Bioproduction of 2-Phenylethanol by Yarrowia lipolytica on Sugar Beet Molasses as a Low-Cost Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Media and Growth Conditions
2.2. Strains and Plasmid Construction
2.2.1. Marker Recovery
2.2.2. Vector Construction through Golden Gate Assembly
2.2.3. Transformations of Y. lipolytica
2.3. Fermentation Conditions
2.3.1. Growth Media Composition and Culture Conditions
2.3.2. Optimization of Culture Media Composition: Design of Experiment
2.4. Analytical Methods: OD, 2-PE, Sugar Consumption
2.5. Elemental Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Strain Construction and Validation of Invertase Activity
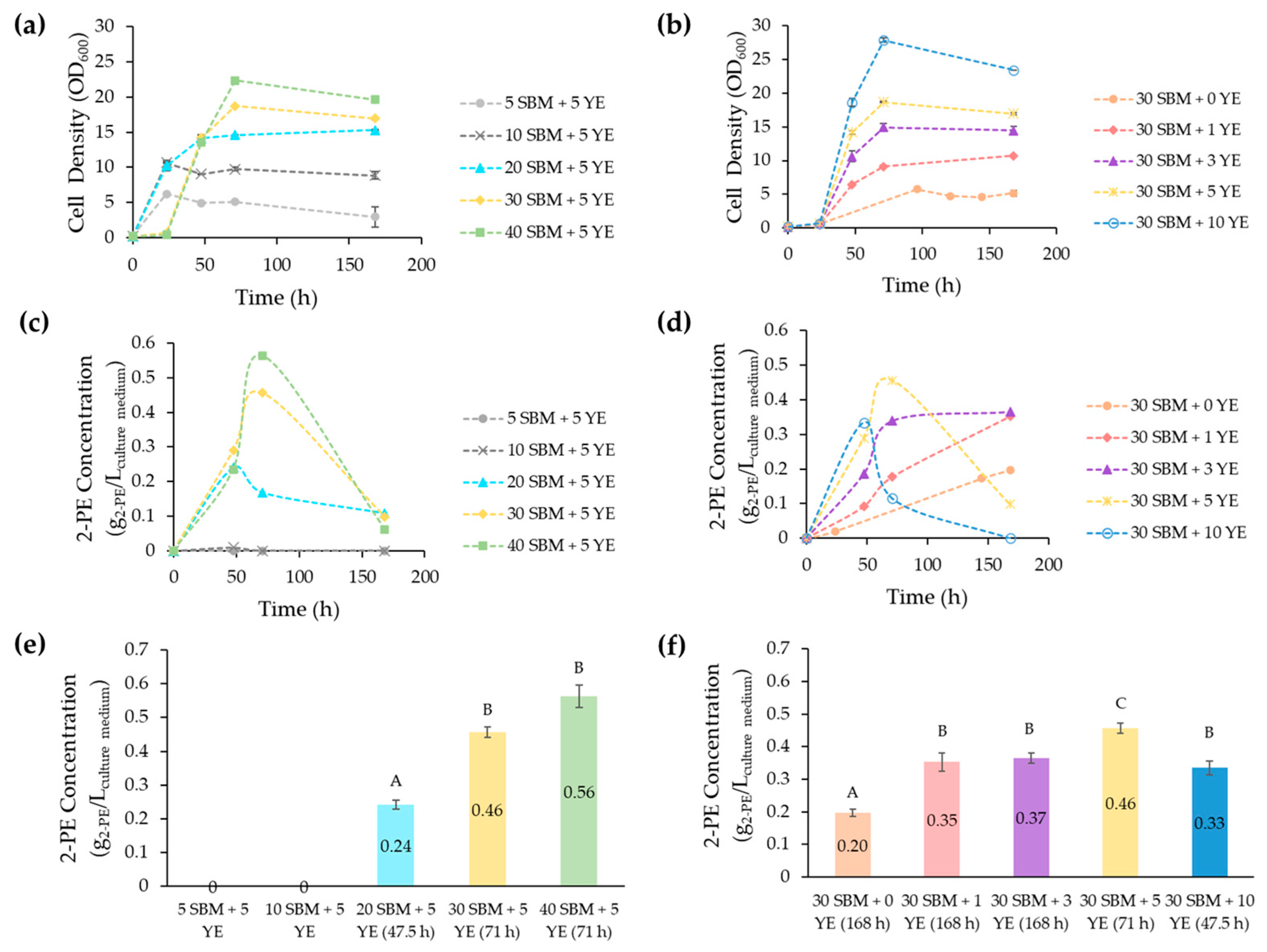
3.2. Impact of SBM and YE on Y. lipolytica’s Growth and 2-PE Production
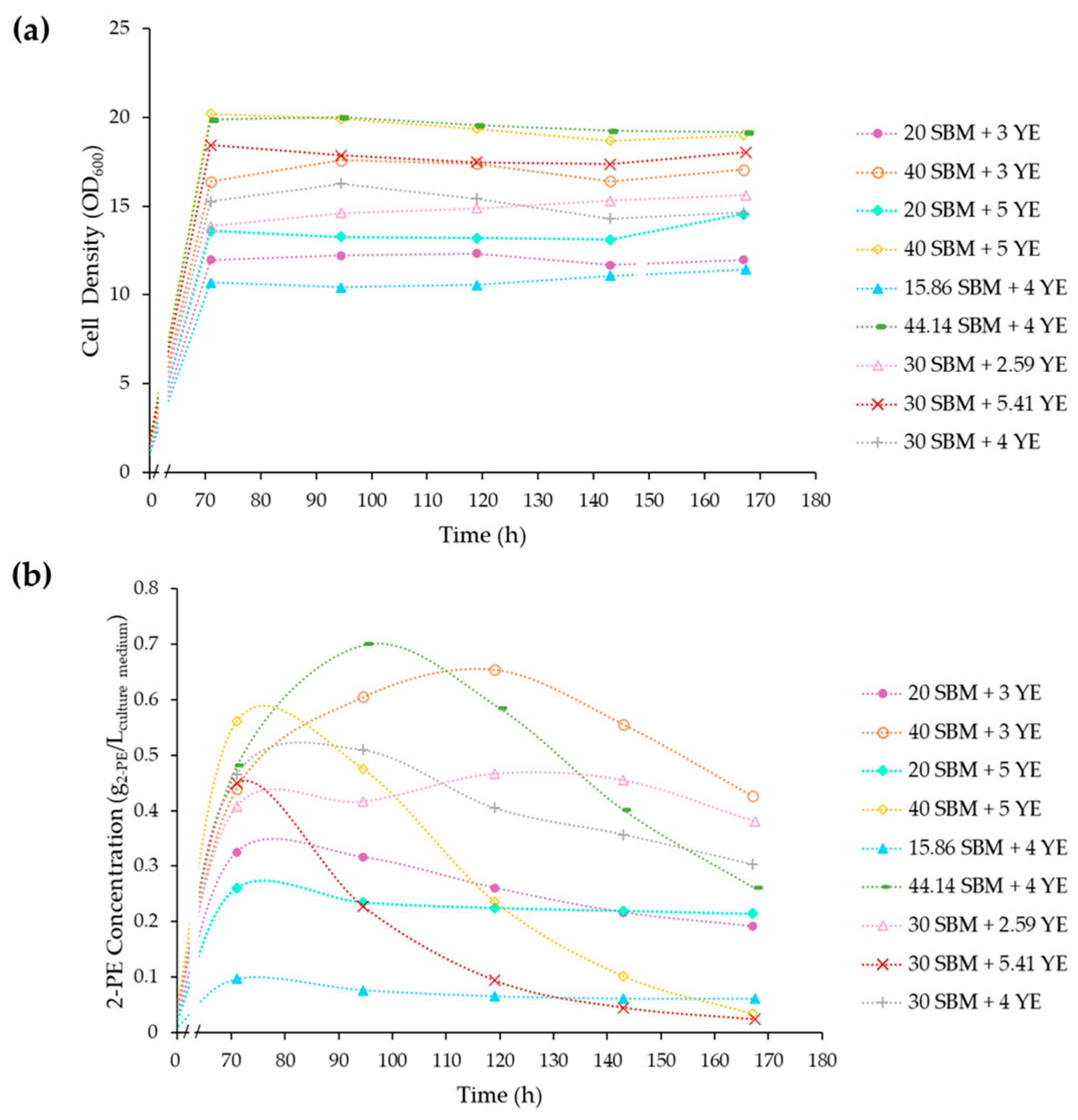
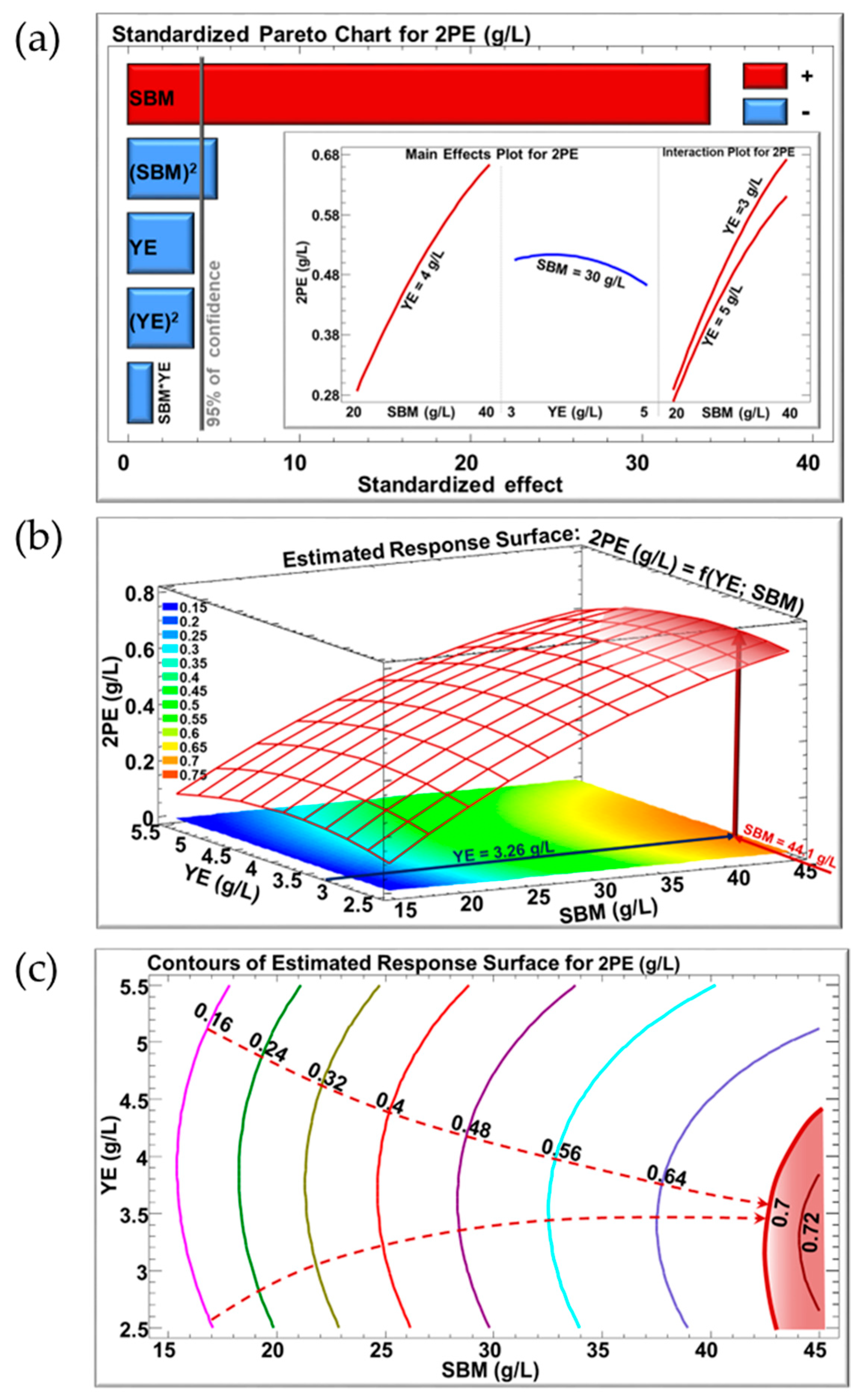
3.3. Optimization of the SBM-Based Culture Media Composition
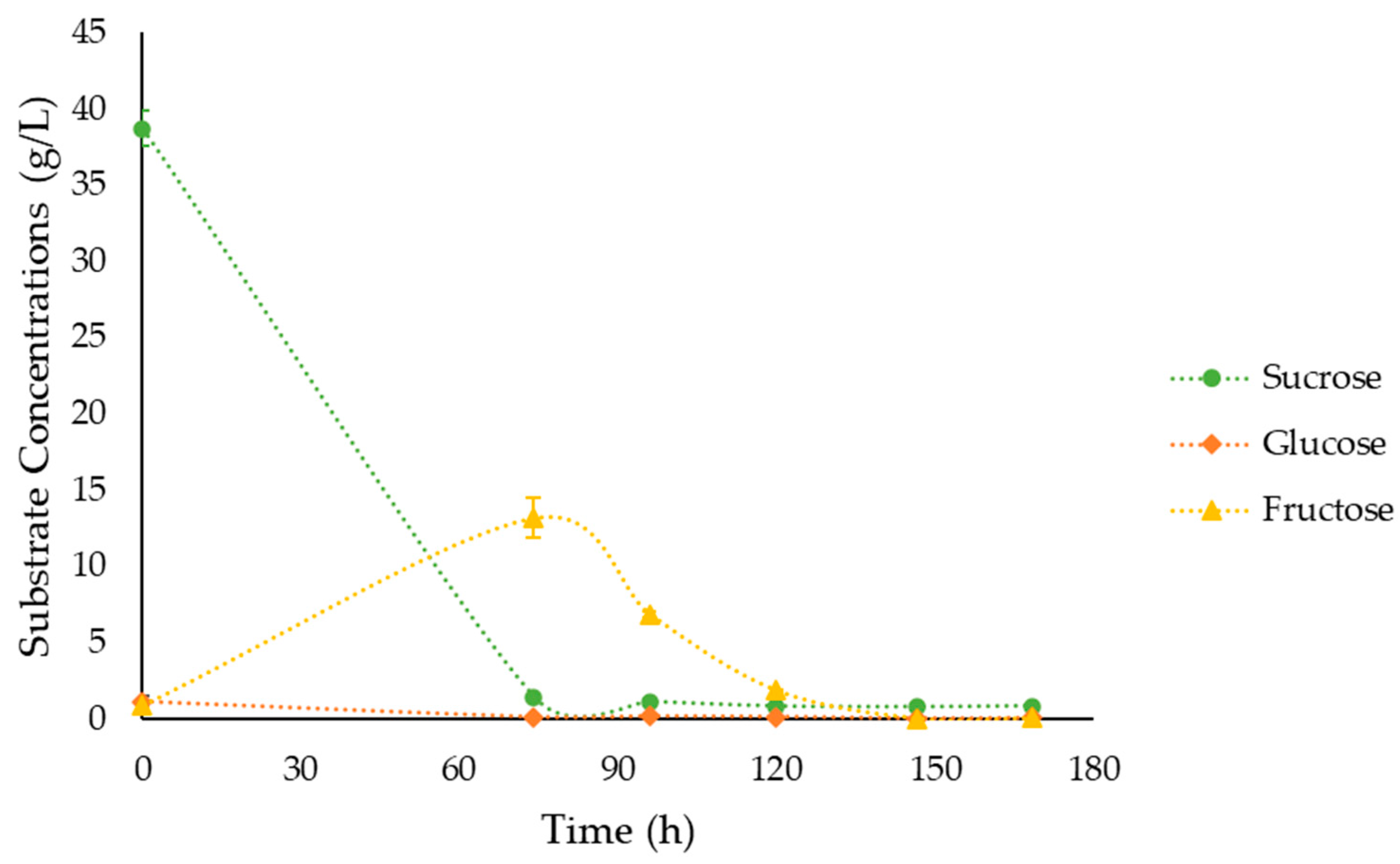
3.4. Fermentations under the Optimal Culture Conditions
| Strain | Carbon Source | Supplementation with L-Phe | 2-PE Production | Reference |
|---|---|---|---|---|
| NCYC3825 (GMO) | 40 g/L Glucose | 7 g/L (Added after 73 h of cultivation) | 1.98 g/L | [25] |
| po1fk7P (GMO) | 40 g/L Glucose | 4 g/L | 2.67 g/L | [55] |
| CH 1/5 (non-GMO) | 40 g/L Crude Glycerol | 8 g/L (at t = 0 h), with an additional 4 g/L added after 80 h of fermentation. | 3.2 g/L | [9] |
| JMY9398 (GMO) | 44.14 g/L SBM | 0 g/L | 0.71 g/L | This study |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, R.; Song, Q.; Xia, H.; Song, N.; Yang, Q.; Zhang, X.; Yao, L.; Yang, S.; Dai, J.; Chen, X. Isolation and Identification of Non-Saccharomyces Yeast Producing 2-Phenylethanol and Study of the Ehrlich Pathway and Shikimate Pathway. J. Fungi 2023, 9, 878. [Google Scholar] [CrossRef]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance Material Review on Phenylethyl Alcohol. Food Chem. Toxicol. 2012, 50, S224–S239. [Google Scholar] [CrossRef] [PubMed]
- Drężek, K.; Kozłowska, J.; Detman, A.; Mierzejewska, J. Development of a Continuous System for 2-Phenylethanol Bioproduction by Yeast on Whey Permeate-Based Medium. Molecules 2021, 26, 7388. [Google Scholar] [CrossRef] [PubMed]
- Lukito, B.R.; Basri, N.; Thong, A.; Hermansen, C.; Weingarten, M.; Peterson, E.C. Co-Culture of Kluyveromyces Marxianus and Meyerozyma Guilliermondii with In Situ Product Recovery of 2-Phenylethanol. J. Agric. Food Chem. 2023, 23, 8991–8997. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Hu, X.; Liu, X.; Zhao, Y.; Xu, Y. Production of 2-Phenylethanol by Microbial Mixed Cultures Allows Resource Recovery of Cane Molasses Wastewater. Fresenius Environ. Bull. 2014, 23, 1356–1365. [Google Scholar]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological Production of 2-Phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [CrossRef]
- Holyavkin, C.; Turanlı-Yıldız, B.; Yılmaz, Ü.; Alkım, C.; Arslan, M.; Topaloğlu, A.; Kısakesen, H.İ.; de Billerbeck, G.; François, J.M.; Çakar, Z.P. Genomic, Transcriptomic, and Metabolic Characterization of 2-Phenylethanol-Resistant Saccharomyces Cerevisiae Obtained by Evolutionary Engineering. Front. Microbiol. 2023, 14, 1148065. [Google Scholar] [CrossRef]
- Zhu, N.; Xia, W.; Wang, G.; Song, Y.; Gao, X.; Liang, J.; Wang, Y. Engineering Corynebacterium Glutamicum for de Novo Production of 2-Phenylethanol from Lignocellulosic Biomass Hydrolysate. Biotechnol. Biofuels Bioprod. 2023, 16, 75. [Google Scholar] [CrossRef]
- Braga, A.; Freitas, B.; Cordeiro, A.; Belo, I. Valorization of Crude Glycerol as Carbon Source for the Bioconversion of L-Phenylamine to 2-Phenylethanol by Yarrowia Species. J. Chem. Technol. Biotechnol. 2021, 96, 2940–2949. [Google Scholar] [CrossRef]
- Alonso-Vargas, M.; Téllez-Jurado, A.; Gómez-Aldapa, C.A.; Ramírez-Vargas, M.D.R.; Conde-Báez, L.; Castro-Rosas, J.; Cadena-Ramírez, A. Optimization of 2-Phenylethanol Production from Sweet Whey Fermentation Using Kluyveromyces Marxianus. Fermentation 2022, 8, 39. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. Bioprocesses for 2-Phenylethanol and 2-Phenylethyl Acetate Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 9991–10004. [Google Scholar] [CrossRef] [PubMed]
- Mitri, S.; Salameh, S.J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- El Kantar, S.; Koubaa, M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 284. [Google Scholar] [CrossRef]
- Gao, H.; Wang, H.; Zhang, Y.; Wang, Y.; Liu, G.; Zhao, Q.; Yu, Z.; Xin, F.; Zhang, W. Design and Optimization of Artificial Light-Driven Microbial Consortia for the Sustainable Growth and Biosynthesis of 2-Phenylethanol. Chem. Eng. J. 2023, 466, 143050. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Dulermo, T.; Nicaud, J.M. Engineering Yarrowia lipolytica to Produce Biodiesel from Raw Starch. Biotechnol. Biofuels 2015, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology; Springer: Berlin/Heidelberg, Germany, 1996; pp. 313–388. [Google Scholar]
- Eszterbauer, E.; Németh, Á. Investigations for a Yarrowia-Based Biorefinery: In Vitro Proof-of-Concept for Manufacturing Sweetener, Cosmetic Ingredient, and Bioemulsifier. Fermentation 2023, 9, 793. [Google Scholar] [CrossRef]
- Carsanba, E.; Agirman, B.; Papanikolaou, S.; Fickers, P.; Erten, H. Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation 2023, 9, 687. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R. What Makes Yarrowia lipolytica Well Suited for Industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.; Mesquita, D.P.; Cordeiro, A.; Belo, I.; Ferreira, E.C.; Amaral, A.L. Monitoring Biotechnological Processes through Quantitative Image Analysis: Application to 2-Phenylethanol Production by Yarrowia lipolytica. Process Biochem. 2023, 129, 221–229. [Google Scholar] [CrossRef]
- Martínez, O.; Sánchez, A.; Font, X.; Barrena, R. Bioproduction of 2-Phenylethanol and 2-Phenethyl Acetate by Kluyveromyces Marxianus through the Solid-State Fermentation of Sugarcane Bagasse. Appl. Microbiol. Biotechnol. 2018, 102, 4703–4716. [Google Scholar] [CrossRef]
- Carlquist, M.; Gibson, B.; Karagul Yuceer, Y.; Paraskevopoulou, A.; Sandell, M.; Angelov, A.I.; Gotcheva, V.; Angelov, A.D.; Etschmann, M.; de Billerbeck, G.M.; et al. Process Engineering for Bioflavour Production with Metabolically Active Yeasts—A Mini-Review. Yeast 2015, 32, 123–143. [Google Scholar] [CrossRef]
- Kong, S.; Pan, H.; Liu, X.; Li, X.; Guo, D. De Novo Biosynthesis of 2-Phenylethanol in Engineered Pichia Pastoris. Enzym. Microb. Technol. 2020, 133, 109459. [Google Scholar] [CrossRef]
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods 2022, 11, 109. [Google Scholar] [CrossRef]
- Celińska, E.; Kubiak, P.; Białas, W.; Dziadas, M.; Grajek, W. Yarrowia lipolytica: The Novel and Promising 2-Phenylethanol Producer. J. Ind. Microbiol. Biotechnol. 2013, 40, 389–392. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia Kudriavzevii through Solid-State Fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Lazar, Z.; Dulermo, T.; Neuvéglise, C.; Crutz-Le Coq, A.M.; Nicaud, J.M. Hexokinase—A Limiting Factor in Lipid Production from Fructose in Yarrowia lipolytica. Metab. Eng. 2014, 26, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.T.; Song, H.; Raschbauer, M.; Emerstorfer, F.; Omann, M.; Stelzer, F.; Neureiter, M. Utilization of Desugarized Sugar Beet Molasses for the Production of Poly(3-Hydroxybutyrate) by Halophilic Bacillus megaterium Uyuni S29. Process Biochem. 2019, 86, 9–15. [Google Scholar] [CrossRef]
- Saric, L.; Filipcev, B.; Simurina, O.; Plavsic, D.; Saric, B.; Lazarevic, J.; Milovanovic, I. Sugar Beet Molasses: Properties and Applications in Osmotic Dehydration of Fruits and Vegetables. Food Feed Res. 2016, 43, 135–144. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Beigbeder, J.B.; de Medeiros Dantas, J.M.; Lavoie, J.M. Optimization of Yeast, Sugar and Nutrient Concentrations for High Ethanol Production Rate Using Industrial Sugar Beet Molasses and Response Surface Methodology. Fermentation 2021, 7, 86. [Google Scholar] [CrossRef]
- Grigs, O.; Didrihsone, E.; Bolmanis, E. Investigation of a Broad-Bean Based Low-Cost Medium Formulation for Bacillus Subtilis MSCL 897 Spore Production. Fermentation 2023, 9, 390. [Google Scholar] [CrossRef]
- Larroude, M.; Nicaud, J.M.; Rossignol, T. Yarrowia lipolytica Chassis Strains Engineered to Produce Aromatic Amino Acids via the Shikimate Pathway. Microb. Biotechnol. 2021, 14, 2420–2434. [Google Scholar] [CrossRef]
- Fickers, P.; Le Dall, M.T.; Gaillardin, C.; Thonart, P.; Nicaud, J.M. New Disruption Cassettes for Rapid Gene Disruption and Marker Rescue in the Yeast Yarrowia lipolytica. J. Microbiol. Methods 2003, 55, 727–737. [Google Scholar] [CrossRef]
- Larroude, M.; Park, Y.K.; Soudier, P.; Kubiak, M.; Nicaud, J.M.; Rossignol, T. A Modular Golden Gate Toolkit for Yarrowia lipolytica Synthetic Biology. Microb. Biotechnol. 2019, 12, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.C.; Beckerich, J.M.; Gaillardin, C. One-Step Transformation of the Dimorphic Yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 1997, 48, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Gajdoš, P.; Nicaud, J.M.; Rossignol, T.; Čertík, M. Single Cell Oil Production on Molasses by Yarrowia lipolytica Strains Overexpressing DGA2 in Multicopy. Appl. Microbiol. Biotechnol. 2015, 99, 8065–8074. [Google Scholar] [CrossRef]
- Spagnuolo, M.; Hussain, M.S.; Gambill, L.; Blenner, M. Alternative Substrate Metabolism in Yarrowia lipolytica. Front. Microbiol. 2018, 9, 320134. [Google Scholar] [CrossRef]
- Lazar, Z.; Rossignol, T.; Verbeke, J.; Crutz-Le Coq, A.M.; Nicaud, J.M.; Robak, M. Optimized Invertase Expression and Secretion Cassette for Improving Yarrowia lipolytica Growth on Sucrose for Industrial Applications. J. Ind. Microbiol. Biotechnol. 2013, 40, 1273–1283. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B. Process Optimization of Biological Hydrogen Production from Molasses by a Newly Isolated Clostridium Butyricum W5. J. Biosci. Bioeng. 2009, 107, 138–144. [Google Scholar] [CrossRef]
- Al Sahyouni, W.; El Kantar, S.; Khelfa, A.; Park, Y.K.; Nicaud, J.M.; Louka, N.; Koubaa, M. Optimization of Cis-9-Heptadecenoic Acid Production from the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 245. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, Y.; Ke, C.; Bai, Y.; Liu, X.; Li, S. Production of Welan Gum from Cane Molasses by Sphingomonas Sp. FM01. Carbohydr. Polym. 2020, 244, 116485. [Google Scholar] [CrossRef]
- Eliodório, K.P.; de Gois e Cunha, G.C.; de Oliveira Lino, F.S.; Sommer, M.O.A.; Gombert, A.K.; Giudici, R.; Basso, T.O. Physiology of Saccharomyces Cerevisiae during Growth on Industrial Sugar Cane Molasses Can Be Reproduced in a Tailor-Made Defined Synthetic Medium. Sci. Rep. 2023, 13, 10567. [Google Scholar] [CrossRef] [PubMed]
- Taskin, M.; Ortucu, S.; Aydogan, M.N.; Arslan, N.P. Lipid Production from Sugar Beet Molasses under Non-Aseptic Culture Conditions Using the Oleaginous Yeast Rhodotorula Glutinis TR29. Renew. Energy 2016, 99, 198–204. [Google Scholar] [CrossRef]
- Olbrich, H. The Molasses. Princ. Sugar Technol. 1963, 3, 511–697. [Google Scholar]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Mouret, J.R. Combined Effects of Nutrients and Temperature on the Production of Fermentative Aromas by Saccharomyces Cerevisiae during Wine Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- de Jesús Rodríguez-Romero, J.; Aceves-Lara, C.A.; Silva, C.F.; Gschaedler, A.; Amaya-Delgado, L.; Arrizon, J. 2-Phenylethanol and 2-Phenylethylacetate Production by Nonconventional Yeasts Using Tequila Vinasses as a Substrate. Biotechnol. Rep. 2020, 25, e00420. [Google Scholar] [CrossRef]
- Wittmann, C.; Hans, M.; Bluemke, W. Metabolic Physiology of Aroma-Producing Kluyveromyces Marxianus. Yeast 2002, 19, 1351–1363. [Google Scholar] [CrossRef]
- Conde-Báez, L.; Castro-Rosas, J.; Villagómez-Ibarra, J.R.; Páez-Lerma, J.B.; Gómez-Aldapa, C. Evaluation of Waste of the Cheese Industry for the Production of Aroma of Roses (Phenylethyl Alcohol). Waste Biomass Valorization 2017, 8, 1343–1350. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; Jin, Y.; Liu, H.; Zhang, H.; Sun, Y.; Liu, G. Biosynthesis of 2-Phenylethanol Using Tobacco Waste as Feedstock. Biocatal. Biotransform. 2013, 31, 292–298. [Google Scholar] [CrossRef]
- Etschmann, M.M.W.; Sell, D.; Schrader, J. Screening of Yeasts for the Production of the Aroma Compound 2-Phenylethanol in a Molasses-Based Medium. Biotechnol. Lett. 2003, 25, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, P. Recent Advances in Biotechnological Production of 2-Phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Arias, S.; Olivera, E.R.; Arcos, M.; Naharro, G.; Luengo, J.M. Genetic Analyses and Molecular Characterization of the Pathways Involved in the Conversion of 2-Phenylethylamine and 2-Phenylethanol into Phenylacetic Acid in Pseudomonas Putida U. Environ. Microbiol. 2008, 10, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ma, J.; Zhu, Y.; Xu, P. Refactoring Ehrlich Pathway for High-Yield 2-Phenylethanol Production in Yarrowia lipolytica. ACS Synth. Biol. 2020, 9, 623–633. [Google Scholar] [CrossRef]
- Celińska, E.; Ledesma-Amaro, R.; Larroude, M.; Rossignol, T.; Pauthenier, C.; Nicaud, J.M. Golden Gate Assembly System Dedicated to Complex Pathway Manipulation in Yarrowia lipolytica. Microb. Biotechnol. 2017, 10, 450–455. [Google Scholar] [CrossRef]



| Strain Number | Description/Genotype | Reference | |
|---|---|---|---|
| E. coli | JME547 | pUB4-CRE (2.1 kb Cre fragment in pUB4) | [35] |
| JME1657 (DH5α) | ZetapTEF-SUC2-LEU2ex | [38] | |
| JME5742 | ZetaUP-URA3-8UASpTEF–AMG-8UASpTEF-ScAro3-8UASpTEF-α amylase-tLip2-Zeta Down | This study | |
| Y. lipolytica | JMY8032 | Po1d + URA3ex-YlARO1-YlARO2 + LEU2ex(recovered)ScARO4K229L-ScARO7T226I + LEU2ex-YlTKL + NATex-YlARO8-YlARO10 | [34] |
| JMY9390 | Po1d + URA3ex(recovered)-YlARO1-YlARO2 + LEU2ex(recovered)ScARO4K229L-ScARO7T226I + LEU2ex(recovered)-YlTKL + NATex(recovered)-YlARO8-YlARO10 | This study | |
| JMY9392 | JMY9390 + pTEF-SUC2-LEU2ex | This study | |
| JMY9398 | JMY9392 + ZetaUP-URA3-8UASpTEF–AMG-8UASpTEF-ScAro3-8UASpTEF-α amylase-tLip2-Zeta Down | This study |
| Trial | SBM (g/L of Sucrose) | YE (g/L) |
|---|---|---|
| 1 | 5 | 5 |
| 2 | 10 | 5 |
| 3 | 20 | 5 |
| 4 | 30 | 5 |
| 5 | 40 | 5 |
| 6 | 30 | 1 |
| 7 | 30 | 3 |
| 8 | 30 | 10 |
| 9 | 30 | 0 |
| Experiment | SBM (g/L of Sucrose) | YE (g/L) | 2-PE Produced (g/L) | SD | |||
|---|---|---|---|---|---|---|---|
| Coded Variable | Decoded Variable | Coded Variable | Decoded Variable | ||||
| 1 | n = 2 | −1 | 20 | −1 | 3 | 0.33 | 0.01 |
| 2 | n = 2 | +1 | 40 | −1 | 3 | 0.65 | 0.01 |
| 3 | n = 2 | −1 | 20 | +1 | 5 | 0.26 | 0.03 |
| 4 | n = 2 | +1 | 40 | +1 | 5 | 0.56 | 0.01 |
| 5 | n = 2 | −α (−1.41) | 15.86 | 0 | 4 | 0.10 | 0.02 |
| 6 | n = 2 | +α (+1.41) | 44.14 | 0 | 4 | 0.70 | 0.01 |
| 7 | n = 2 | 0 | 30 | –α (−1.41) | 2.59 | 0.47 | 0.01 |
| 8 | n = 2 | 0 | 30 | +α (+1.41) | 5.41 | 0.45 | 0.02 |
| 9 | n = 3 | 0 | 30 | 0 | 4 | 0.51 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitri, S.; Louka, N.; Rossignol, T.; Maroun, R.G.; Koubaa, M. Bioproduction of 2-Phenylethanol by Yarrowia lipolytica on Sugar Beet Molasses as a Low-Cost Substrate. Fermentation 2024, 10, 290. https://doi.org/10.3390/fermentation10060290
Mitri S, Louka N, Rossignol T, Maroun RG, Koubaa M. Bioproduction of 2-Phenylethanol by Yarrowia lipolytica on Sugar Beet Molasses as a Low-Cost Substrate. Fermentation. 2024; 10(6):290. https://doi.org/10.3390/fermentation10060290
Chicago/Turabian StyleMitri, Sara, Nicolas Louka, Tristan Rossignol, Richard G. Maroun, and Mohamed Koubaa. 2024. "Bioproduction of 2-Phenylethanol by Yarrowia lipolytica on Sugar Beet Molasses as a Low-Cost Substrate" Fermentation 10, no. 6: 290. https://doi.org/10.3390/fermentation10060290









