Abstract
Microbial factories, including microalgae biofactories, have the enormous potential to produce biochemicals for manufacturing diverse bioproducts. A strategic approach to biofactories is maintaining cultures in bioreactors with sufficient resource inputs to optimize biochemical precursors for manufacturing bioproducts. Exploiting synergies that use the waste output from a bioreactor containing one microbial culture as a resource input to another bioreactor with a different microbe can lead to overall efficiencies in biofactories. In this paper, two synergies are evaluated. The first is between yeast and algae bioreactors, where data are presented on oxygen (O2) uptake by aerobic yeast cultures and their production of carbon dioxide (CO2) and the uptake of CO2 by algae and their production of O2. The second focuses on a carbon capture reactor, which is utilized to increase CO2 levels to promote higher algal production. This approach of waste as a resource for bioreactor cultures is a novel synergy that can be important to bioreactor designs and, ultimately, to the production of bioproducts.
1. Introduction
Microbial factories are unique microbial cultures grown in bioreactors to produce precursor chemicals for manufacturing bioproducts. A similar concept is that of biorefineries, which promotes a cascading approach to harvesting and using microbial biochemicals [1,2]. One goal of microbial factories is to create large value chains that replace fossil fuels and synthetic chemicals. Another primary goal of microbial factories is to produce more sustainable products. Products can be made more sustainable by designing them from biodegradable materials that can be used as technical and biological nutrients in a cradle-to-cradle cycle, illustrated by bioproducts in Table 1. On Earth, bioproducts that replace fossil fuels can become essential to a bio-circular economy. In space, they can provide the raw materials and resources needed to manufacture components on space stations at a much lower cost than if these materials are transported from Earth. Microalgae biofactories also have the potential to sequester carbon dioxide both in Earth’s atmosphere [3] and on space stations [4].

Table 1.
Bionutrients from microalgae.
A variety of biological pathways can be used by microbes to produce the precursor chemicals for bioproducts (Table 2). The types of bioproducts that can be derived from algae, fungi, bacteria, and plants range from electronics and optics to biofuels, structural components (i.e., plastics and carbon fiber), and industrial chemicals (Table 3). This list of bioproducts will surely expand as research on microbial factories expands.

Table 2.
Different metabolic strategies to produce precursor chemicals.
The bioproducts and bionutrients listed in Table 1 and Table 3 were produced from pure cultures confined to one bioreactor and supplied by outside resources, including gases, nutrients, and in the case of algae, light. However, synergies can also provide such resources, further increasing bioreactor efficiencies. State of the art for microbial synergies focuses on combining microbial cultures as a co-culture. The symbiotic approach of co-cultures is to mix two or more pure microbial cultures in one bioreactor [12,13,14]. Co-cultures have been shown to improve yields of biomass and high-value products via the metabolism of both microbial strains. Examples are a co-culture of bacteria and microalgae that increases nutrient recycling [15] and floc-forming bacteria that enable sedimentation of microalgae [16], which is especially pertinent to wastewater treatment plants [17,18] and sludge digestion [19]. Polycultures of microalgae can develop a symbiosis, exchanging nutrients and metabolites with heterotrophic organisms [15]. Organic carbon produced by bacteria can enhance algal production [20]. Two yeasts, Saccharomyces cerevisiae and Kluyveromyces marxianus, co-produce inulinase, and co-cultures of the yeasts of Trichoderma sp. and S. cerevisiae can produce bioethanol [21]. Bacteria have been shown to act as chemical mediators of photosynthetic oxygen, siderophores, and vitamin B12 to promote microalgal metabolites for bioproducts [22].
Despite the synergies documented for co-cultures, recycling of wastes as resources for pure cultures is not considered in any of these studies. Here, we use waste outputs from a bioreactor containing one microbial culture as an essential resource for a second bioreactor with a different microbe. We, thereby, create a synergy where wastes are recycled, and two pure cultures can be simultaneously grown to produce two or more bioproducts. One synergy in this paper is between algal and yeast bioreactors, where CO2 uptake and O2 production by pure cultures of algae are evaluated for O2 uptake and CO2 production by pure cultures of yeast.

Table 3.
Bioproducts from algae and other microbes.
Table 3.
Bioproducts from algae and other microbes.
| Bioproduct | Nutrient | Organism | Source | Process | Ref. |
|---|---|---|---|---|---|
| Ceramics | Silicon polymers Biominerals | Algae | Exoskeleton | Photobioreactors | [23] [24,25] |
| Insulators | Polyvinyl alcohol (PVA) | Bacteria | Biopolymer | Heterotrophic bioreactor | [26] |
| Liquid crystals | Biominerals | Algae, vascular plants | Biopolymer | Photobioreactor | [27] |
| Optics | 2-methyl pentanol | Bacteria | Enzymatic | Trade secret-Codexis | [28] |
| Semiconductors | Silica frustules | Algae | Exoskeleton | Photobioreactor | [29] |
| Polyvinyl alcohol (PVA) | Algae, vascular plants | Biopolymer | Photobioreactor | [27] | |
| Medical sensors | Glycerides | abfp 1 | Fat-degrade enzymes | Bioreactors | [30] |
| Polymers | [31] | ||||
| Biodiesel | Triglycerides | Algae | Fat droplets | Photobioreactor | [32] |
| Various alcohols | Butanol, propanol | Fungi (yeasts) | Differing pathways | Bioreactor | [33] |
| Ethanol | Various sugars | Fungi (yeasts) | Differing pathways | Bioreactors | [34,35] |
| Cellulosic material | Vascular plants, | Cutin | Photobioreactor | [36] | |
| Lignin | Vascular plants | Cutin | Fermenter | [37] | |
| Glycerol | algae, vascular plants | Fat droplets | Photobioreactor | [38] | |
| Cellulosic material | Fungi | Cell wall | Bioreactors | [39] | |
| Hydrogen | Organics | Bacteria Algae | Biomass Biomass | Dark fermenters | [40,41] |
| Lignins | Vascular plants | Cutin | Vacuum pyrolysis, catalyst | [42] | |
| Glycerol | Algae, vascular plants | Fat droplets | High temperature, pressure, metal catalyst | [43] | |
| Waste glycerol | Bacteria | Biomass | Microbial fuel cell | [44,45] | |
| Ethane | Organic waste | Bacteria | Biomass | Digester, single-multi-stage | [46] |
| Lipids | Algae | Fat droplets | Anaerobic digester | [47] | |
| Other oils | Lipids | Algae, | Fat droplets | Fluidized beds—pyrolysis | [9] |
| vascular plants | Pyrolysis, gasification | [48] | |||
| Solar cells | Silica frustules | Diatoms | Exoskeleton | Photobioreactor | [29] |
| Membranes | PVA hydrogels | Bacteria | Chitosan, Gelatin | Bioreactors | [49] |
| Surfactants | Biopolymers | abfp 1 | Various | Various | [50] |
| Nano- composites | Nano-carbon | Algae, vascular plants | Biopolymers | Photobioreactor | [51] [52] |
| Adhesives | Algae Bacteria Fungi | Biopolymers | Bioreactors | [53,54] [55] [56] | |
| Nanocrystals | Cellulose fibers | Bacteria, vascular plants | Cell wall | Bioreactors | [57] |
| Nanofibers | Proteins, peptides | Gluconacetobacter xylinus | Biopolymer | Bioreactors | [58] |
| Structures | Carbon fiber | Algae | Glycerol | Photobioreactor | [59] |
| Acrylic | Acrylic acid | Algae, vascular plants | Fat droplets | Photobioreactor | [38] |
| PET, PLA | Glycols | Algae, vascular plants | Fat droplets | Photobioreactors | [60,61] |
| Cellulose fibers | Vascular plants | Cell wall | Photobioreactor | [62] | |
| Polyvinyl | Ethanol | Algae, vascular plants | Fat droplets | Photobioreactor | [63] |
| Cement | Calcium, Silica | Algae Vascular plants | Exoskeletons Biomass | Photobioreactor Photobioreactor | [64] [65] |
| Nano-cement nuclei | Calcium carbonate | Algae–cocci | Exoskeletal | Photobioreactor | [66] |
1 abfp are algae, bacteria, fungi, and vascular plants. Photobioreactors include plant cultivation systems.
Another synergy evaluated in this paper is carbon capture and use (CCU), and specifically, direct air capture of CO2 and use by pure cultures of algae. State of the art for carbon capture has evolved from metal oxides, zeolites, ionic liquids, activated carbons, fluorinated solvents, membranes, and molecular sieves, to more advanced metal–organic frameworks (MOFs) and covalent organic ligands [67,68]. Organo-ligands and graphene-type materials have high adsorption capacities, ten times higher than that of specific types of activated carbon, zeolites, and metal–organic frameworks, and are used in direct carbon capture from the atmosphere [69]. Microalgal bioreactors have not been used with these direct carbon capture technologies. Rather, the captured carbon has been geo-sequestrated and stored either in mines, basalts, or aquatic systems [70].
Microalgal bioreactors have been used to remove CO2 from flue gas [71]. However, this differs from direct air capture in that it is a post-combustion technology [72] where monoethanolamine is still the most common carbon sorbent. Unfortunately, monoethanolamine has a high regeneration energy, although more novel solvents with amine blends and lower regeneration energy are being implemented [69]. The future of intensified absorption technologies combined with algal bioreactors can produce a hybrid, synergistic process where added-value bioproducts are manufactured [73].
Here, we show a novel approach for synergistic bioreactors. In exploring the algae–yeast and CCU synergies, data are used to calculate the production and consumption rates of waste resources. These rate processes are then used to suggest how bioreactor operations can foster synergies between bioreactors and ultimately lead to pathways for different bioproducts. To our knowledge, this is the first time data on recycling waste between bioreactors have been published, making the synergistic approach in this study novel and significantly different from co-cultures and direct air carbon capture experiments synergies in the literature.
2. Materials and Methods
Algal and yeast mini-bioreactors consisted of peristaltic pumps connected to each bioreactor to circulate cells at a 30 mL/min rate through hollow fiber membranes (PMDSXA 100 cm2, MedArray Inc., Ann Arbor, MI, USA.). The internal volume of the membrane was 8.0 mL, and with tubing and pumps, the total volume of each bioreactor was 10 mL. The membranes were highly effective chambers for growing cells and for gas exchange of CO2 and O2 [74]. Prior to the experiment, tubing and glassware were autoclaved at 121 °C and 1034 mbar (15 psi) for 20 min and assembled under a laminar flow hood. Membranes were flushed with 50 mL of 70% ethanol and left overnight (≈10 h) to sterilize, after which the membranes were rinsed with 100 mL of filtered deionized water, and the remaining parts of the system assembled under a laminar flow hood. After bioreactors were assembled, they were flushed with their respective media and then filled with approximately 10 mL of medium containing the parent cultures. At this point, the time series started.
The green algae Tetraselmis sp. was obtained from Roscoff culture collection (RCC2604) and grown in the algae bioreactor under a photosynthetic photon flux density (PPFD) of 520 μE m−2 s−1 using an LED light array tuned to the cell’s pigment absorption spectrum [6]. The f/2 algal medium of Guillard [75] was prepared in 1 liter deionized water as stock solutions containing 30 g Na2CO3 plus a metal stock solution of 3.15 g FeCl3 6(H2O), 9.8 g CuSO4 5(H2O), 6.3 g Na2MoO4 2(H2O), 22 g ZnSO4 7(H2O). 20 g CoCl2 6(H2O), and 180 g MnCl2 4(H2O), all chelated with 4.36 g Na2(EDTA)2(H2O) and containing vitamins (200 mg vitamin B1, 0.1 g Vitamin H, 1 g vitamin B12) but no nitrate. The nitrogen source was Aurin™ (a urine product used to simulate recycled human waste) containing 26.3 g NH4-H and 31.9 g NO3-N. The stock solution of f/2 was diluted 1:1000 mL in 33 ppt seawater (Instant OceanTM, Spectrum Brands, Commerce, VA, USA) for the initial inoculum and 1:100 mL for the pulsed feed.
A culture of Saccharomyces cerevisiae (BY4742,MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0) was grown in the yeast bioreactor. The yeast medium was Synthetic Complete (SC) consisting of 5 g L−1 of (NH4)2SO4, 17 g L−1 of YNB, two nucleic acids (18 m g L−1 of Adenine and 76 mg L−1 of Uracil), and twelve amino acids (176 mg L−1 of Leucine, and 76 mg L−1 of each of the following: arginine; asparagine; cysteine; glutamine; glycine; histidine; lysine; methionine; phenylalanine; serine; threonine; tyrosine; and valine). The carbon source was 7.5 g L−1 D-glucose. For pulsed feeding of the yeast bioreactor, a 10-fold concentrated medium was used [76].
Coinciding with reduced biomass growth on day 4 in the algae bioreactor and approximately every two days in the yeast bioreactor, the bioreactors were given a pulse of concentrated medium to prevent nutrient limitation and extend the duration of the experiments. Using a syringe, 1 mL of suspension was sampled from each bioreactor, and 1 mL of concentrated media was injected into each bioreactor; thus, a constant volume was maintained in the bioreactors. The cell samples (i.e., suspension) were counted on a flow cytometer (Accuri C6, BD Biosciences, New York, CA, USA) and filtered onto 0.25 mm GF/F pre-weighed filters. Filters were dried at 105 °C for 1 h, cooled to room temperature in a desiccator, and weighed to determine biomass.
For both bioreactors, time series of pressure, temperature, dissolved oxygen (DO), pH, and optical density (for biomass) were recorded every 5 min over 10 days of the experiments using the SRF optical array (PreSens, Precision Sensing GmbH, DE) in line and downstream of the PMDSXA membranes. Briefly, this optical system uses chemically doped spots to measure pH and DO. Spots were glued inside a 2 mL volume optical chamber [76], which was aligned with the optical sensors of the SRF array. A second set of times series was also run for 1–2 days and data are statistically presented. DO and pH of the SRF were post-calibrated. Hanna Instruments standard solutions of pH 4, 9.21, and 10 were used for the pH calibration. For the DO calibration, air was bubbled in the media at 4 L min−1 until the media was saturated. The deoxygenated medium was obtained by adding NaSO3 until DO was below the detection limit, near zero [76]. For all experiments, DO was corrected for changes in temperature and atmospheric pressure.
The specific growth rate was calculated from the slopes of linear regressions as
where N corresponds to cell biomass at times ti+1 and ti, where ti+1 − ti are the time periods of abrupt changes in biomass trends.
The oxygen transfer rate, KLa, of the hollow-fiber filters was calculated by Granata et al. [74] as
where Q is the fluid flow rate through the membrane (43 L d−1); DOBr is the oxygen concentration in the bioreactor in mg L−1; DOs is the saturated oxygen concentration in mg L−1, and A is the surface area of the membrane (100 cm2). The KLa of the membrane was 1872 d−1 [74].
The oxygen uptake rate, OUR, was calculated by Granata et al. [74] as
Equation (3) was used for calculating the O2 production rate by algae, P. DO per unit biomass, Λ, was calculated for each time point and averaged over the time period of interest.
Missing values from the time series were interpolated. However, outliers with more than 4 standard deviations from a moving average were deleted and interpolated over neighboring values. Missing and aberrant data points were less than 3% of the time series.
In a separate experiment on CCU, calcium hydroxide was saturated in deionized water in a 100 mL flask. After 5 min of air bubbling at the rate of 4 L min−1, solid calcium carbonate formed in the deionized water. The solution was acidified, releasing 100% CO2, mixed with compressed air to 10% CO2, and bubbled at a flow rate of 10 L min−1 into a 500 mL flask with algae and medium. Grab samples of 1 mL volume were taken periodically during the CCU experiments. Samples were used to determine the partial pressure of CO2 (pCO2) in the culture, the biomass, and the number of cells.
The concentration of CO2 in the bioreactors was determined using a Mettler-Toledo InPro5000i sensor (Mettler-Toledo LLC, Columbus, OH, USA). Unfortunately, the pCO2 sensor was too large to integrate with the small bioreactors during the yeast and algae time series. Therefore, the pCO2 of these cultures was only determined on grab samples. The pCO2 data were temperature corrected and calibrated from 0.04% (i.e., air) to 100% CO2 (compressed CO2) and for pH values of 4, 7, and 9.21. Carbonate species and total alkalinity were calculated from equilibrium constants constrained by pH and pCO2 measurements [77]. Since barometric pressure was steady during the CCU experiments, a constant value of 1013.25 mbars was used to correct the pCO2 measurements.
Analysis of Variance (ANOVA) and General Linear Models (GLM) were conducted on data using SPSS v29.0.2. Tukey test and t-tests were used to discriminate differences between means. Statistical tests were run for both equal and unequal variances with alpha values set to 0.05. Means values, plus and minus one standard deviation, are used in this paper. Slopes of the natural log of biomass (i.e., growth curves) were statistically compared as Univariate–GLM interactions for each time period.
3. Results
3.1. Results of Yeast and Algae Bioreactors
3.1.1. Yeast Time Series
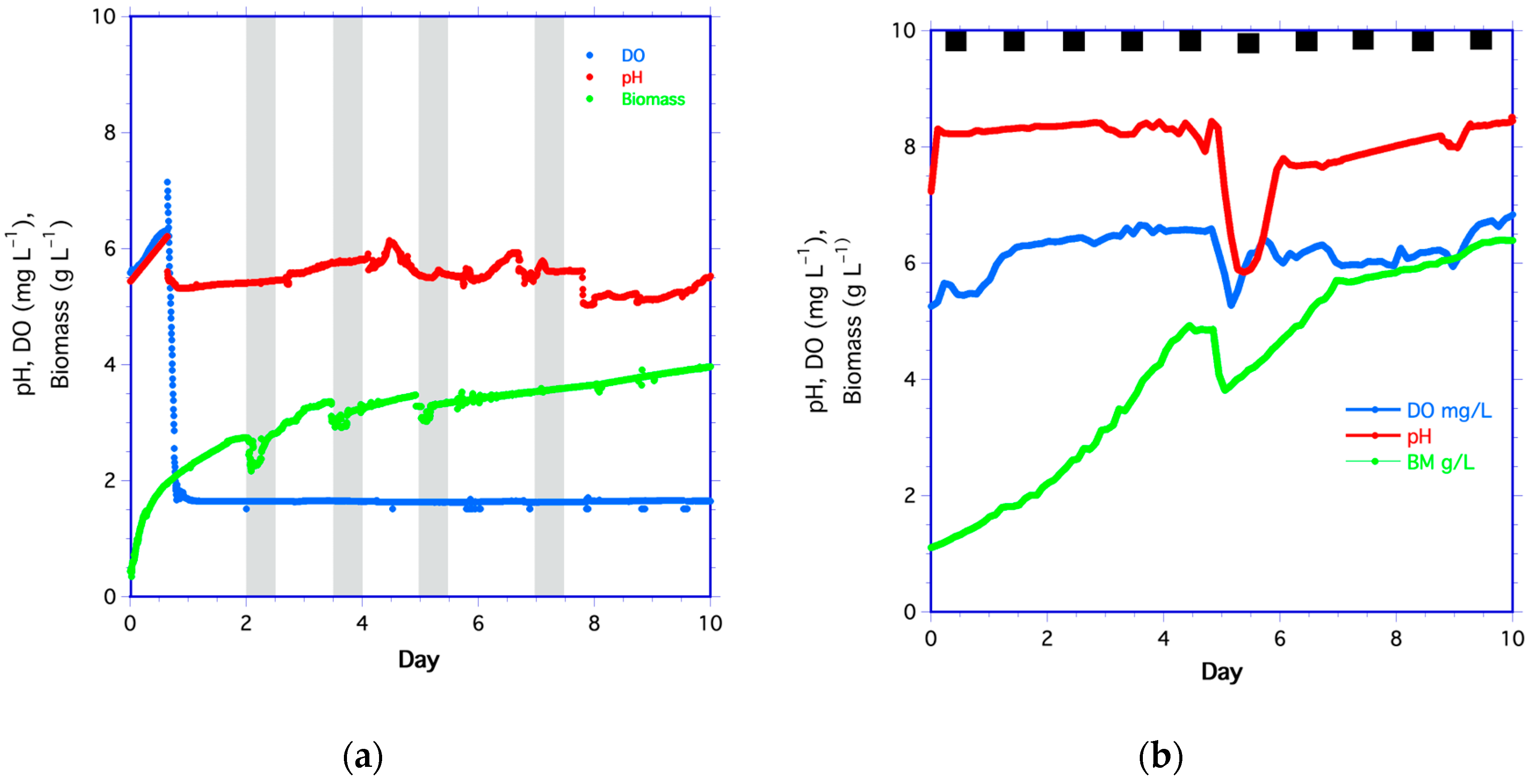
The yeast time series was divided into five periods coinciding with the pulsed feeding schedule (Figure 1a). For each period, biomass was reduced as a result of the dilution but increased as cells grew throughout the remaining period. During most periods, pH was low but increased gradually as biomass increased. The exceptions were at days 4.5, 5, and 6.2 when pH increased soon after the medium pulses (Figure 1a). There was an abrupt decrease in pH on day 7.9, which is unexplained but may have been caused by a bubble in the optical window. Even after this drop in pH, values continued to increase as biomass increased. The fact that pH did not decrease as biomass increased and more cells respired can be attributed to the membrane’s high transport rate of CO2 [78,79,80], which was 5.4 times that of oxygen [81].
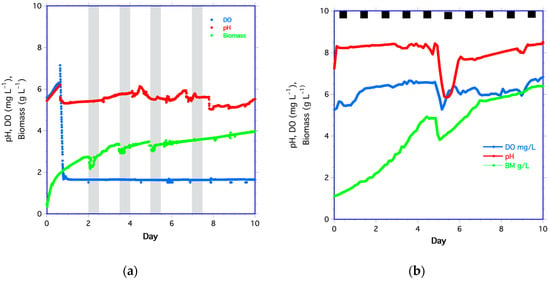
Figure 1.
Time series of dissolved oxygen (DO in blue), pH (in red), and biomass (in green) for (a) yeast and (b) algae. The shaded columns in (a) are the four periods of pulsed medium addition. The black squares in (b) are the dark periods for algae. Algae were pulse-fed on day 4.8.
After the initial inoculation of yeast and medium, DO decreased sharply (Figure 1a). After day 1, DO remained steady at 1.64 ± 0.02 mg L−1 (about 21% of saturation) despite the increase in biomass, suggesting that the yeast respired oxygen at the same rate it diffused through the membrane. Although the yeast medium had higher DO (6.3 mg L−1) than in the bioreactor, DO did not increase after the pulsed medium addition, even though an increase of 1.9 mg L−1 was expected. The most likely explanation is that the higher biomass concentration rapidly respired the available oxygen. The second yeast time series followed the same biomass, DO, and pH trends as in Figure 1a.
3.1.2. Algae Time Series
Algal biomass increased from the start of the experiment (Figure 1b). By day 4.4, biomass leveled off, indicating that the nutrients may have been limiting. A pulse of medium was added on day 4.8 to increase the duration of the experiment. The pH increased abruptly within 2 h of the start of the experiment and then gradually increased thereafter. DO increased rapidly until day 1, then rose at a slower rate. This likely resulted from DO production equilibrating with atmospheric oxygen transport through the membrane. There were decreases in biomass, DO, and pH after the medium pulse on day 4.8, after which biomass increased again. However, by day 7, the rate of biomass increase was slower than growth from days 0 to 4 and 4.8 to 6.2, as indicated by the slopes of these lines.
The algae medium had a DO of 5.8 mg L−1, lower than the DO in the bioreactor. The lower DO accounts for the decrease in DO after the medium pulse on day 4.8. The dilution of biomass and pH by adding medium also accounts for decreases in cell biomass and pH. By day 5, biomass increased, and pH steadily rose. After day 5, DO increased rapidly and then fluctuated until algal growth rates declined on day 7. However, the average biomass, pH, and DO all trended upward until day 10.0. For the second algae time series, biomass, pH, and DO all followed the same trend as in Figure 1b, increasing over time.
3.1.3. Growth Rates, DO, and CO2 Rates
Yeast
The average specific growth rates for yeast during the first 24 h were high, averaging 1.8 ± 0.18 per day for the two independent yeast time series. For the remaining periods (1 to 5), specific growth rates progressively decreased from 0.21 d−1 and 0.19 d−1 for the first two periods to 0.04 d−1 and 0.05 d−1 for the last two periods (Table 4). Slopes of growth rates had r2 > 0.94, showing a good fit for the linear model. Growth rates were significantly different for all periods (df = 5, F = 5771, p < 0.01), indicating that growth was not steady-state.

Table 4.
Rates for time series for yeast and algae.
The changes in pH correlated with changes in pCO2, although other metabolites produced during growth could have contributed to pH [76]. It is certain that the two peaks in pH on days 4.5 and 6.5 were the result of dilution caused by the lower CO2aq concentration of the medium. Therefore, the decrease in pH over the next 0.5 days was likely the result of yeast respiring CO2 at a rate of 0.57 g (L d)−1. This is within the range of 0.43–2.4 g (L d)−1 CO2 respired by Saccharomyces cerevisiae in fermenters during exponential growth [81].
The means of the ratio of dissolved oxygen per unit biomass, Λ, decreased over time for yeast and statistically differed for all periods (df = 4, F = 5083, p < 0.001). However, since there was no change in DO during these periods, changes in the ratios only reflect changes in biomass concentration. The mean of the ratio over all periods was 0.51 mg DO per g of yeast.
The oxygen uptake rate, OUR (Equation (2)), was estimated at 8.6 g (L d)−1 based on the difference between the DO saturated medium (6.2 mg O2 L−1) and the DO depleted bioreactor (1.6 mg O2 L−1) and accounting for oxygen transfer through the membrane (Table 4). Using the same bioreactor set-up but for a continuous culture, Granata et al. [76] showed that yeast had a maximum OUR of 14.3 g (L d)−1, which was 40% higher than the yeast cultures in Figure 1a. The higher OUR in their experiments was probably the result of higher growth rates (4.8 d−1) and the operation of the bioreactor in steady-state conditions (i.e., continuous culture).
Algae
The average specific growth rates for Tetraselmis during the first 24 h were high, with a mean of 0.45 ± 0.15 d−1 for both algae time series. For the next two periods (1–2.5 days and 2.5–4 days), growth rates were lower (0.32 d−1 and 0.34 d−1) and decreased further (0.21 d−1 and 0.05 d−1) from days 5–7 and 7–10 (Table 4). Slopes of growth curves had r2 > 0.97, indicating a good fit for the regression model. Slopes were statistically different (df = 4; F = 108,630; p < 0.001) for all four periods such that specific growth was not in a steady state. Even though algae growth rates were reduced after medium pulses, they were consistent with rates of 0.35 d−1 [82,83,84] and 0.16 d−1 [85] for Tetraselmis cultures under similar light and nutrient concentrations.
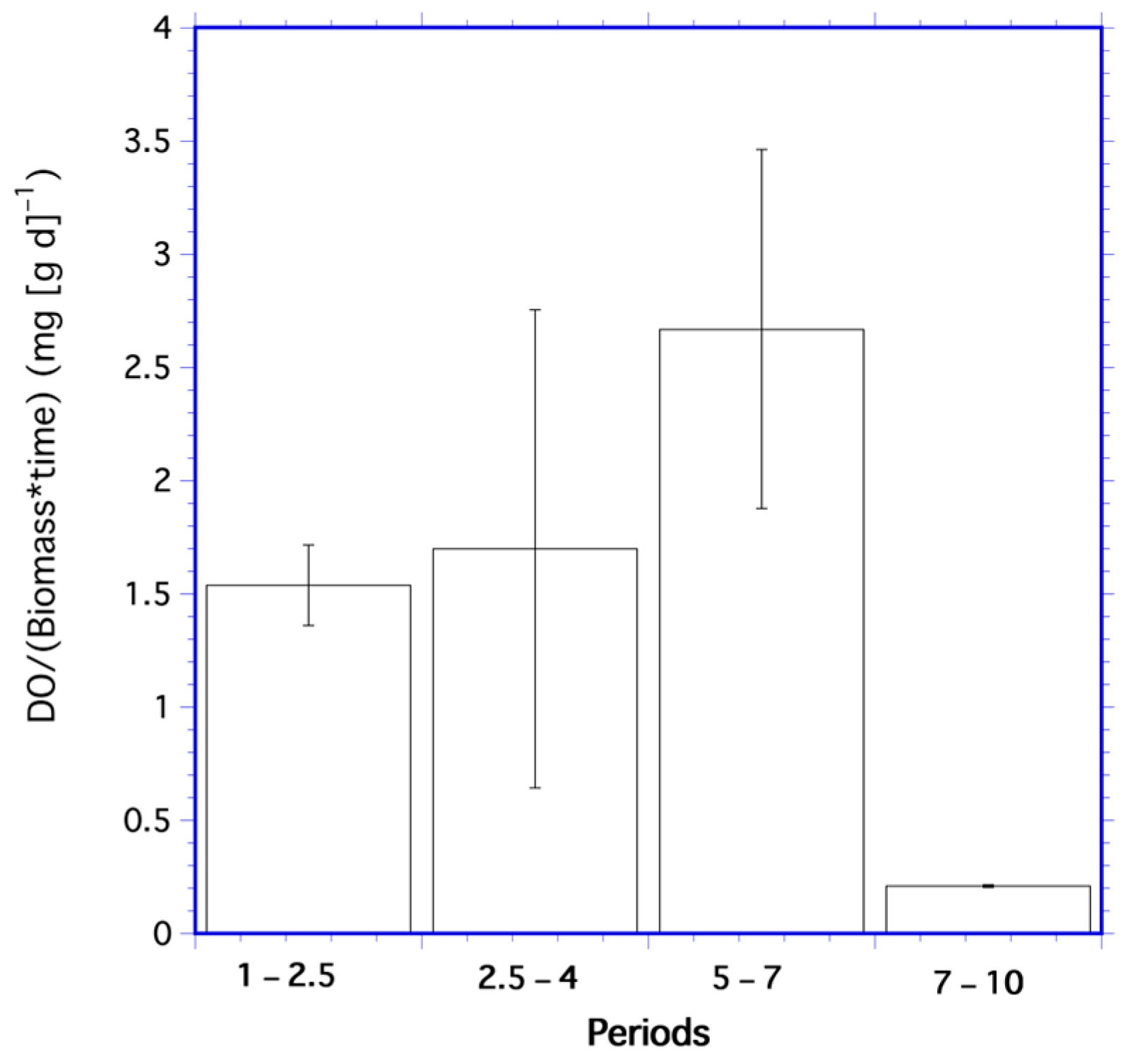
Ratios of mean dissolved oxygen per unit biomass from Table 4 statistically differed over all periods (df = 3; F = 18,516; p < 0.001). The mean of Λ over all periods was 1.8 mg DO per g of algae. The rate of change in these ratios (Figure 2) was also significantly different for each period (df = 3; F = 18,516; p < 0.001), with the mean over all periods of 1.5 mg DO (g d)−1.
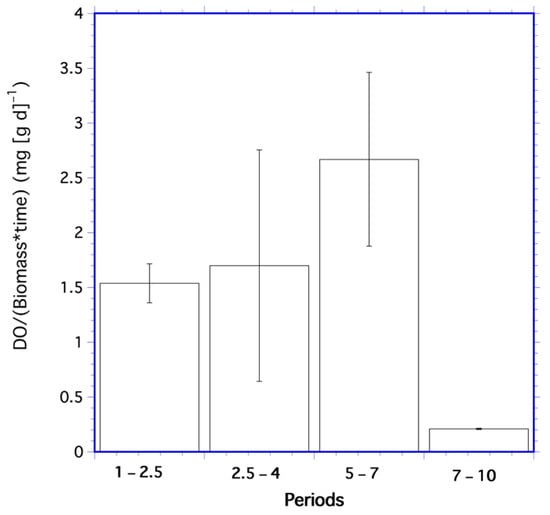
Figure 2.
Mean oxygen per unit biomass rates for the periods 1–2.5 days, 2.5–4 days, 5–7 days, and 7–10 days in the algae time series. Error bars are one standard deviation.
The mean oxygen production rate, P, was 1.3 g (L d)−1 based on the mean DO in the bioreactor (6.5 mg DO L−1), which was more saturated than the medium (5.8 mg L−1). Average cellular oxygen production was 288 pg DO (cell d)−1, similar to the upper range of 228 pg DO (cell d)−1 for algae cultures under identical light and nutrient conditions [86].
3.2. Results from CO2 Capture and Use by Algae
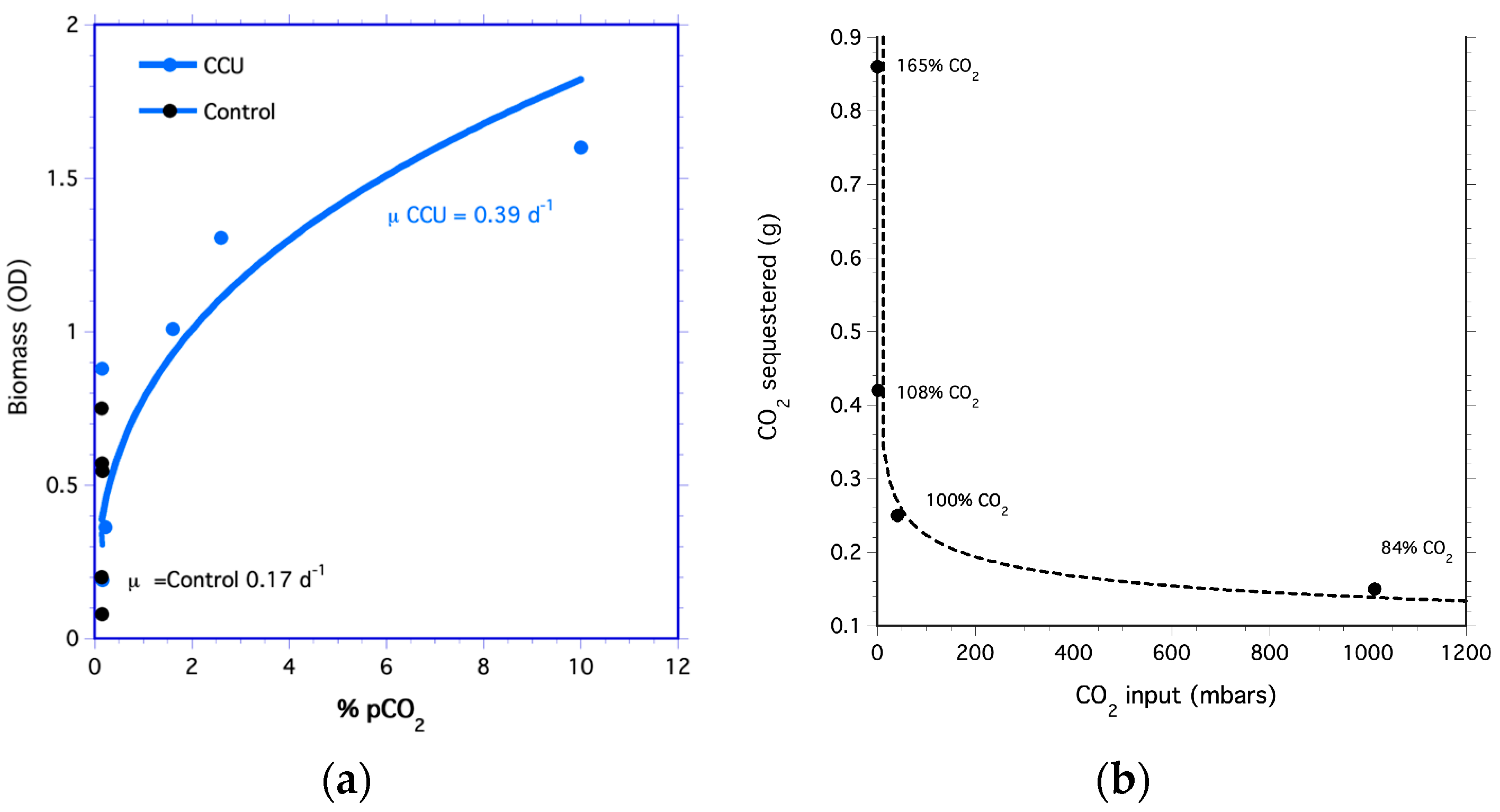
Using membranes, high concentrations of CO2 were hydrated into the algal medium at >99%; thus, all the captured CO2 was made available to the algae culture during the CCU experiment. As the concentrations of CO2 transferred to the culture increased, so did the biomass (Figure 3a). Tetraselmis cells in the medium hydrated with a 10% CO2-air mixture had a growth rate of 0.38 d−1, which was higher than the growth rate of 0.17 d−1 of the control that used filtered air (i.e., 0.04% CO2). These results are similar to studies showing increased algae growth on sequestered CO2 [87,88].
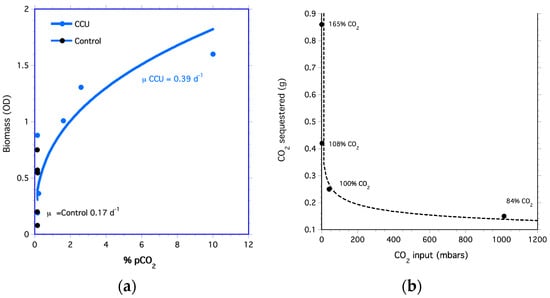
Figure 3.
Carbon capture and uptake by algae. (a) Biomass optical density of Tetraselmis for cells grown on captured carbon (CCU treatment) and cells grown on air (Control). (b) Grams of CO2 sequestered. Percentages are CO2 released after acidification of CO2 sequestered by Ca(OH)2.
The algal growth rate for the aerated bioreactor (control treatment) was lower than the growth rate for cells grown on captured CO2 (CCU treatment). Grams of CO2 sequestered decreased as pCO2 of air-CO2 mixtures increased (Figure 3b). This is because the small amounts of Ca(OH)2 used in the experiment to sequester CO2 were consumed at the higher CO2 concentration. Since CO2 sequestered is directly proportional to the mass of Ca(OH)2, more CO2 can be sequestered by increasing the amount of Ca(OH)2.
Metabolic products and CO2 regulated pH levels in bioreactors. In general, low pH levels can inhibit microbial activity. CO2 gas transport into bioreactor media hydrates dissolved CO2, rapidly converting it to bicarbonate (HCO3−). This process also depends on whether the bioreactor is open or closed to the atmosphere. Changes in pH also depend on the total alkalinity to resist acidification of the system. Consequently, it was important to monitor not only the pH of the bioreactors but also the concentration of CO2 during carbon capture, uptake by algae, and generation of CO2 by yeast. Table 5 shows the carbon chemistry of yeast, algae, and CCU bioreactors. As CO2 is added to a bioreactor, pH decreases, which is consistent with the yeast bioreactor time series and the CCU bioreactor experiment (Table 5). Alternatively, as CO2 is consumed, pH increases, as in the algae time series. Additionally, the yeast and algae bioreactors mimic an open system, which reflects the nature of the gas exchange by the membranes.

Table 5.
Bioreactor carbonate chemistry.
4. Discussion
CO2 and O2 Processes and Resource Synergies
In theory, C6H12O6 + 6O2 => 6CO2 + 6H2O, so heterotrophs use one mole of glucose and six moles of oxygen to produce six moles of CO2. For every gram of glucose, 1.4 g of CO2 and 1.1 g of O2 would be produced. As a first-order approach that neglects cell maintenance and energy demands, we assume that for 50 mg of yeast, 50 mg of glucose is needed. On very short time scales, uptake would be 0.42 g glucose d−1 (24 h/d × 0.35 g glucose/h/g yeast × 0.05 g yeast), requiring 0.46 g of O2 d−1 (0.42 g glucose/d × 1.1 g O2/g glucose) and releasing 0.59 g CO2 d−1 (0.42 g glucose/d × 1.4 g CO2/g glucose).
In the time series for yeast and algae, the ratio of OUR to P was 6.6:1 (8.6:1.3), indicating that the production of oxygen by algae would need to be 6.6× higher to supply the minimum oxygen level for yeast production given the conditions presented in this paper. The mean oxygen to biomass ratio was 0.51 mg DO per g of yeast, while for algae, it was 1.8 mg DO per g of algae, about 3.5× that of yeast. This should be considered when scaling up large microbial factories since yeast uptake of oxygen can be balanced by either high algae biomass concentrations or large algal bioreactor volumes that increase the total biomass and, thus, increase oxygen levels. The downside of large algal cultivation is that biomass production tends to decrease with increased volume [89]. The main reason for this lower production is that most large volumes of algae are cultured in ponds and raceways in which temperature, CO2, and contamination are hard to control, and, thus, production cannot be optimized. Large bioreactors also have a lower surface area of illumination, resulting in less light reaching cells. Additionally, high biomass concentrations increase light attenuation, reducing cell growth. However, bioreactor systems can be better designed to mitigate these problems and optimize production, which will be critical to achieving functional microbial factories in the future.
High CO2 concentrations are problematic on space stations and are already a grave concern in the Earth’s atmosphere. Carbon capture and use can mitigate elevated CO2 levels by coupling reactors that concentrate sequestered CO2 with algal bioreactors that consume it, resulting in bioreactors with increasing production for biomaterials—a win–win synergy. As well as recycling respired CO2 on space stations to assist with life support, microalgal factories can also produce O2. The maximum O2 consumption for an astronaut in space with a body weight of 75 g is 4.6 × 103 L d−1 [90], which equates to 3 kg O2 d−1 at NTP. An algal bioreactor producing 1.3 g O2 (L d)−1 would need to have a volume of 2.3 m3 to provide sufficient O2 for the astronaut. The same size astronaut on a space station would produce 7.4 kg CO2 d−1 [90]. This can be mitigated in a 17 L carbon capture reactor and, in the process, produce precursor chemicals for manufacturing bioproducts by coupling to an algal bioreactor.
The above discussion shows the feasibility of synergies between yeast and algae bioreactors and CCU. As shown in Table 1, Table 2 and Table 3, different biochemical pathways and chemical composition of the cells will produce a diverse range of bioproducts. These processes usually depend on bioreactor design and operations for both heterotrophic and autotrophic cultures. Hence, the synergies discussed in this paper could be utilized to produce many different products. An example is biofuel, where algae can produce biodiesel from lipids, as well as carbon fiber from the glycerol byproduct [59], while yeast can produce unsaturated fatty acids for propanol and butanol [91]. The latter two can be reduced and/or dehydrated to propane and butane [34,35].
The design and operation of bioreactors can also reduce stress on cultures, making them more productive. For most microbes, extremes and fluctuations in temperature, micronutrient concentrations, oxygen levels, and mixing rates can stress cells, affecting the cells’ chemical composition and yields of precursor chemicals. For example, high sugar concentrations increase the growth rates of yeast, but very high concentrations cause osmotic stress [92]. Oxygen limitation causes alcohol fermentation as cultures become anoxic [78,93], which results in cells with fewer proteins. A lack of nitrogen leads to lower protein production [94] since amino acids are inhibited for algae and yeast, as well as most microbes [95,96].
Bioreactors can be operated as batch, fed-batch, and continuous cultures to favor the desired biochemical composition of cells, specific growth rates, and biomass concentration. For algae, high light levels above those saturating the photosystem, as well as the spectral quality of the light, can either inhibit or stimulate growth and lipid production, depending on the species of algae and their pigment composition [6]. A non-limiting nutrient supply is also critical for bioreactor operations. In this study, bioreactors were pulsed with nutrients when biomass began to decline. This is similar to a fed-batch process except that the volume of the cultures remained the same. Granata et al. [76] operated the same bioreactors as continuous cultures to maintain steady-state growth rates for yeast at 4.8 d−1. Although no biochemical data were collected in their study, the side scatter from flow cytometry showed that cells were more densely packed, meaning that they had higher intracellular granularity than cells in this study. This occurred even though the size of the cells was the same based on forward scatter (Granata unpublished data).
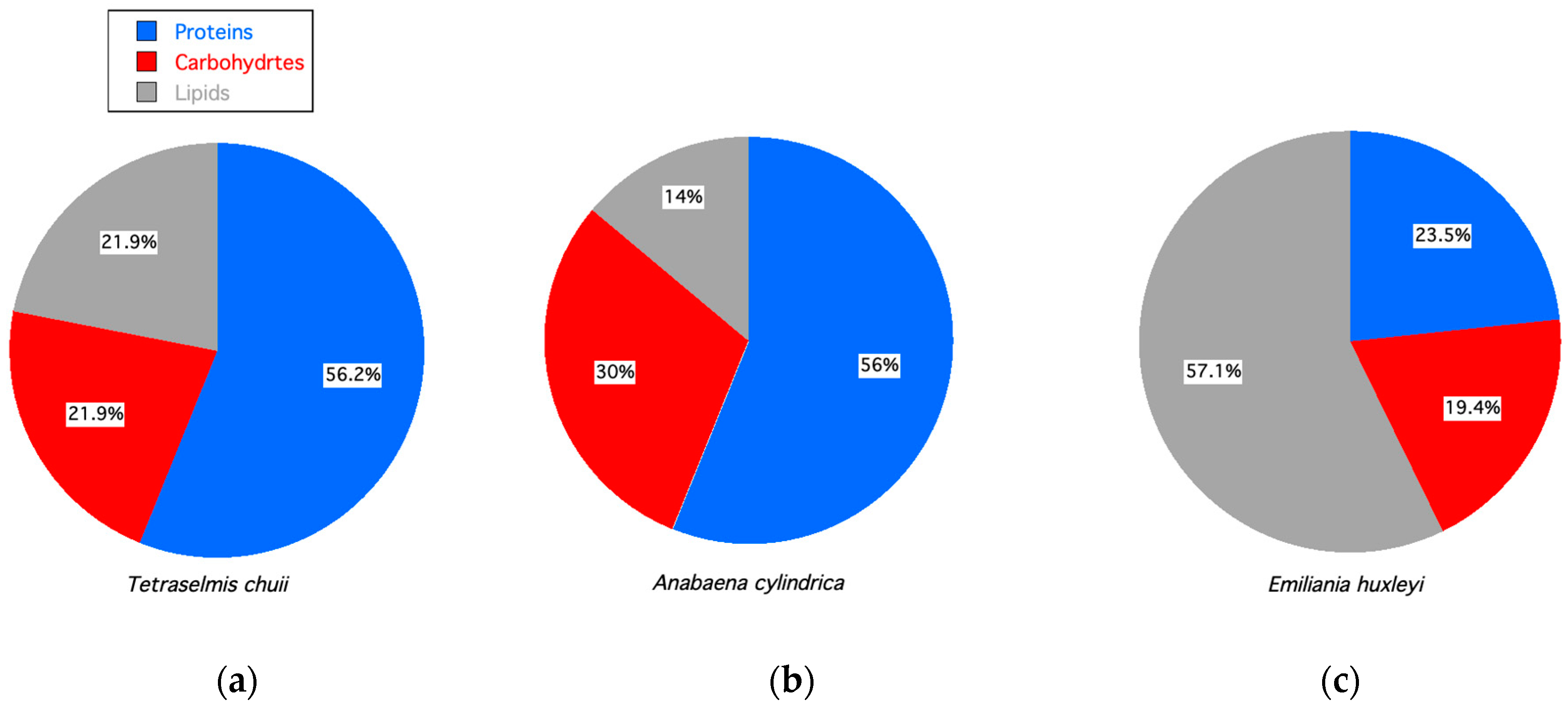
In addition to bioreactor operations, biochemical precursors for bioproducts are also dependent on the species cultured, as illustrated in Figure 4. These three microalgae were grown in similar conditions and sampled at the same growth stage (i.e., stationary) but had different biochemical compositions with variations in lipids (fats), carbohydrates (e.g., sugars, starches), and proteins.
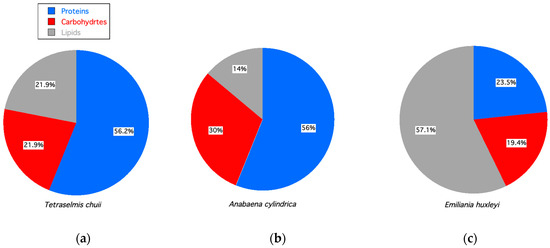
Figure 4.
Percent proteins, carbohydrates, and lipids for microalgae in stationary growth phase. (a) Tetraselmis chuii [84]. (b) Anabeana cylindrica [84]. (c) Emiliania huxleyi [97].
Even the types of proteins, carbohydrates, and lipids can vary as a function of growth conditions. For example, chrysophytes, eustigmatophytes, dinophytes, and xanthophytes produce triacylglycerols, TAGS, when stressed, although some species do so in steady-state growth conditions [98]. Granata et al. [6] found that Tetraselmis sp. and Emiliania huxleyi (a haptophyte) can produce higher neutral lipid concentrations in high light under steady-state growth conditions. It has also been shown that phosphate-limited cultures of Tetraselmis produce more TAGS rich in c16:0 and C18:1 contents [99]. All these factors will affect the types of bioproducts produced by biofactories.
5. Conclusions
In summary, synergistic bioreactors can be operated to recycle waste streams to reduce the input of virgin resources. This has been demonstrated in two cases. First, by capturing CO2 to enhance algae growth. Second, the production of O2 by algae that is available for uptake by yeast, and, conversely, the production of CO2 by yeast to drive algal photosynthesis. On a space station, algae bioreactors could release O2 into the cabin while reducing ambient CO2 levels. Yeast could also use ambient O2 not only to produce biomaterials but also food ingredients [100] and, when deprived of oxygen, to yield consumptive alcohols [101]. On Earth, CO2 capture and use by algae would mitigate atmospheric levels and, combined with an algae–yeast synergy, would contribute to both yeast and algae bioproducts for the bioeconomy, including food supplements and ingredients, bioplastics, and biofuels (just to name a few), some of which are already commercially available. The efficacy of microbial factories in producing a large variety of bioproducts depends on identifying specific strains of microbes that will produce the required precursor chemicals and then designing and operating bioreactors to optimize the chemical composition and yields of cultures.
Author Contributions
Conceptualization, all authors; methodology, T.G. and B.R.; validation and analysis, T.G.; writing—original draft preparation, T.G.; writing—review and editing, M.E., B.R., and F.K.; project administration, B.R. and T.G.; funding, M.E. and B.R. All authors have read and agreed to the published version of the manuscript.
Funding
The development and testing of the bioreactor for time series measurements was funded by the European Space Agency through PRODEX. The CCU experiments were internally funded at the Hochschule Luzern.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to the lead author.
Acknowledgments
We would like to thank PRODEX for funding.
Conflicts of Interest
The authors have no conflict of interest. PRODEX had no role in the design of this study but funded a part of the bioreactor design and validation. The collection, analyses, or interpretation of data and the writing of this manuscript were performed by the Space Biology group and the Algae group at the Hochschule Luzern.
References
- Khoo, C.G.; Dasan, Y.K.; Lam, M.K.; Lee, K.T. Algae biorefinery: Review on a broad spectrum of downstream processes and products. Bioresour. Technol. 2019, 292, 121964. [Google Scholar] [CrossRef]
- Bastiaens, L.; Van Roy, S.; Thomassen, G.; Elst, K. Biorefinery of algae: Technical and economic considerations. In Microalgae-Based Biofuels and Bioproducts from Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing Series in Energy; Woodhead: Sawston, UK, 2017; pp. 327–345. [Google Scholar]
- Rumbold, K.; van Buijsen, H.J.J.; Overkamp, K.M.; van Groenestijn, J.W.; Punt, P.J.; Werf, M.J.v.d. Microbial production host selection for converting second-generation feedstocks into bioproducts. Microb. Cell Fact. 2009, 8, 64. [Google Scholar] [CrossRef]
- Granata, T.; Egli, M. Biological Nutrients: Sustainable Materials Concept. In ESA Report 2016 (AO/1-7707/13/NL/R), T324-001QT; Volume WP 2100 + WP 2200 Milestone Report V02R01; ESA: Paris, France, 2016; pp. 1–25. [Google Scholar]
- Barbosa, M. Microalgal Photobioreactors: Scale-Up and Optimisation; Wageningen University: Wageningen, The Netherlands, 2003. [Google Scholar]
- Granata, T.; Habermacher, P.; Härri, V.; Egli, M. The influence of bio-optical properties of Emiliania huxleyi and Tetraselmis sp. on biomass and lipid production when exposed to different light spectra and intensities of an adjustable LED array and standard light sources. SN Appl. Sci. 2019, 1, 524. [Google Scholar] [CrossRef]
- Chi, Z.-M.; Liu, G.; Zhao, S.; Li, J.; Peng, Y. Marine yeasts as biocontrol agents and producers of bio-products. Appl. Microbiol. Biotechnol. 2010, 86, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- de Jong, E.; Higson, A.; Walsh, P. Bio-Based Chemicals—Value Added Products from Biorefineries; Volume Task 42, Biorefinery; IEA Bioenergy: Paris, France, 2015; p. 36. [Google Scholar]
- Harmsen, J.; Powell, J.B.; Venderbosch, R.H.; Prins, W. Fast Pyrolysis of Biomass for Energy and Chemicals: Technologies at Various Scales; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Mülhaupt, R. Green polymer chemistry and bio-based plastics: Dreams and reality. Macromol. Chem. Phys. 2013, 214, 159–174. [Google Scholar] [CrossRef]
- Rajagopal, R. Bio-Based Chemicals, Specialities and Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Au, S.H.; Shihm, S.C.C.; Wheeler, A.R. Integrated microbioreactor for culture and analysis of bacteria, algae and yeast. Biomed Microdevices 2011, 13, 41–50. [Google Scholar] [CrossRef]
- Rosero-Chasoy, G.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Buitrón, G.; Chairez, I.; Ruiz, H.A. Microbial co-culturing strategies for the production high-value compounds, a reliable framework towards sustainable biorefinery implementation—An overview. Bioresour. Technol. 2021, 321, 124458. [Google Scholar] [CrossRef]
- Sheppard, V.C.; Scheffel, A.; Poulsen, N.; Kröger, N. Live Diatom Silica Immobilization of Multimeric and Redox-Active Enzymes. Appl. Environ. Microbiol. 2012, 78, 211–218. [Google Scholar] [CrossRef]
- Alam, M.A.; Wan, C.; Tran, D.T.; Mofijur, M.; Ahmed, S.F.; Mehmood, M.A.; Shaik, F.; Vo, D.-V.N.; Xu, J. Microalgae binary culture for higher biomass production, nutrients recycling, and efficient harvesting: A review. Environ. Chem. Lett. 2022, 20, 1153–1168. [Google Scholar] [CrossRef]
- Liao, B.Q.; Allen, D.G.; Droppo, I.; Leppard, G.; Liss, S. Surface properties of sludge and their role in bioflocculation and settleability. Water Res. 2001, 35, 339–350. [Google Scholar] [CrossRef]
- Sial, A.; Zhang, B.; Zhang, A.; Liu, K.; Imtiaz, S.A.; Yashir, N. Microalgal–Bacterial Synergistic Interactions and Their Potential Influence in Wastewater Treatment: A Review. BioEnergy Res. 2021, 14, 723–738. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.R.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef]
- Sun, L.; Ma, J.; Li, L.; Tian, Y.; Zhang, Z.; Liao, H.; Li, J.; Tang, W.; He, D. Exploring the essential factors of performance improvement in sludge membrane bioreactor technology coupled with symbiotic algae. Water Res. 2020, 181, 115843. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, W.; Zeng, W.; Yan, Z.; Jia, B.; Li, G.; Liang, H. Organic carbon promotes algae proliferation in membrane-aeration based bacteria-algae symbiosis system (MA-BA). Water Res. 2020, 176, 115736. [Google Scholar] [CrossRef]
- Swain, M.R.; Mishra, J.; Thatoi, H. Bioethanol production from sweet potato (Ipomoea batatas L.) flour using co-culture of Trichoderma sp. and Saccharomyces cerevisiae in solid-state fermentation. Braz. Arch. Biol. Technol. 2013, 56, 171–179. [Google Scholar] [CrossRef]
- Tong, C.Y.; Honsa, K.; Derek, C.J.C. A review on microalgal-bacterial co-culture: The multifaceted role of beneficial bacteria towards enhancement of microalgal metabolite production. Environ. Res. 2023, 228, 15872. [Google Scholar] [CrossRef]
- Rogato, A.; De Tommasi, E. Physical, Chemical, and Genetic Techniques forDiatom Frustule Modification: Applications in Nano technology. Appl. Sci. 2020, 10, 8738. [Google Scholar] [CrossRef]
- Renne, G. Biomineralization. In S&TR; Lawrence Livermore National Lab.: Livermore, CA, USA, 2006; pp. 1–7. [Google Scholar]
- Launey, M.E.; Munch, E.; Alsem, D.H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. A novel biomimetic approach to the design of high-performance ceramic–metal composites. J. R. Soc. Interface 2009, 7, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Bennerman, A.D.; Liu, J.; Wan, W. A ‘degradable’ poly(vinyl alcohol) iron oxide nanoparticle hydrogel. Acta Biomater. 2017, 58, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Bao, Z. Biomaterials-based organic electronic devices. Polym. Int. 2010, 59, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.; Demain, A. Microbial Enzymes: Tools for Biotechnological Processes. Biomolecules 2014, 4, 117–139. [Google Scholar] [CrossRef]
- Bradbury, J. Nature’s Nanotechnologists: Unveiling the Secrets of Diatoms. PLoS Biol. 2004, 2, e306. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Chapter 1—Enzymes in Lipid Modification: An Overview. In Lipid Modification by Enzymes and Engineered Microbes; Volume Enzymes in Lipid Modification: An Overview; AOCS Press: Champaign, IL, USA, 2018; pp. 1–9. [Google Scholar]
- Muskovich, M.; Bettinger, C.J. Biomaterials-Based Electronics: Polymers and Interfaces for Biology and Medicine. Adv. Healthc. Mater. 2012, 1, 248–266. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefin. 2010, 4, 287–295. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Wan, J.U.N. Yeast Species Mediated Bioprocesses and Bio-Products for Biotechnological Application. J. Biotechnol. Biomed. Sci. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Elliston, A.; Le Gall, G.; Colquhoun, I.J.; Collins, S.R.A.; Dicks, J.; Roberts, I.N.; Waldron, K.W. Yeast diversity in relation to the production of fuels and chemicals. Sci. Rep. 2017, 7, 14259. [Google Scholar] [CrossRef] [PubMed]
- Kavscek, M.; Strazar, M.; Curk, T.; Natter, K.; Petrovic, U. Yeast as a cell factory: Current state and perspectives. Microb. Cell Fact. 2015, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Xiao, Y.; Xia, X.X.; Zhao, X.Q.; Peng, L.; Srinophakun, P.; Bai, F.W. Cellulosic ethanol production: Progress, challenges and strategies for solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I.; Farmer, T.J. The integration of green chemistry into future biorefineries. Biofuels Bioprod. Biorefin. 2009, 3, 72–90. [Google Scholar] [CrossRef]
- Van Haveren, J.; Scott, E.L.; Sanders, J. Bulk chemicals from biomass. Biofuels Bioprod. Biorefin. 2008, 2, 41–57. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Cullen, D.; Hibbett, D.; Goodwin, S.B.; Jeffries, T.W.; Kubicek, C.P.; Kuske, C.; Magnuson, J.K.; Martin, V.; Spatafora, J.; et al. Fueling the Future with Fungal Genomics; Contract No. DE-AC02-05CH11231; U.S. Department of Energy: Washington, DC, USA, 2011.
- Khanna, N.; Das, D. Biohydrogen production by dark fermentation. Interdiscip. Rev. Energy Environ. 2012, 2, 363–472. [Google Scholar] [CrossRef]
- Benemann, J.; Rocheleu, R. Biohydrogen Production. In Hydrogen Program, Final Summary Report 1996–2000; U.S. Department of Energy: Washington, DC, USA, 2000. [Google Scholar]
- Mohanty, P.; Pant, K.K.; Mittal, R. Hydrogen generation from biomass materials: Challenges and opportunities. WIREs Energy Environ. 2015, 4, 139–155. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Papadopoulou, C.; Matralis, H.; Verykios, X. Production of renewable hydrogen by reformation of biofuels. WIREs Energy Environ. 2014, 3, 231–253. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Dolfin, E.; Scott, J.R.; Edwards, S.R.; Jones, C.; Curtis, T.P. Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 2013, 97, 6979–6989. [Google Scholar]
- Monnet, F. An Introduction to Anaerobic Digestion of Organic Wastes; Remade Scotland: Crieff, UK, 2003. [Google Scholar]
- Uggetti, E.; Sialve, B.; Trably, E.; Steyer, J.P. Integrating microalgae production with anaerobic digestion: A biorefinery approach. Biofuels Bioprod. Biorefin. 2014, 8, 516–529. [Google Scholar] [CrossRef]
- Kar, Y.; Şen, N. Catalytic pyrolysis of the oily seeds of Styrax officinalis L. for bio-fuels and valuable industrial chemicals. Environ. Prog. Sustain. Energy 2012, 31, 619–627. [Google Scholar] [CrossRef]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(Vinyl Alcohol) Cryogels for Biomedical Applications. In Advances in Polymer Science; Künkel, A., Battagliarin, G., Winnacker, M., Rieger, B., Coates, G., Eds.; Springer Link: Berlin/Heidelberg, Germany, 2014; Volume 263, pp. 283–321. [Google Scholar]
- Wool, R.; Sun, S.X. Affordable Resins and Adhesives from Optimized Soybean Varieties; DEFC07-01ID14217 DOE Final Technical Report 2003; U.S. Department of Energy: Washington, DC, USA, 2004.
- Baker, S.; Harini, B.P.; Rakshith, D.; Satish, S. Marine microbes: Invisible nanofactories. J. Pharm. Res. 2013, 6, 383–388. [Google Scholar] [CrossRef]
- Li, C.; Mezzenga, R. The interplay between carbon nanomaterials and amyloid fibrils in bio-nanotechnology. Nanoscale 2013, 5, 6207–6218. [Google Scholar] [CrossRef]
- Bitton, R.; Bianco-Peled, H. Novel biomimetic adhesives based on algae glue. Macromol. Biosci. 2008, 8, 393–400. [Google Scholar] [CrossRef]
- Molino, P.J.; Chiovitti, A.; Higgins, M.J.; Dugdale, T.M.; Wetherbee, R. Diatom Adhesives: Molecular and Mechanical Properties; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Bamford, N.C.; Howe, P.L. Adhesive Bacterial Exopolysaccharides; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Epstein, L.; Nicholson, R. Adhesion and Adhesives of Fungi and Oomycetes; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Spaic, M.; Small, D.P.; Cook, J.R.; Wan, W. Characterization of anionic and cationic functionalized bacterial cellulose nanofibres for controlled release applications. Cellulose 2014, 21, 1529–1540. [Google Scholar] [CrossRef]
- Mathijsen, D. Algae to sustainably produce carbon fiber and simultaneously take CO2 out of the atmosphere. Reinf. Plast. 2020, 64, 50–53. [Google Scholar] [CrossRef]
- Shen, L.; Worrell, E.; Patel, M.K. Comparing life cycle energy and GHG emissions of bio-based PET, recycled PET, PLA, and man-made cellulosics. Biorefining 2012, 6, 625–639. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Er, A.C.; Aiyub, K.; Mohd Yasin, N.H.; Ngan, S.L.; Chew, K.W.; Khoo, K.S.; Ling, T.C.; Juan, J.C.; Ma, Z.; et al. Current status and perspectives of algae-based bioplastics: A reviewed potential for sustainability. Algal Res. 2023, 71, 103078. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Alvarenga, R.A.F.; Dewulf, J.; De Meester, S.; Wathelet, A.; Villers, J.; Thommeret, R.; Hruska, Z. Life cycle assessment of bioethanol-based PVC. Biofuels Bioprod. Biorefin. 2013, 7, 386–395. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. 10—Calcium Silicate–Based Cements. In Mineral Trioxide Aggregate: Properties and Clinical Applications; Torabinejad, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 342. [Google Scholar]
- Rieger, J.; Kellermeier, M.; Nicoleau, L. Die Bildung von Nanopartikeln und Nanostrukturen—CaCO3, Zement und Polymere aus Sicht der Industrie. Angew. Chem. 2014, 126, 12586–12603. [Google Scholar] [CrossRef]
- Lu, X. Polymer/Calcium Carbonare Nanocomponents; Woodhead Publishing: Sawston, UK, 2006. [Google Scholar]
- Pettinari, C.; Tombesi, A. Metal–organic frameworks for carbon dioxide capture. MRS Energy Sustain. 2020, 7, 35. [Google Scholar] [CrossRef]
- Sevilla, M.; Falco, C.; Titirici, M.-M.; Fuertes, A.B. High-performance CO2 sorbents from algae. J. Mater. Chem. A 2019, 7, 17466. [Google Scholar] [CrossRef]
- Osman, A.I.; Hefny, M.; Maksoud, M.I.A.; Elgarahy, A.M.; Rooney, D.W. Recent advances in carbon capture storage and utilization technologies: A review. Environ. Chem Lett. 2021, 19, 797–849. [Google Scholar] [CrossRef]
- IPCC. Special Report on Carbon Dioxide Capture and Storage; Metz, B., Davidson, O., de Coninck, H.C., Loos, M., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2021; p. 450. [Google Scholar]
- Song, C.; Liu, Q.; Qi, Y.; Chen, G.; Song, Y.; Kansha, Y.; Kitamura, Y. Absorption-microalgae hybrid CO2 capture and biotransformation strategy—A review. Int. J. Greenhouse Gas Control 2019, 88, 109–111. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Cardias, B.B.; de Morais, M.G.; Costa, J.A.V. CO2 conversion by the integration of biological and chemical methods: Spirulina sp. LEB 18 cultivation with diethanolamine and potassium carbonate addition. Bioresour. Technol. 2018, 267, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Granata, T.; Follonier, C.; Burkhardt, C.; Rattenbacher, B. Methods for Oxygenation of Continuous Cultures of Brewer’s Yeast, Saccharomyces cerevisiae. Fermentation 2021, 7, 282. [Google Scholar] [CrossRef]
- Guillard, R. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W., Chanley, M., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Granata, T.; Rattenbacher, B.; John, G. Micro-Bioreactors in Space: Case Study of a Yeast (Saccharomyces cerevisiae) Bioreactor with a Non-Invasive Monitoring Method. Front. Space Technol. 2022, 2, 773814. [Google Scholar] [CrossRef]
- Snoeyink, V.L.; Jenkis, D. Water Chemistry; Johm Wiley & Sons: Hoboken, NJ, USA, 1980; p. 463. [Google Scholar]
- Ibrahim, M.H.; El-Naas, M.H.; Zhang, Z.; Van der Bruggen, B. CO2 Capture Using Hollow Fiber Membranes: A Review of Membrane Wetting. Energy Fuels 2018, 32, 963–978. [Google Scholar] [CrossRef]
- Montoya, P. Membrane Gas Exchange; MedArray, Inc.: Ann Arbor, MI, USA, 2010; pp. 1–7. [Google Scholar]
- Hafeez, S.; Safdar, T.; Pallari, E.; Manos, G.; Aristodemou, E.; Zhang, Z.; Al-Salem, S.M.; Achilleas Constantinou, A. CO2 capture using membrane contactors: A systematic literature review. Front. Chem. Sci. Eng. 2021, 15, 720–754. [Google Scholar] [CrossRef]
- Rosenfeld, E.; Beauvoit, B.; Rigoulet, M.; Salmon, J.M. Non-respiratory oxygen consumption pathways in anaerobically-grown Saccharomyces cerevisiae: Evidence and partial characterization. Yeast 2002, 19, 1299–1321. [Google Scholar] [CrossRef] [PubMed]
- Abiusi, F.; Sampietro, G.; Marturano, G.; Biondi, N.; Rodolfi, L.; D’Ottavio, M.; Tredici, M. Growth, photosynthetic efficiency, and biochemical composition of Tetraselmis suecica F&M-M33 grown with LEDs of different colors. Biotechnol. Bioeng. 2013, 111, 956–964. [Google Scholar] [PubMed]
- Pereira, H.; Páramo, J.; Silva, J.; Marques, A.; Barros, A.; Maurício, D.; Santos, T.; Schulze, P.; Barros, R.; Gouveia, L.; et al. Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO2 mitigation: From an agar plate to 100-m3 industrial photobioreactors. Sci. Rep. 2018, 8, 5112. [Google Scholar] [CrossRef] [PubMed]
- Rafay, R.; Uratani, J.M.; Hernandez, H.H.; Rodríguez, J. Growth and Nitrate Uptake in Nannochloropsis gaditana and Tetraselmis chuii Cultures Grown in Sequential Batch Reactors. Front. Mar. Sci. 2020, 7, 77. [Google Scholar] [CrossRef]
- Lee, W.-K.; Ryu, Y.-K.; Choi, W.-Y.; Kim, T.; Park, A.; Lee, Y.-J.; Jeong, Y.; Lee, C.-G.; Kang, D.-H. Year-Round Cultivation of Tetraselmis sp. for Essential Lipid Production in a Semi-Open Raceway System. Mar. Drugs 2021, 19, 314. [Google Scholar] [CrossRef] [PubMed]
- Nanninga, H.; Tyrrell, E. Importance of light for the formation of algal blooms by Emiliania huxleyi. Mar. Ecol. Prog. Ser. 1996, 136, 195–203. [Google Scholar] [CrossRef]
- Kim, H.-W.; Cheng, J.; Rittmann, B.E. Direct membrane-carbonation photobioreactor producing photoautotrophic biomass via carbon dioxide transfer and nutrient removal. Bioresour. Technol. 2016, 204, 32–37. [Google Scholar] [CrossRef]
- Lai, J.; Yusoff, F.M.; Shariff, M. Large-scale culture of a tropical marine microalga Chaetoceros calcitrans Paulsen Takano 1968 at different temperatures using annular photobioreactors. Pak. J. Biol. Sci. 2012, 15, 635–640. [Google Scholar] [CrossRef][Green Version]
- Granata, T. Dependency of Microalgal Production on Biomass and the Relationship to Yield and Bioreactor Scale-up for Biofuels: A Statistical Analysis of 60+ Years of Algal Bioreactor Data. Bioenergy Res. 2017, 10, 267–287. [Google Scholar] [CrossRef]
- Scott, J.P.R.; Green, D.A.; Weerts, G.; Cheuvront, S.N. Body size and its implications upon resource utilization during human space exploration missions. Nat. Sci. Rep. 2020, 10, 13836. [Google Scholar] [CrossRef]
- Verbelen, P.J.; Depraetere, S.A.; Winderickx, J.; Delvaux, F.R.; Delvaux, F. The influence of yeast oxygenation prior to brewery fermentation on yeast metabolism and the oxidative stress response. FEMS Yeast Res. 2009, 9, 226–239. [Google Scholar] [CrossRef]
- Gomar-Alba, M.; Morcillo-Parra, M.A.; Olmo, M.L. Response of yeast cells to high glucose involves molecular and physiological differences when compared to other osmostress conditions. FEMS Yeast Res. 2015, 15, fov039. [Google Scholar] [CrossRef]
- Vieira, E.D.; Andrietta, M.-d.-G.; Andrietta, S.R. Yeast biomass production: A new approach in glucose-limited feeding strategy. Braz. J. Microbiol. 2013, 44, 551–558. [Google Scholar] [CrossRef]
- Herrero, E.; Ros, J.; Bellí, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta 2008, 1780, 1217–1235. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Takagi, H. Metabolic regulatory mechanisms and physiological roles of functional amino acids and their applications in yeast. Biosci. Biotechnol. Biochem. 2019, 83, 1449–1462. [Google Scholar] [CrossRef]
- Fernandez, E.; Blanch, W.M.; Marañonm, E.; Holligan, P. High rates of lipid biosynthesis in cultured, mesocosm and coastal populations of the coccolithophore Emiliania huxleyi. Mar. Ecol. Prog. Ser. 1994, 114, 13–22. [Google Scholar] [CrossRef]
- Jusoh, M.; Loh, S.H.; Chuah, T.S.; Aziz, A.; Cha, T.S. Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 2015, 111, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; Williams, T.C.; Goold, H.D.; Pretorius, I.S.; Paulsen, I.T. Harnessing bioengineered microbes as a versatile platform for space nutrition. Nat. Commun. 2022, 13, 6177. [Google Scholar] [CrossRef] [PubMed]
- Nutts, R.U. Reinheitsgebot; Duke, W.-I., Ed.; Munich, 1516; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).