Beneficial Effects of Probiotics on Liver Injury Caused by Chronic Alcohol Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Probiotic Bacteria
2.2. Animals and Treatment
2.3. Biochemical Analysis of Serum and Liver
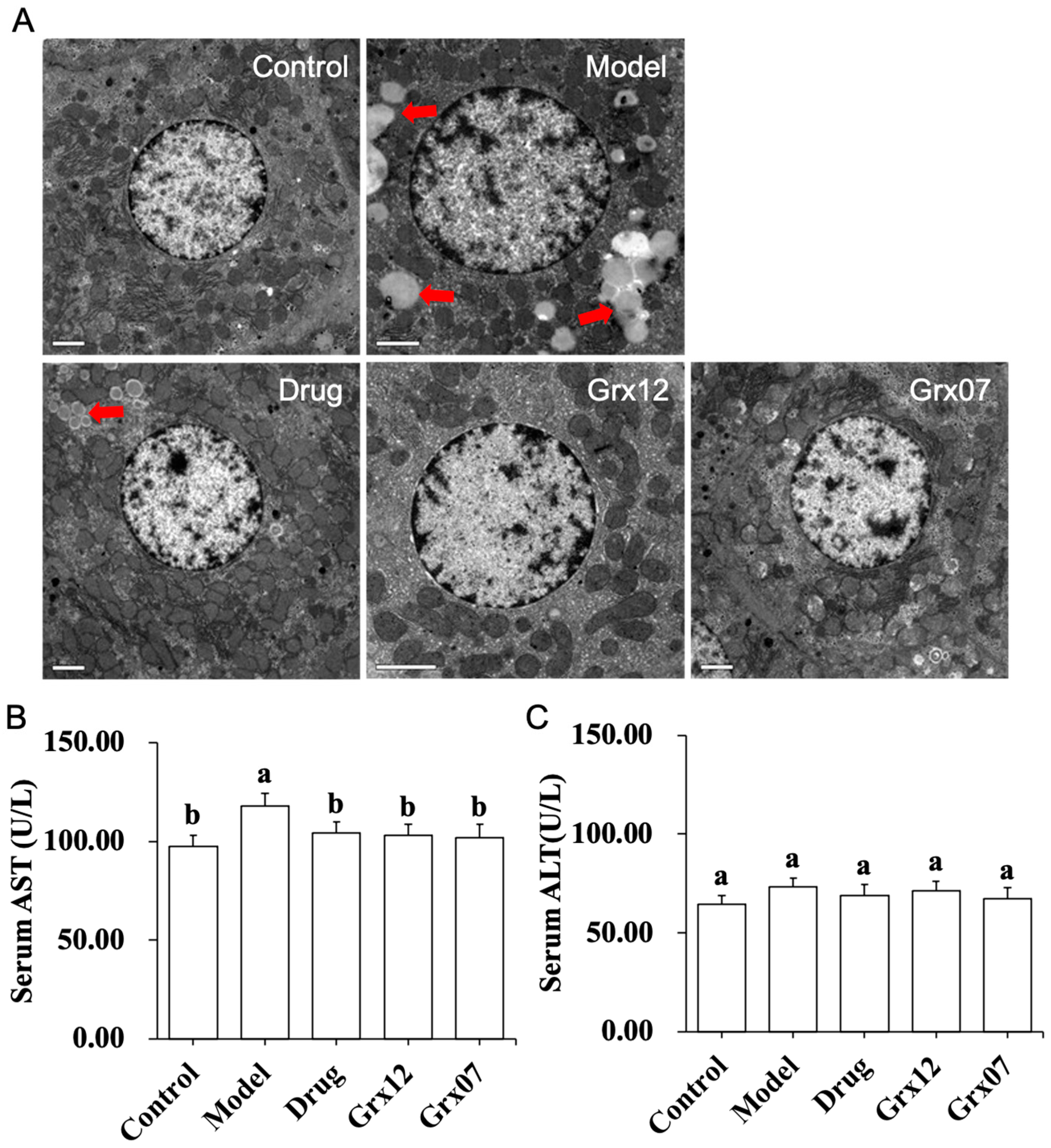
2.4. Pathological Observation of the Rat Liver
2.5. Determination of Liver Cell Index
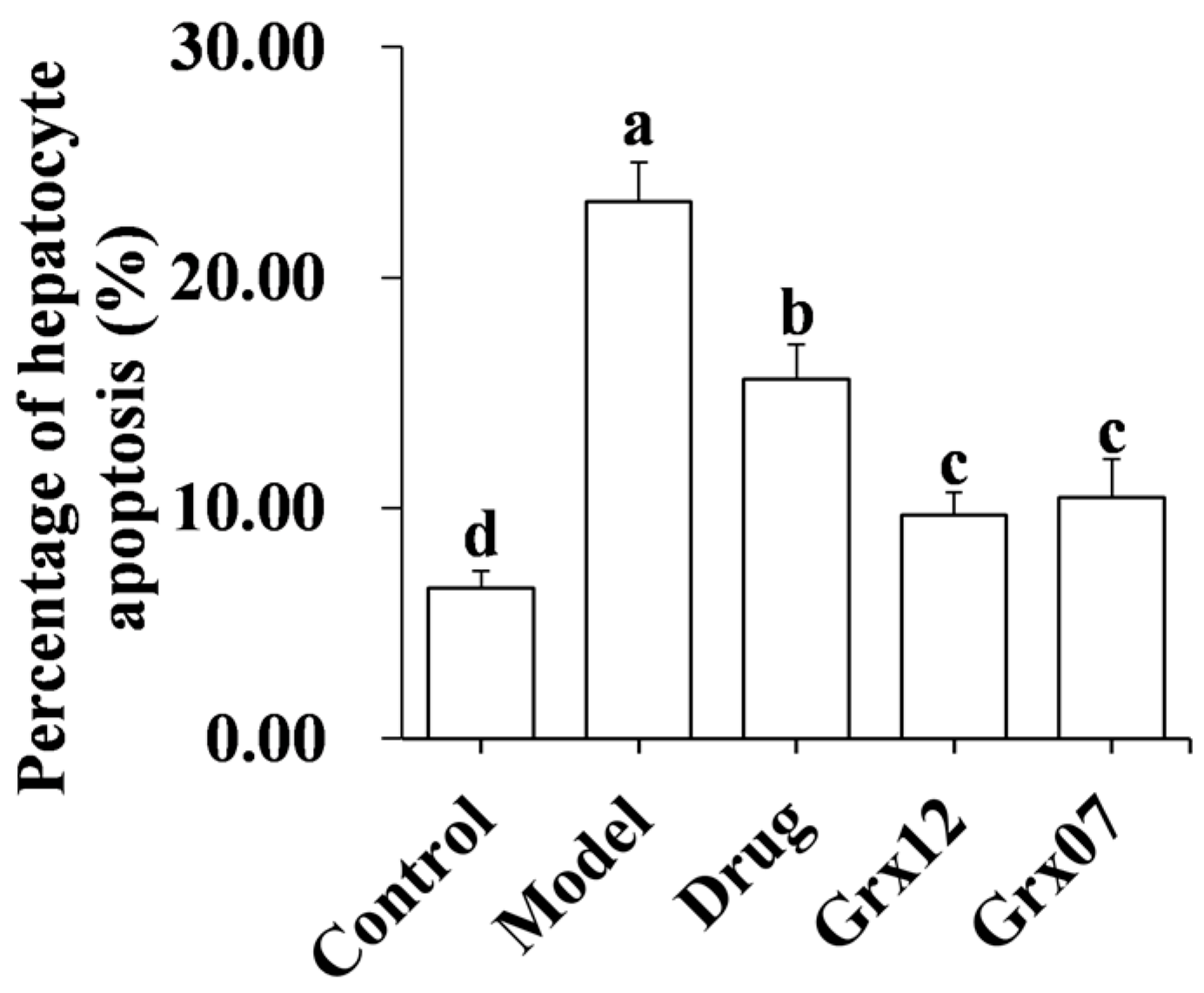
2.6. Hepatocyte Apoptosis
2.7. Determination of Antioxidant Specific Protein Nrf2 in Rat Liver
2.8. Statistical Analysis
3. Results
3.1. General State of Rats
3.2. Human-Derived Probiotics Alleviate the Liver Injury
3.3. Human-Derived Probiotics Reduce the Hepatocyte Apoptosis
3.4. Human-Derived Probiotics Restore ADH and ATPase
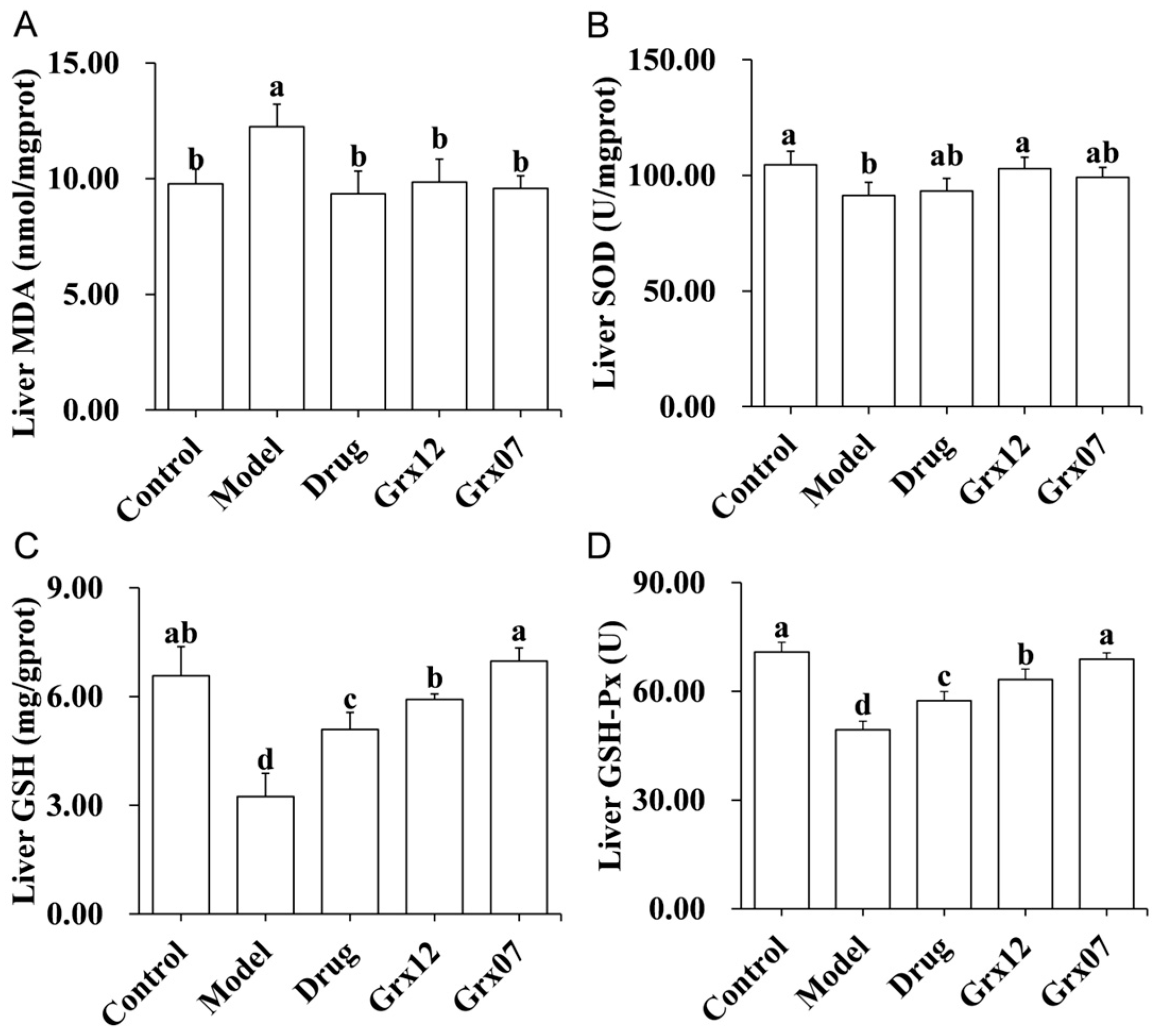
3.5. Human-Derived Probiotics Alleviate Liver Oxidative Stress
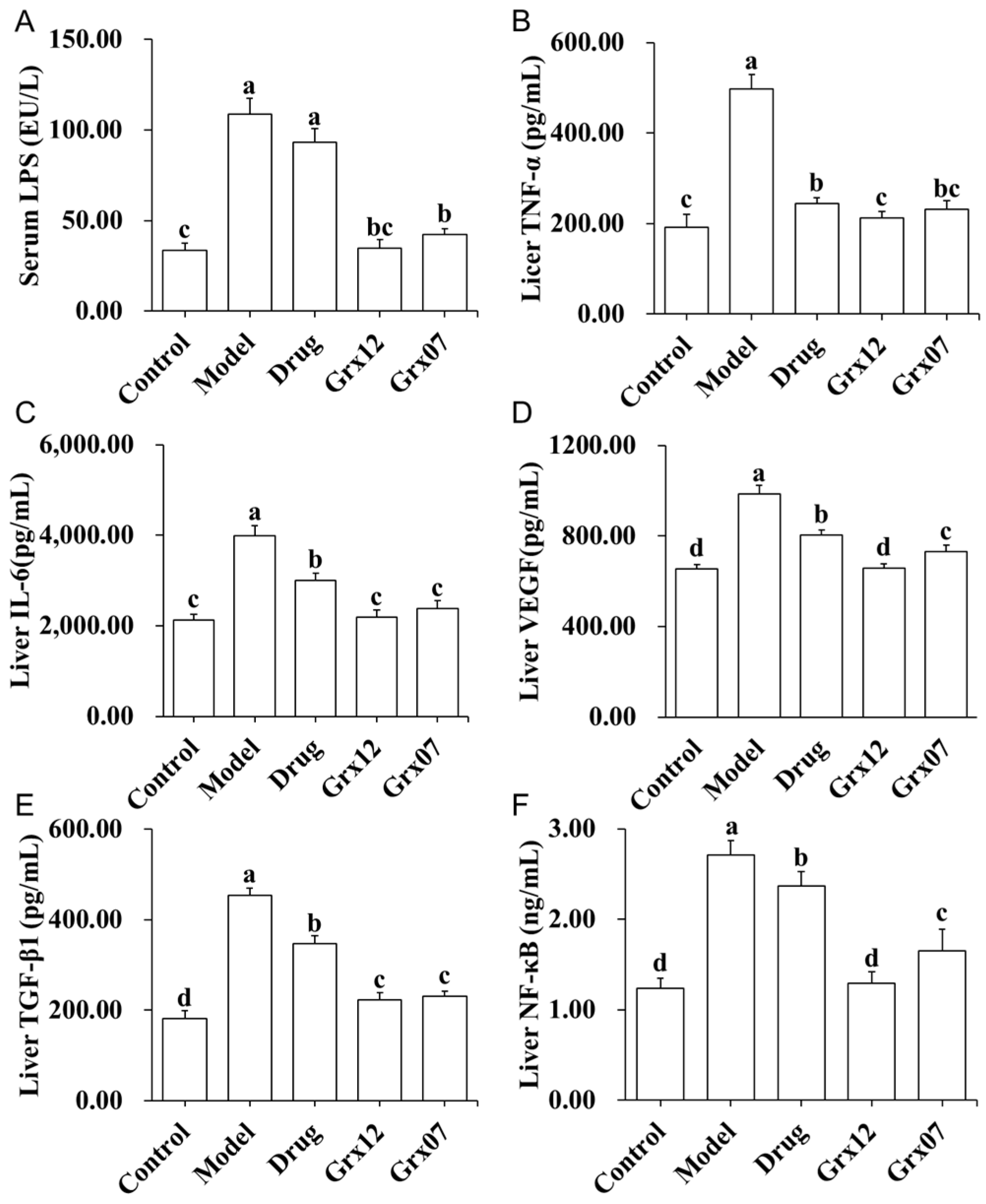
3.6. Human-Derived Probiotics Alleviate Liver Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malnick, S.D.H.; Alin, P.; Somin, M.; Neuman, M.G. Fatty Liver Disease-Alcoholic and Non-Alcoholic: Similar but Different. Int. J. Mol. Sci. 2022, 23, 16226. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Wong, N.-K.; Lv, Y.; Liu, Y.; Zhong, J.; Chen, S.; Li, W.; Koike, K.; Liu, X.; et al. Epidemiological Realities of Alcoholic Liver Disease: Global Burden, Research Trends, and Therapeutic Promise. Gene Expr. 2020, 20, 105–118. [Google Scholar] [CrossRef]
- Seitz, H.K.; Neuman, M.G. The History of Alcoholic Liver Disease: From an Unrecognized Disease to One of the Most Frequent Diseases in Hepatology. J. Clin. Med. 2021, 10, 858. [Google Scholar] [CrossRef]
- Mackus, M.; Van De Loo, A.J.; Garssen, J.; Kraneveld, A.D.; Scholey, A.; Verster, J.C. The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover. J. Clin. Med. 2020, 9, 3421. [Google Scholar] [CrossRef]
- Slevin, E.; Baiocchi, L.; Wu, N.; Ekser, B.; Sato, K.; Lin, E.; Ceci, L.; Chen, L.; Lorenzo, S.R.; Xu, W.; et al. Kupffer Cells: Inflammation Pathways and Cell-Cell Interactions in Alcohol-Associated Liver Disease. Am. J. Pathol. 2020, 190, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- Ohashi, K.; Pimienta, M.; Seki, E. Alcoholic liver disease: A current molecular and clinical perspective. Liver Res. 2018, 2, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-Z.; Chandimali, N.; Han, Y.-H.; Lee, D.-H.; Kim, J.-S.; Kim, S.-U.; Kim, T.-D.; Jeong, D.K.; Sun, H.-N.; Lee, D.S.; et al. Pathogenesis, Early Diagnosis, and Therapeutic Management of Alcoholic Liver Disease. Int. J. Mol. Sci. 2019, 20, 2712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 15. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Mishra, G.; Singh, P.; Molla, M.; Yimer, Y.S.; Dinda, S.C.; Chandra, P.; Singh, B.K.; Dagnew, S.B.; Assefa, A.N.; Ewunetie, A. Harnessing the potential of probiotics in the treatment of alcoholic liver disorders. Front. Pharmacol. 2023, 14, 1212742. [Google Scholar] [CrossRef]
- Nanji, A.A.; Khettry, U.; Sadrzadeh, S.M. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc. Soc. Exp. Biol. Med. 1994, 205, 243–247. [Google Scholar] [CrossRef]
- He, Q.; Yang, C.; Kang, X.; Chen, Y.; Zhang, T.; Zhang, H.; Kwok, L.-Y. Intake of Bifidobacterium lactis Probio-M8 fermented milk protects against alcoholic liver disease. J. Dairy. Sci. 2022, 105, 2908–2921. [Google Scholar] [CrossRef]
- Gan, Y.; Tong, J.; Zhou, X.; Long, X.; Pan, Y.; Liu, W.; Zhao, X. Hepatoprotective Effect of Lactobacillus plantarum HFY09 on Ethanol-Induced Liver Injury in Mice. Front. Nutr. 2021, 8, 684588. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, H.; Liu, X.; Li, Q.; Liu, Y.; Wang, W.; Chen, D.; Xiao, L.; Gu, R. Comparison and selection of probiotic Lactobacillus from human intestinal tract and traditional fermented food in vitro via PCA, unsupervised clustering algorithm, and heat-map analysis. Food Sci. Nutr. 2022, 10, 4247–4257. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Gu, R.; Su, W. Screening of antioxidative lactic acid bacteria from the intestinal tract of the longevity village people of Rugao. Sci. Technol. Food Ind. 2014, 35, 153–157. [Google Scholar]
- Sun, F.; Xie, M.; Zhu, L.; Xue, J.; Gu, Z. Inhibitory effect of osthole on alcohol-induced fatty liver in mice. Dig. Liver Dis. 2009, 41, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.H.; Li, J.P.; Liu, S.L.; Zou, X.; Fang, L.H.; Zhou, J.Y.; Wu, J.; Xi, S.Y.; Chen, Y.; Zhang, Y.Y.; et al. Autophagy Protects from Raddeanin A (RA)-Induced Apoptosis in 2 SGC-7901 Human Gastric Cancer Cells. Evid.-Based Complement. Altern. Med. 2016, 2016, 9406758. [Google Scholar] [CrossRef] [PubMed]
- Qing, R.; Huang, Z.; Tang, Y.; Xiang, Q.; Yang, F. Cordycepin alleviates lipopolysaccharide-induced acute lung injury via Nrf2/HO-1 pathway. Int. Immunopharmacol. 2018, 60, 18–25. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Zahid, M.; Arif, M.; Rahman, M.A.; Mujahid, M. Hepatoprotective and antioxidant activities of Annona squamosa seed extract against alcohol-induced liver injury in Sprague Dawley rats. Drug Chem. Toxicol. 2020, 43, 588–594. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Zhang, M.; Fang, G.; Cheng, C.-J.; Wang, M.-K.; Han, Y.-M.; Hou, X.-T.; Hao, E.-W.; Hou, Y.-Y.; Bai, G. Ursolic acid reduces hepatocellular apoptosis and alleviates alcohol-induced liver injury via irreversible inhibition of CASP3 in vivo. Acta Pharmacol. Sin. 2020, 42, 1101–1110. [Google Scholar] [CrossRef]
- Teschke, R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines 2018, 6, 106. [Google Scholar] [CrossRef]
- Begum, R.; Thota, S.; Abdulkadir, A.; Kaur, G.; Bagam, P.; Batra, S. NADPH oxidase family proteins: Signaling dynamics to disease management. Cell Mol. Immunol. 2022, 19, 660–686. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Kim, H.H.; Choi, S.E.; Jeong, W.I. Oxidative stress and glutamate excretion in alcoholic steatosis: Metabolic synapse between hepatocyte and stellate cell. Clin. Mol. Hepatol. 2020, 26, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.A.; Khasawneh, M.K. Cancer metabolism and oxidative stress: Insights into carcinogenesis and chemotherapy via the non-dihydrofolate reductase effects of methotrexate. BBA Clin. 2015, 3, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Xiong, X.; Zhu, H.; Chen, R.; Zhang, S.; Chen, G.; Jian, Z. Nrf2 Regulates Oxidative Stress and Its Role in Cerebral Ischemic Stroke. Antioxidants 2022, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Primers 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, X.R. Kinetic analysis of interaction between lipopolysaccharide and biomolecules. Chin. J. Anal. Chem. 2007, 35, 677–680. [Google Scholar] [CrossRef]
- Sana, M.; Rashid, M.; Rashid, I.; Akbar, H.; Gomez-Marin, J.E.; Dimier-Poisson, I. Immune response against toxoplasmosis-some recent updates RH: Toxoplasma gondii immune response. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221078436. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, H.-G.; Han, J.-M.; Lee, J.-S.; Lee, H.-W.; Choi, M.-K.; Son, C.-G. The herbal formula CGX ameliorates the expression of vascular endothelial growth factor in alcoholic liver fibrosis. J. Ethnopharmacol. 2013, 150, 892–900. [Google Scholar] [CrossRef]
- Zhang, C.; Ellis, J.L.; Yin, C. Inhibition of vascular endothelial growth factor signaling facilitates liver repair from acute ethanol-induced injury in zebrafish. Dis. Model. Mech. 2016, 9, 1383–1396. [Google Scholar] [CrossRef]
- Buko, V.; Kuzmitskaya, I.; Kirko, S.; Belonovskaya, E.; Naruta, E.; Lukivskaya, O.; Shlyahtun, A.; Ilyich, T.; Zakreska, A.; Zavodnik, I. Betulin attenuated liver damage by prevention of hepatic mitochondrial dysfunction in rats with alcoholic steatohepatitis. Physiol. Int. 2019, 106, 323–334. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S.J.C.M. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]


| Group | Gavage (0.1 mL/10 g Rat Body Weight) | |
|---|---|---|
| Morning | Afternoon | |
| Control | saline | saline |
| Model | Chinese liquor | saline |
| Drug | Chinese liquor | Dongbaogangtai (0.06 g/mL methionine and chline bitartrate solution) |
| Grx12 | Chinese liquor | 109 CFU/Grx12 bacterial suspension |
| Grx07 | Chinese liquor | 109 CFU/mL Grx07 bacterial suspension |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, J.; Qu, H.; Liu, D.; Wa, Y.; Chen, D.; Chen, X.; Gu, R.; Huang, Y. Beneficial Effects of Probiotics on Liver Injury Caused by Chronic Alcohol Consumption. Fermentation 2024, 10, 127. https://doi.org/10.3390/fermentation10030127
Sang J, Qu H, Liu D, Wa Y, Chen D, Chen X, Gu R, Huang Y. Beneficial Effects of Probiotics on Liver Injury Caused by Chronic Alcohol Consumption. Fermentation. 2024; 10(3):127. https://doi.org/10.3390/fermentation10030127
Chicago/Turabian StyleSang, Jian, Hengxian Qu, Dong Liu, Yunchao Wa, Dawei Chen, Xia Chen, Ruixia Gu, and Yujun Huang. 2024. "Beneficial Effects of Probiotics on Liver Injury Caused by Chronic Alcohol Consumption" Fermentation 10, no. 3: 127. https://doi.org/10.3390/fermentation10030127






