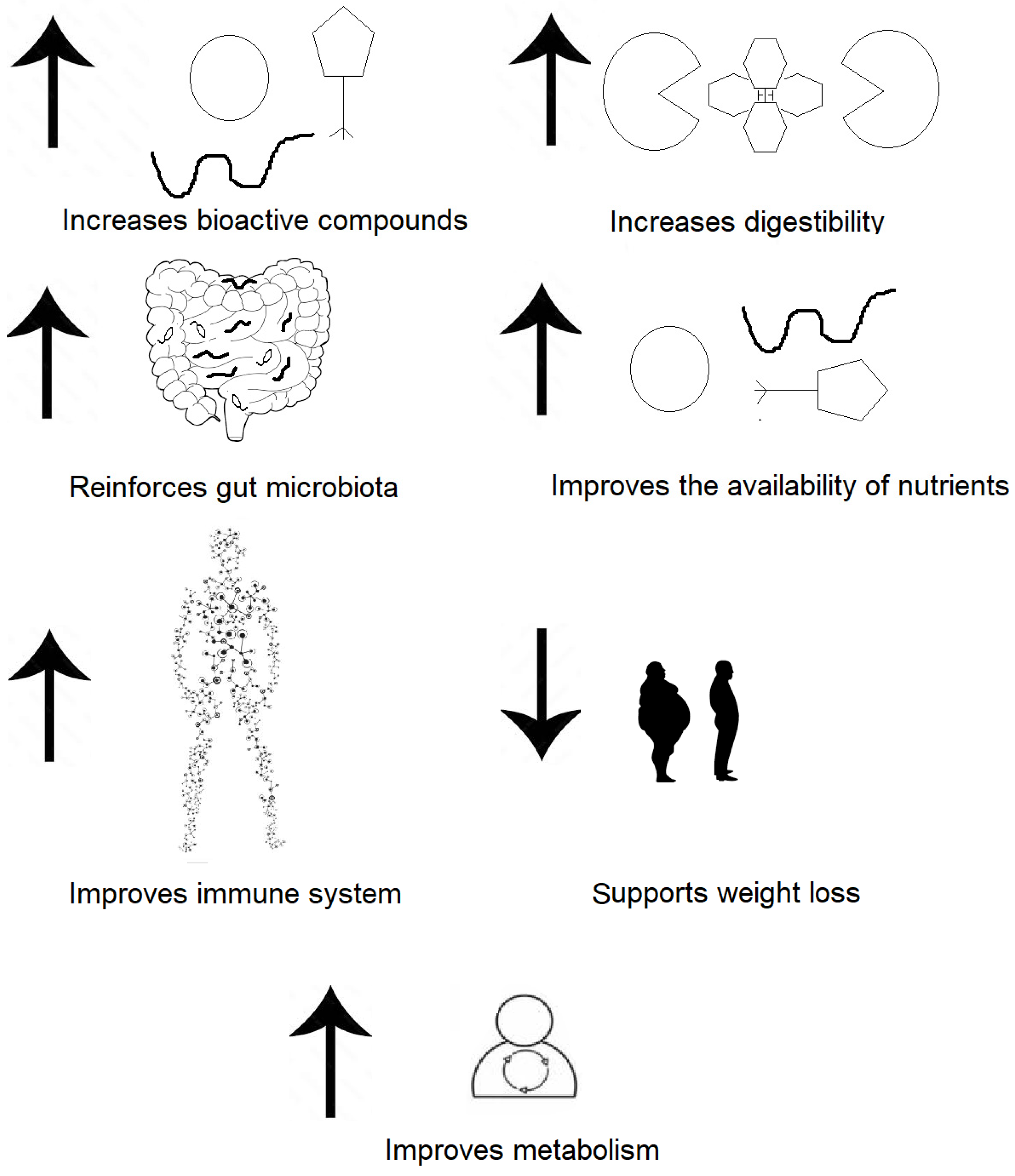
3.1. The Importance of Fermented Products for Consumer Health
3.2. Health Benefits of Fermented Vegetables
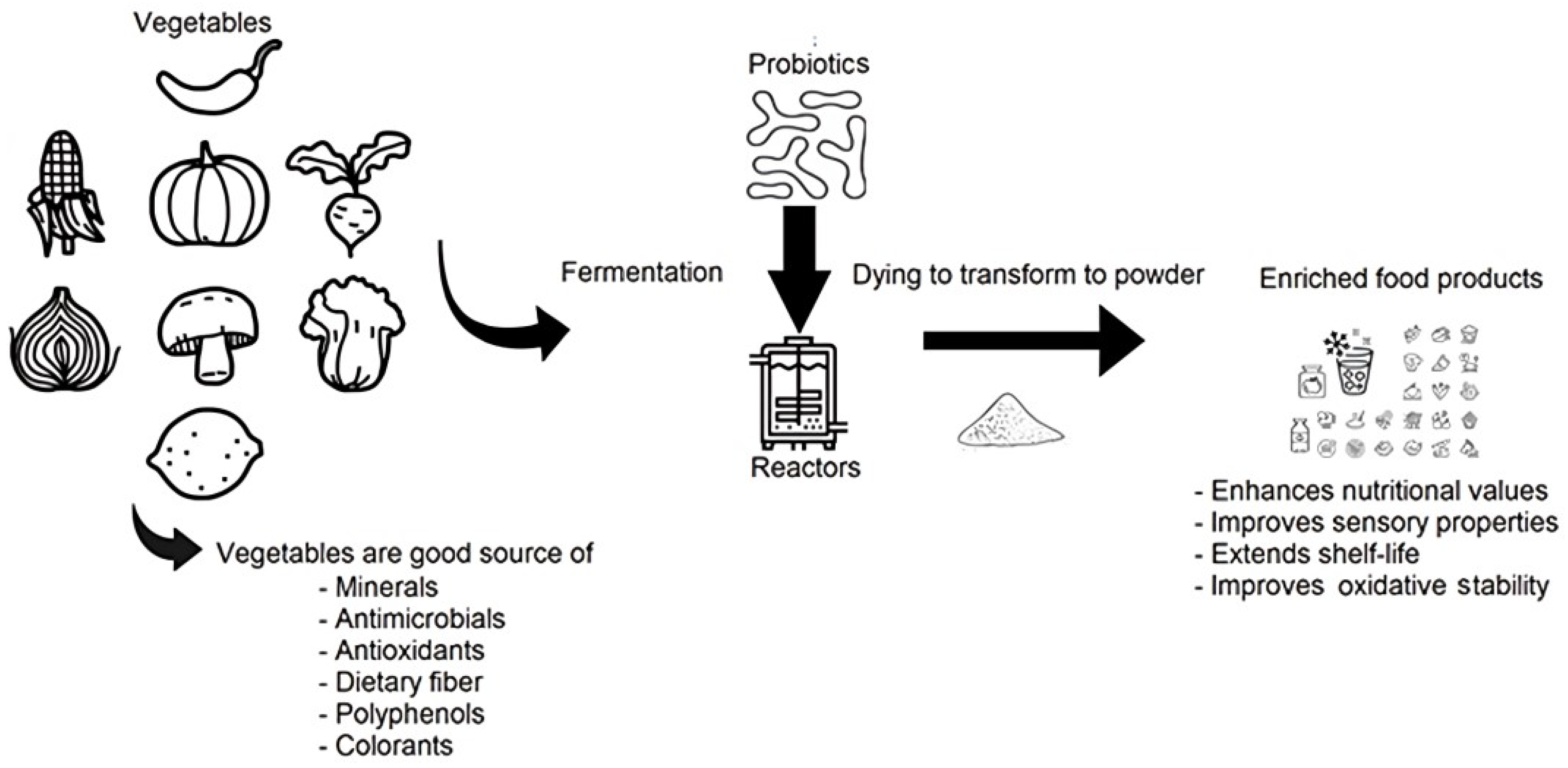
3.3. Solid-State Vegetable Fermentation for Improvement of the Nutritional and Functional Profile
3.4. Solid-State Vegetable Fermentation to Obtain Extracts with Antioxidant Properties
3.5. Solid-State Vegetable Fermentation to Obtain Enzymes and Other Metabolites of Interest
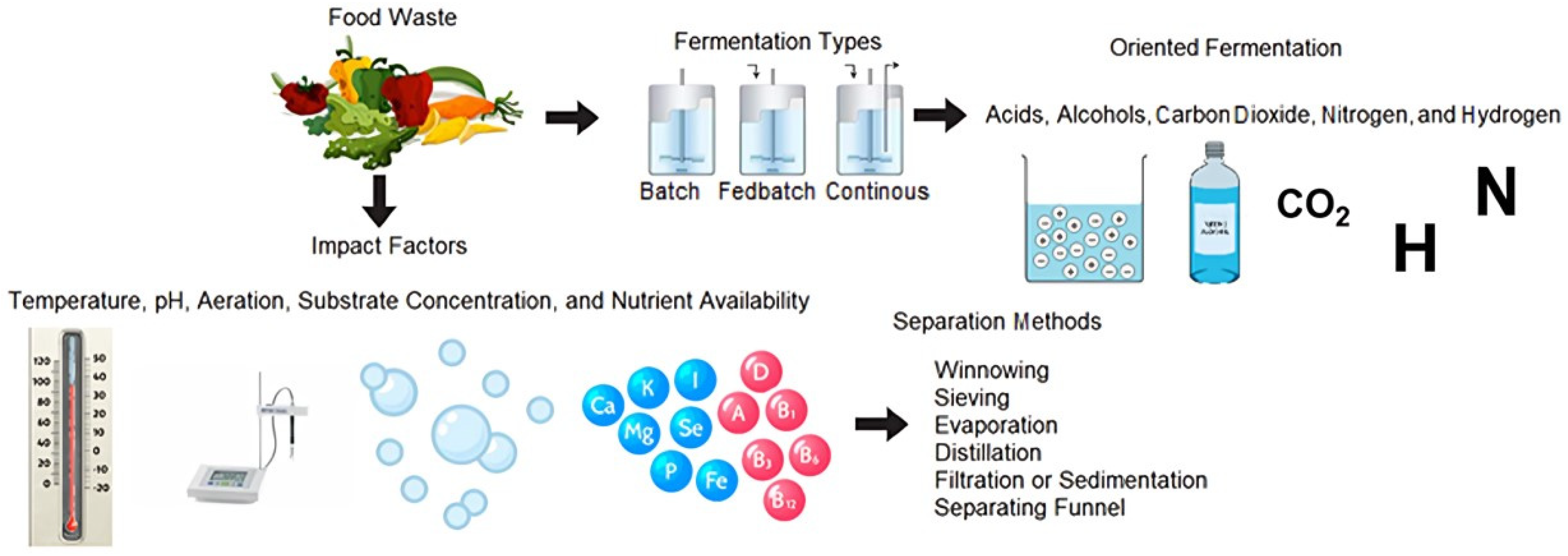
4. Vegetable Fermentation as a Sustainability Strategy
4.1. Parameters of Relevance in Vegetable Fermentation
4.1.1. pH
4.1.2. Carbon Source
4.1.3. Nitrogen Source
4.1.4. Temperature
4.1.5. Cell Density
4.2. Fermentation Method
- Cell immobilization and recirculation: LAB cells can be recirculated or immobilized on solid supports in different modes to increase cell density. However, this has kept lactic acid yield and productivity the same. On the other hand, the recirculation of cells has given higher concentrations of lactic acid and equal or greater yields [106].
- Fed-batch fermentations: The batch process consists of carrying out an initial feeding with all the nutrients necessary for the microorganism inside the reactor so that it reproduces exponentially until the substrate is exhausted. Subsequently, the fermentation reaches the stationary. In contrast, the fed-batch methodology supplies nutrients to the microorganism during fermentation without removing the spent medium. This feed stream can be supplied continuously, with variable or constant flows, or discontinuously in pulses [112].
4.3. Microencapsulation in Improving the Viability of Microorganisms in the Development of Products in Matrices of Plant Origin
5. Trends and Challenges in Vegetable-Fermented Products
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.C.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef]
- Torres-Sánchez, R.; Martínez-Zafra, M.T.; Castillejo, N.; Guillamón-Frutos, A.; Artés-Hernández, F. Real-time monitoring system for shelf life estimation of fruit and vegetables. Sensors 2020, 20, 1860. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Szutowska, J. Functional properties of lactic acid bacteria in fermented fruit and vegetable juices: A systematic literature review. Eur. Food Res. Technol. 2020, 246, 357–372. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Peréz-Dıaz, I.M.; Breidt, F.; Buescher, R.W.; Arroyo-López, F.N.; Jiménez-Dıaz, R.; Garrido-Fernández, A.; Johanningsmeire, S. Fermented and acidified vegetables. In Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association: Washington, DC, USA, 2013; pp. 521–532. [Google Scholar]
- Das, R.; Pandey, H.; Das, B.; Sarkar, S. Fermentation and its application in vegetable preservation: A review. Int. J. Food Ferment. Technol. 2016, 6, 207–217. [Google Scholar] [CrossRef]
- Swain, M.R.; Anandharaj, M.; Ray, R.C.; Rani, R.P. Fermented fruits and vegetables of Asia: A potential source of probiotics. Biotechnol. Res. Int. 2014, 2014, 250424. [Google Scholar] [CrossRef] [PubMed]
- Divya, J.B.; Varsha, K.K.; Nampoothiri, K.M. Newly isolated lactic acid bacteria with probiotic features for potential application in food industry. Appl. Biochem. Biotechnol. 2012, 167, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Mora-Adames, W.I.; Fuenmayor, C.A.; Benavides-Martín, M.A.; Algecira-Enciso, N.A.; Quicazán, M.C. Bee pollen as a novel substrate in pilot-scale probiotic-mediated lactic fermentation processes. Lebensm.-Wiss. Technol. 2021, 141, 110868. [Google Scholar] [CrossRef]
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One health, fermented foods, and gut microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef]
- Zabat, M.A.; Sano, W.H.; Wurster, J.I.; Cabral, D.J.; Belenky, P. Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, S.H.; Jeon, C.O. Microbial community dynamics during fermentation of doenjang-meju, traditional Korean fermented soybean. Int. J. Food Microbiol. 2014, 185, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Tamang, B. Role of Lactic Acid Bacteria in Fermentation and Biopreservation of Traditional Vegetable Products. Ph.D. Dissertation, University of North Bengal, West Bengal, India, 2006. [Google Scholar]
- Sonar, N.R.; Halami, P.M. Phenotypic identification and technological attributes of native lactic acid bacteria present in fermented bamboo shoot products from North-East India. J. Food Sci. Technol. 2014, 51, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Pontonio, E.; Buchin, S.; De Angelis, M.; Lattanzi, A.; Valerio, F.; Gobbeti, M.; Calasso, M. Diversity of the lactic acid bacterium and yeast microbiota in the switch from firm-to liquid-sourdough fermentation. Appl. Environ. Microbiol. 2014, 80, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.H.; Cho, Y.M.; Noh, G.M.; Om, A.S. Cancer preventive potential of kimchi lactic acid bacteria (Weissella cibaria, Lactobacillus plantarum). J. Cancer Prev. 2014, 19, 253–258. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Siragusa, S.; De Angelis, M.; Paradiso, A.; Minervini, F.; De Gara, L.; Gobbetti, M. Selection and use of autochthonous mixed starter for lactic acid fermentation of carrots, French beans or marrows. Int. J. Food Microbiol. 2008, 127, 220–228. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Sada, A.; Orlando, P. Fermentative ability of alginate-prebiotic encapsulated Lactobacillus acidophilus and survival under simulated gastrointestinal conditions. J. Funct. Foods 2009, 1, 319–323. [Google Scholar] [CrossRef]
- Filannino, P.; Cardinali, G.; Rizzello, C.G.; Buchin, S.; De Angelis, M.; Gobbetti, M.; Di Cagno, R. Metabolic responses of Lactobacillus plantarum strains during fermentation and storage of vegetable and fruit juices. Appl. Environ. Microbiol. 2014, 80, 2206–2215. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Tabanelli, G.; Montanari, C.; Gardini, F.; Lanciotti, R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiol. 2015, 47, 74–84. [Google Scholar] [CrossRef]
- Di Cagno, R.; Surico, R.F.; Minervini, G.; De Angelis, M.; Rizzello, C.G.; Gobbetti, M. Use of autochthonous starters to ferment red and yellow peppers (Capsicum annum L.) to be stored at room temperature. Int. J. Food Microbiol. 2009, 130, 108–116. [Google Scholar] [CrossRef]
- Caplice, E.; Fitzgerald, G.F. Food fermentations: Role of microorganisms in food production and preservation. Int. J. Food Microbiol. 1999, 50, 131–149. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic fermented fruit or vegetable juices: Past, present and future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Monika, K.; Malik, T.; Gehlot, R.; Rekha, K.; Kumari, A.; Sindhu, R.; Rohilla, P. Antimicrobial property of probiotics. Environ. Conserv. J. 2021, 22, 33–48. [Google Scholar]
- Lillo-Pérez, S.; Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Probiotics in fruit and vegetable matrices: Opportunities for nondairy consumers. Lebensm.-Wiss. Technol. 2021, 151, 112106. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Monnin, L.; Zheng, J.; Zhang, L.; Coton, M.; Sicard, D.; Walter, J. Starter Culture Development and Innovation for Novel Fermented Foods. Annu. Rev. Food Sci. Technol. 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cui, Y.; Qu, X. Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch. Microbiol. 2018, 200, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Pobiega, K.; Piwowarek, K.; Kot, A.M. Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules 2021, 26, 1858. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.G.R.; Gasga, V.M.Z.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Burgos, J.A.S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Premi, M.; Khan, K.A. Antioxidants in Fruits and Vegetables: Role in the Prevention of Degenerative Diseases. In Processing of Fruits and Vegetables: From Farm to Fork; Apple Academic Press: Oakville, ON, Canada, 2019; pp. 3–22. [Google Scholar]
- Thakur, A.; Sharma, R. Health promoting phytochemicals in vegetables: A mini review. Int. J. Food Ferment. Technol. 2018, 8, 107–117. [Google Scholar] [CrossRef]
- Luckow, T.; Sheehan, V.; Delahunty, C.; Fitzgerald, G. Determining the odor and flavor characteristics of probiotic, health-promoting ingredients and the effects of repeated exposure on consumer acceptance. J. Food Sci. 2005, 70, S53–S59. [Google Scholar] [CrossRef]
- Braga, H.F.; Conti-Silva, A.C. Papaya nectar formulated with prebiotics: Chemical characterization and sensory acceptability. LWT-Food Sci. Technol. 2015, 62, 854–860. [Google Scholar] [CrossRef]
- Fleming, H.P.; Etchells, J.L.; Bell, T.A. Vapor analysis of fermented Spanish-type green olives by gas chromatography. J. Food Sci. 1969, 34, 419–422. [Google Scholar] [CrossRef]
- Aurand, L.W.; Singleton, J.A.; Bell, T.A.; Etchells, J.L. Identification of Volatile Constituents from Pure-Culture Fermentations of Brined Cucumbers. J. Food Sci. 1965, 30, 288–295. [Google Scholar] [CrossRef]
- Karki, T.; Okada, S.; Baba, T.; Itoh, H.; Kozaki, M. Studies on the Microflora of Nepalese Pickles Gundruk Studies on “Microorganisms and their Role in Gundruk Fermentation” Part I. Nippon. Shokuhin Kogyo Gakkaishi 1983, 30, 357–367. [Google Scholar] [CrossRef]
- Thompson, R.L.; Fleming, H.P.; Hamann, D.D.; Monroe, R.J. Method for Determination of Firmness in Cucumber Slices 1. J. Texture Stud. 1982, 13, 311–324. [Google Scholar] [CrossRef]
- Gil-Pena, M.L.; Sardinero, E.; Garcia-Serrano, P.; Schnabel, I.; Garrido, J. Continuous production of volatile fatty acids by acidogenesis of sugar beet vinasse. Environ. Technol. 1986, 7, 479–486. [Google Scholar] [CrossRef]
- McFeeters, R.F.; Fleming, H.P. pH effect on calcium inhibition of softening of cucumber mesocarp tissue. J. Food Sci. 1991, 56, 730–732. [Google Scholar] [CrossRef]
- Etchells, J.L.; Borg, A.F.; Kittel, I.D.; Bell, T.A.; Fleming, H.P. Pure culture fermentation of green olives. Appl. Microbiol. 1966, 14, 1027–1041. [Google Scholar] [CrossRef]
- Jeon, I.J.; Breene, W.M.; Munson, S.T. Texture of cucumbers: Correlation of instrumental and sensory measurements. J. Food Sci. 1973, 38, 334–337. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.I.; Garrido-Fernandez, J. Chlorophyll and carotenoid presence in olive fruit (Olea europaea). J. Agric. Food Chem. 1989, 37, 1–7. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martínez-Avila, G.; Montañez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Cetin, I.; Koletzko, B.; Moreno, L.A.; Matthys, C. Relevance of European alignment for micronutrients’ recommendation regarding pregnant and lactating women, infants, children and adolescents: An insight into preliminary steps of EURRECA. Matern. Child Nutr. 2010, 6 (Suppl. S2), 3–4. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Vijayendra, S.V.N.; Halami, P.M. Health benefits of fermented vegetable products. In Health Benefits of Fermented Foods and Beverages; Prakash Tamang, J., Ed.; Taylor & Francis Group: Boca Raton, FL, USA; London, UK, 2015; pp. 297–324. [Google Scholar]
- Krishna, C. Solid-state fermentation systems—An overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzmán, N.; Ascacio-Valdés, J.; Serna-Cock, L.; dos Santos Correia, M.T.; Contreras-Esquivel, J.C.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger to enhance the phenolic contents and antioxidative activity of Mexican mango seed: A promising source of natural antioxidants. Lebensm.-Wiss. Technol. 2019, 112, 108236. [Google Scholar] [CrossRef]
- Subramaniyam, R.; Vimala, R. Solid state and submerged fermentation for the production of bioactive substances: A comparative study. Int. J. Sci. Nat. 2012, 3, 480–486. [Google Scholar]
- Lizardi-Jiménez, M.A.; Hernández-Martínez, R. Solid state fermentation (SSF): Diversity of applications to valorize waste and biomass. 3 Biotech 2017, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, L. Fundamentos de la fermentación en estado sólido y aplicación a la industria alimentaria. Ciencia y tecnología alimentaria 1996, 1, 4–12. [Google Scholar] [CrossRef]
- Gulsunoglu, Z.; Purves, R.; Karbancioglu-Guler, F.; Kilic-Akyilmaz, M. Enhancement of phenolic antioxidants in industrial apple waste by fermentation with Aspergillus spp. Biocatal. Agric. Biotechnol. 2020, 25, 101562. [Google Scholar] [CrossRef]
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka and their antioxidant activity. J. Funct. Foods 2017, 32, 185–194. [Google Scholar] [CrossRef]
- Bier, M.C.J.; Medeiros, A.B.P.; De Kimpe, N.; Soccol, C.R. Evaluation of antioxidant activity of the fermented product from the biotransformation of R-(+)-limonene in solid-state fermentation of orange waste by Diaporthe sp. Biotechnol. Res. Innov. 2019, 3, 168–176. [Google Scholar] [CrossRef]
- Chi, C.H.; Cho, S.J. Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. and Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2016, 68, 619–625. [Google Scholar] [CrossRef]
- Alejando-Paredes, L.; Flores-Fernández, C.N.; Zavaleta, A.I. Optimización del medio para la producción de proteasas extracelulares por Pseudomonas sp. M211 en fermentación sumergida. Rev. Soc. Química Perú 2017, 83, 449–462. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- El-Katony, T.M.; Nour El-Dein, M.M.; El-Fallal, A.A.; Ibrahim, N.G.; Mousa, M.M. Substrate–fungus interaction on the enzymatic and non-enzymatic antioxidant activities of solid state fermentation system. Bioresour. Bioprocess. 2020, 7, 28. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Sousa, B.A.; Correia, R.T.P. Phenolic content, antioxidant activity and antiamylolytic activity of extracts obtained from bioprocessed pineapple and guava wastes. Braz. J. Chem. Eng. 2012, 29, 25–30. [Google Scholar] [CrossRef]
- Santos, V.A.Q.; Nascimento, C.G.; Schmidt, C.A.; Mantovani, D.; Dekker, R.F.; da Cunha, M.A.A. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. Lebensm.-Wiss. Technol. 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Correa Deza, M.A.; Rodríguez de Olmos, A.; Selva Garro, M. Fermentación en sustrato sólido utilizando cultivos lácticos para la obtención de productos vegetales bioenriquecidos con isoflavonas agliconas. Rev. Argent. Microbiol. 2019, 51, 201–207. [Google Scholar] [PubMed]
- de Olmos, A.R.; Garro, M.S. Metabolic profile of Lactobacillus paracasei subsp. paracasei CRL 207 in solid state fermentation using commercial soybean meal. Food Biosci. 2020, 35, 100584. [Google Scholar]
- Cobaxin Márquez, M.J. Mejoramiento de las Características Funcionales de una Pasta de Soya Mediante Fermentación. Ph.D. Dissertation, Universidad Veracruzana, Instituto de Ciencias Básicas, Xalapa, Mexico, 2011. [Google Scholar]
- Xing, Q.; Dekker, S.; Kyriakopoulou, K.; Boom, R.M.; Smid, E.J.; Schutyser, M.A. Enhanced nutritional value of chickpea protein concentrate by dry separation and solid state fermentation. Innov. Food Sci. Emerg. Technol. 2020, 59, 102269. [Google Scholar] [CrossRef]
- Muniz, C.E.S.; Santiago, Â.M.; Gusmão, T.A.S.; Oliveira, H.M.L.; de Sousa Conrado, L.; de Gusmão, R.P. Solid-state fermentation for single-cell protein enrichment of guava and cashew by-products and inclusion on cereal bars. Biocatal. Agric. Biotechnol. 2020, 25, 101576. [Google Scholar] [CrossRef]
- Rompato, K.M. Protein enrichment of fruit processing byproducts using solid state fermentation with Saccharomyces cerevisiae and Bacillus subtilis. Biotecnol. Apl. 2015, 32, 4221–4227. [Google Scholar]
- Chandra, P.; Arora, D.S. Production of antioxidant bioactive phenolic compounds by solid-state fermentation on agro-residues using various fungi isolated from soil. Asian J. Biotechnol. 2016, 8, 8–15. [Google Scholar] [CrossRef]
- Sadh, P.K.; Saharan, P.; Duhan, J.S. Bioaugmentation of phenolics and antioxidant activity of Oryza sativa by solid state fermentation using Aspergillus spp. Int. Food Res. J. 2017, 24, 1160–1166. [Google Scholar]
- Santos, T.R.J.; Vasconcelos, A.G.S.; de Aquino Santana, L.C.L.; Gualberto, N.C.; Feitosa, P.R.B.; de Siqueira, A.C.P. Solid-state fermentation as a tool to enhance the polyphenolic compound contents of acidic Tamarindus indica by-products. Biocatal. Agric. Biotechnol. 2020, 30, 101851. [Google Scholar]
- Torino, M.I.; Limón, R.I.; Martínez-Villaluenga, C.; Mäkinen, S.; Pihlanto, A.; Vidal-Valverde, C.; Frias, J. Antioxidant and antihypertensive properties of liquid and solid state fermented lentils. Food Chem. 2013, 136, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Altop, A.; Güngör, E.; Erener, G. Aspergillus niger may improve nutritional quality of grape seed and its usability in animal nutrition through solid-state fermentation. Int. Adv. Res. Eng. J. 2018, 2, 273–277. [Google Scholar]
- Zambrano, C.; Kotogán, A.; Bencsik, O.; Papp, T.; Vágvölgyi, C.; Mondal, K.C.; Krisch, J.; Takó, M. Mobilization of phenolic antioxidants from grape, apple and pitahaya residues via solid state fungal fermentation and carbohydrase treatment. Lebensm.-Wiss. Technol. 2018, 89, 457–465. [Google Scholar] [CrossRef]
- Rashad, M.M.; Mahmoud, A.E.; Ali, M.M.; Nooman, M.U.; Al-Kashef, A.S. Antioxidant and anticancer agents produced from pineapple waste by solid state fermentation. Int. J. Toxicol. Pharmacol. Res. 2015, 7, 287–296. [Google Scholar]
- López, T.; Prado-Barragán, A.; Nevárez-Moorillón, G.V.; Contreras, J.C.; Rodríguez, R.; Aguilar, C.N. Incremento de la capacidad antioxidante de extractos de pulpa de café por fermentación láctica en medio sólido. J. Food CyTA 2013, 11, 359–365. [Google Scholar] [CrossRef]
- Magro, A.E.A.; de Castro, R.J.S. Effects of solid-state fermentation and extraction solvents on the antioxidant properties of lentils. Biocatal. Agric. Biotechnol. 2020, 28, 101753. [Google Scholar]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Budihal, S.R.; Agsar, D. Exploration of agrowastes for the production of cellulase by Streptomyces DSK29 under submerged and solid state systems. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 681–689. [Google Scholar]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Bioproduction of 2-phenylethanol and 2-phenethyl acetate by Kluyveromyces marxianus through the solid-state fermentation of sugarcane bagasse. Appl. Microbiol. Biotechnol. 2018, 102, 4703–4716. [Google Scholar] [CrossRef]
- Thakur, S.A.; Nemade, S.N.; Sharanappa, A. Solid state fermentation of overheated soybean meal (waste) for production of protease using Aspergillus oryzae. IJIRSET 2015, 4, 18456–18461. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Ohara, A.; Nishide, T.G.; Bagagli, M.P.; Dias, F.F.G.; Sato, H.H. A versatile system based on substrate formulation using agroindustrial wastes for protease production by Aspergillus niger under solid state fermentation. Biocatal. Agric. Biotechnol. 2015, 4, 678–684. [Google Scholar] [CrossRef]
- da Silva, L.C.; Honorato, T.L.; Franco, T.T.; Rodrigues, S. Optimization of chitosanase production by Trichoderma koningii sp. under solid-state fermentation. Food Sci. Biotechnol. 2012, 5, 1564–1572. [Google Scholar] [CrossRef]
- Veana, F.; Martínez-Hernández, J.L.; Aguilar, C.N.; Rodríguez-Herrera, R.; Michelena, G. Utilization of molasses and sugar cane bagasse for production of fungal invertase in solid state fermentation using Aspergillus niger GH1. Braz. J. Microbiol. 2014, 45, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Palomino García, L.R.; Biasetto, C.R.; Araujo, A.R.; Del Bianchi, V.L. Enhanced extraction of phenolic compounds from coffee industry’s residues through solid state fermentation by Penicillium purpurogenum. Food Sci. Technol. 2015, 35, 704–711. [Google Scholar] [CrossRef]
- Badui, S. Química de los Alimentos, 4th ed.; Pearson Educación: Upper Saddle River, NJ, USA, 2006; Available online: https://itscv.edu.ec/wp-content/uploads/2019/06/QUIMICA-DE-LOS-ALIMENTOS-4ta-Edicion.pdf (accessed on 31 December 2023).
- López-Cárdenas, F.; Ochoa-Reyes, E.; Baeza-Jiménez, R.; Tafolla-Arellano, J.C.; Ascacio-Valdés, J.A.; Buenrostro-Figueroa, J.J. Solid-State Fermentation as a Sustainable Tool for Extracting Phenolic Compounds from Cascalote Pods. Fermentation 2023, 9, 823. [Google Scholar] [CrossRef]
- Naik, B.; Goyal, S.K.; Tripathi, A.D.; Kumar, V. Screening of agro-industrial waste and physical factors for the optimum production of pullulanase in solid-state fermentation from endophytic Aspergillus sp. Biocatal. Agric. Biotechnol. 2019, 22, 101423. [Google Scholar] [CrossRef]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Valorization of sugarcane bagasse and sugar beet molasses using Kluyveromyces marxianus for producing value-added aroma compounds via solid-state fermentation. J. Clean. Prod. 2017, 158, 8–17. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef]
- Dursun, D.; Dalgıç, A.C. Optimization of astaxanthin pigment bioprocessing by four different yeast species using wheat wastes. Biocatal. Agric. Biotechnol. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Ali, S.R.; Anwar, Z.; Irshad, M.; Mukhtar, S.; Warraich, N.T. Bio-synthesis of citric acid from single and co-culture-based fermentation technology using agro-wastes. Radiat. Res. Appl. Sci. 2016, 9, 57–62. [Google Scholar] [CrossRef]
- Zahan, K.A.; Ismail, N.S.; Leong, C.R.; Ab Rashid, S.; Tong, W.Y. Monascorubin production by Penicillium minioluteum ED24 in a solid-state fermentation using sesame seed cake as substrate. Mater. Today Proc. 2020, 31, 127–135. [Google Scholar] [CrossRef]
- Čertík, M.; Adamechová, Z.; Guothová, L. Simultaneous enrichment of cereals with polyunsaturated fatty acids and pigments by fungal solid state fermentations. J. Biotechnol. 2013, 168, 130–134. [Google Scholar] [CrossRef]
- Čertík, M.; Adamechová, Z.; Laoteng, K. Microbial production of γ-linolenic acid: Submerged versus solid-state fermentations. Food Sci. Biotechnol. 2012, 21, 921–926. [Google Scholar] [CrossRef]
- Vidhyalakshmi, R.; Vallinachiyar, C.; Radhika, R. Production of xanthan from agro-industrial waste. J. Adv. Sci. Res. 2012, 3, 56–59. [Google Scholar]
- Cantatore, V.; Filannino, P.; Gambacorta, G.; De Pasquale, I.; Pan, S.; Gobbetti, M.; Di Cagno, R. Lactic acid fermentation to re-cycle apple by-products for wheat bread fortification. Front. Microbiol. 2019, 10, 2574. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Maoloni, A.; Del Rio, D.; Calani, L.; Bernini, V.; Galavema, G.; Neviani, E.; Lazzi, C. Use of dairy and plant-derived lactobacilli as starters for cherry juice fermentation. Nutrients 2019, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Nanis, I.; Hatzikamari, M.; Katharopoulos, E.; Boukouvala, E.; Ekateriniadou, L.; Litopoulou-Tzanetaki, E.; Gerasopoulos, D. Microbiological and physicochemical changes during fermentation of solid residue of olive mill wastewaters: Exploitation towards the production of an olive paste–type product. Lebensm.-Wiss. Technol. 2020, 117, 108671. [Google Scholar] [CrossRef]
- Karin, H.; Hahn-Hägerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar]
- Ye, K.; Jin, S.; Shimizu, K. Performance improvement of lactic acid fermentation by multistage extractive fermentation. J. Biosci. Bioeng. 1996, 81, 240–246. [Google Scholar] [CrossRef]
- Kashket, E.R. Bioenergetics of lactic acid bacteria: Cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 1987, 3, 233–244. [Google Scholar] [CrossRef]
- Amrane, A.; Yves, P. Growth and lactic acid production coupling for Lactobacillus helveticus cultivated on supplemented whey: Influence of peptidic nitrogen deficiency. J. Biotech. 1997, 55, 1–8. [Google Scholar] [CrossRef]
- Linko, Y.-Y.; Javanainen, P. Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch. Enzyme Microb. Technol. 1996, 19, 118–123. [Google Scholar] [CrossRef]
- Ishizaki, A.; Vonktaveesuk, P. Optimization of substrate feed for continuous production of lactic acid by Lactococcus lactis IO-1. Biotechnol. Lett. 1996, 18, 1113–1118. [Google Scholar] [CrossRef]
- Soto-Navarro, S.A.; Knight, M.H.; Lardy, G.P.; Bauer, M.L.; Caton, J.S. Effect of fiber-based creep feed on intake, digestion, ruminal fermentation, and microbial efficiency in nursing calves. J. Anim. Sci. 2004, 82, 3560–3566. [Google Scholar] [CrossRef] [PubMed]
- Szopa, D.; Mielczarek, M.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Chojnacka, K.; Witek-Krowiak, A. Encapsulation efficiency and survival of plant growth-promoting microorganisms in an alginate-based matrix–A systematic review and protocol for a practical approach. Ind. Crops Prod. 2022, 181, 114846. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Microencapsulation of probiotic cells by means of rennet-gelation of milk proteins. Food Hydrocoll. 2009, 23, 1670–1677. [Google Scholar] [CrossRef]
- Stummer, S.; Salar-Behzadi, S.; Unger, F.M.; Oelzant, S.; Penning, M.; Viernstein, H. Application of shellac for the development of probiotic formulations. Food Res. Int. 2010, 43, 1312–1320. [Google Scholar] [CrossRef]
- de Lara Pedroso, D.; Thomazini, M.; Heinemann, R.J.B.; Favaro-Trindade, C.S. Protection of Bifidobacterium lactis and Lactobacillus acidophilus by microencapsulation using spray-chilling. Int. Dairy J. 2012, 26, 127–132. [Google Scholar] [CrossRef]
- Samborska, K.; Poozesh, S.; Barańska, A.; Sobulska, M.; Jedlińska, A.; Arpagaus, C.; Malekjani, N.; Jafari, S.M. Innovations in spray drying process for food and pharma industries. J. Food Eng. 2022, 321, 110960. [Google Scholar] [CrossRef]
- Ann, E.Y.; Kim, Y.; Oh, S.; Imm, J.Y.; Park, D.J.; Han, K.S.; Kim, S.H. Microencapsulation of Lactobacillus acidophilus ATCC 43121 with prebiotic substrates using a hybridisation system. Int. J. Food Sci. Technol. 2007, 42, 411–419. [Google Scholar] [CrossRef]
- Solanki, H.K.; Pawar, D.D.; Shah, D.A.; Prajapati, V.D.; Jani, G.K.; Mulla, A.M.; Thakar, P.M. Development of microencapsulation delivery system for long-term preservation of probiotics as biotherapeutics agent. Biomed Res. Int. 2013, 2013, 620719. [Google Scholar] [CrossRef] [PubMed]
- López-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R. Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization. Lebensm.-Wiss. Technol. 2019, 110, 102–109. [Google Scholar] [CrossRef]
- Shi, L.E.; Li, Z.H.; Li, D.T.; Xu, M.; Chen, H.Y.; Zhang, Z.L.; Tang, Z.X. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Hongpattarakere, T.; Supansa, U. Bifidogenic characteristic and protective effect of saba starch on survival of Lactobacillus plantarum CIF17AN2 during vacuum-drying and storage. Carbohydr. Polym. 2015, 117, 255–261. [Google Scholar] [CrossRef]
| Microorganism | Genera |
|---|---|
| Lactic acid bacteria (LAB) | Aerococcus, Alloiococcus, Bifidobacterium, Carnobacterium, Dolosigranulum, Enterococcus, Globicatella, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Vagococcus and Weissella |
| Other bacteria | Bacillus |
| Yeast | Candida, Pichia, Saccharomyces and Torulaspora |
| Fungi | Rhizopus and Aspergillus |
| Food | Raw Material | Origin | Microorganisms |
|---|---|---|---|
| Sauerkraut |
| Central Europe |
|
| Kimchi |
| Korea |
|
| Encurtidos |
| World |
|
| Gundruk |
| Nepal, India |
|
| Sink |
| India |
|
| Khalpi |
| Nepal |
|
| Jiang-Gua |
| Taiwan |
|
| Paocai |
| China |
|
| Yan-Dong-Gua |
| Taiwan |
|
| Sayur Asin |
| Indonesia |
|
| Yan-Tsai-Shin |
| Taiwan |
|
| Suan-tsai |
| Taiwan |
|
| Dhamoui |
| Vietnam |
|
| Matrix | Strains | Reference |
|---|---|---|
| Cabbage, lettuce, and garlic | Weissella cibaria and Lactobacillus plantarum | [18] |
| Carrots, beans, and zucchini | Leuconostoc mesenteroides, Lactobacillus plantarum, Weissella soli, Weissella koreensis, Enterococcus faecalis, Pediococcus pentosaceus and Lactobacillus fermentum | [19] |
| Cherry and broccoli | Lactobacillus spp. | [20] |
| Tomato | Lactobacillus acidophilus | [21] |
| Cherries, pineapple, carrot, and tomato | Lactobacillus plantarum | [22] |
| Lettuce and apple | Lactobacillus plantarum | [23] |
| Pepper | Lactobacillus plantarum, Lactobacillus curvatus and Weissella cibaria | [24] |
| Green olives | Lactobacillus pentosus, Lactobacillus plantarum and Candida diddensiae | [25] |
| Yucca (Gari) | Lactobacillus plantarum | [26] |
| Processes | Classification |
|---|---|
| Physical |
|
| Chemical |
|
| Physicochemical |
|
| Microencapsulation Technique | Probiotic | Encapsulating Matrix | Reference |
|---|---|---|---|
| Rennet-gelled protein encapsulation | Bifidobacterium animalis and Lactobacillus paracasei | Milk proteins | [116] |
| Fluid bed | Enterococcus faecium, Bifidobacterium bifidum, and Lactobacillus reuteri | Alginate, shellac, hydroxyoropil methylcellulose, and glycerol | [117] |
| Chilling spray | Bifidobacterium lactis and Lactobacillus acidophilus | Palm interesterified fat | [118] |
| Spray-dried with vacuum and sound | Lactobacillus paracasei and Lactobacillus casei | Maltodextrin and trehalose | [119] |
| Hybridization systems | Lactobacillus acidophilus | Insulin, sorbitol, mannitol, and | [120] |
| Incident aerosol technology | Lactobacillus acidophilus and Lactobacillus rhamnosus | Alginate | [121] |
| Electrocentrifugation | Bifidobacteria sp. | Whey proteins | [122] |
| Electrospray | Lactobacillus plantarum and Bifidobacterium lactis | Alginate, chitosan, inulin, and resistant starch | [123] |
| Extrusion technology | Lactobacillus bulgaricus | Alginate–milk | [124] |
| Vacuum-drying | Lactobacillus plantarum | Resistant starch from unripe saba banana | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleman, R.S.; Montero-Fernández, I.; Marcía, J.A.; Saravia Maldonado, S.A.; Martín-Vertedor, D. Application of Fermentation as a Strategy for the Transformation and Valorization of Vegetable Matrices. Fermentation 2024, 10, 124. https://doi.org/10.3390/fermentation10030124
Aleman RS, Montero-Fernández I, Marcía JA, Saravia Maldonado SA, Martín-Vertedor D. Application of Fermentation as a Strategy for the Transformation and Valorization of Vegetable Matrices. Fermentation. 2024; 10(3):124. https://doi.org/10.3390/fermentation10030124
Chicago/Turabian StyleAleman, Ricardo S., Ismael Montero-Fernández, Jhunior A. Marcía, Selvin A. Saravia Maldonado, and Daniel Martín-Vertedor. 2024. "Application of Fermentation as a Strategy for the Transformation and Valorization of Vegetable Matrices" Fermentation 10, no. 3: 124. https://doi.org/10.3390/fermentation10030124
APA StyleAleman, R. S., Montero-Fernández, I., Marcía, J. A., Saravia Maldonado, S. A., & Martín-Vertedor, D. (2024). Application of Fermentation as a Strategy for the Transformation and Valorization of Vegetable Matrices. Fermentation, 10(3), 124. https://doi.org/10.3390/fermentation10030124








