The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products
Abstract
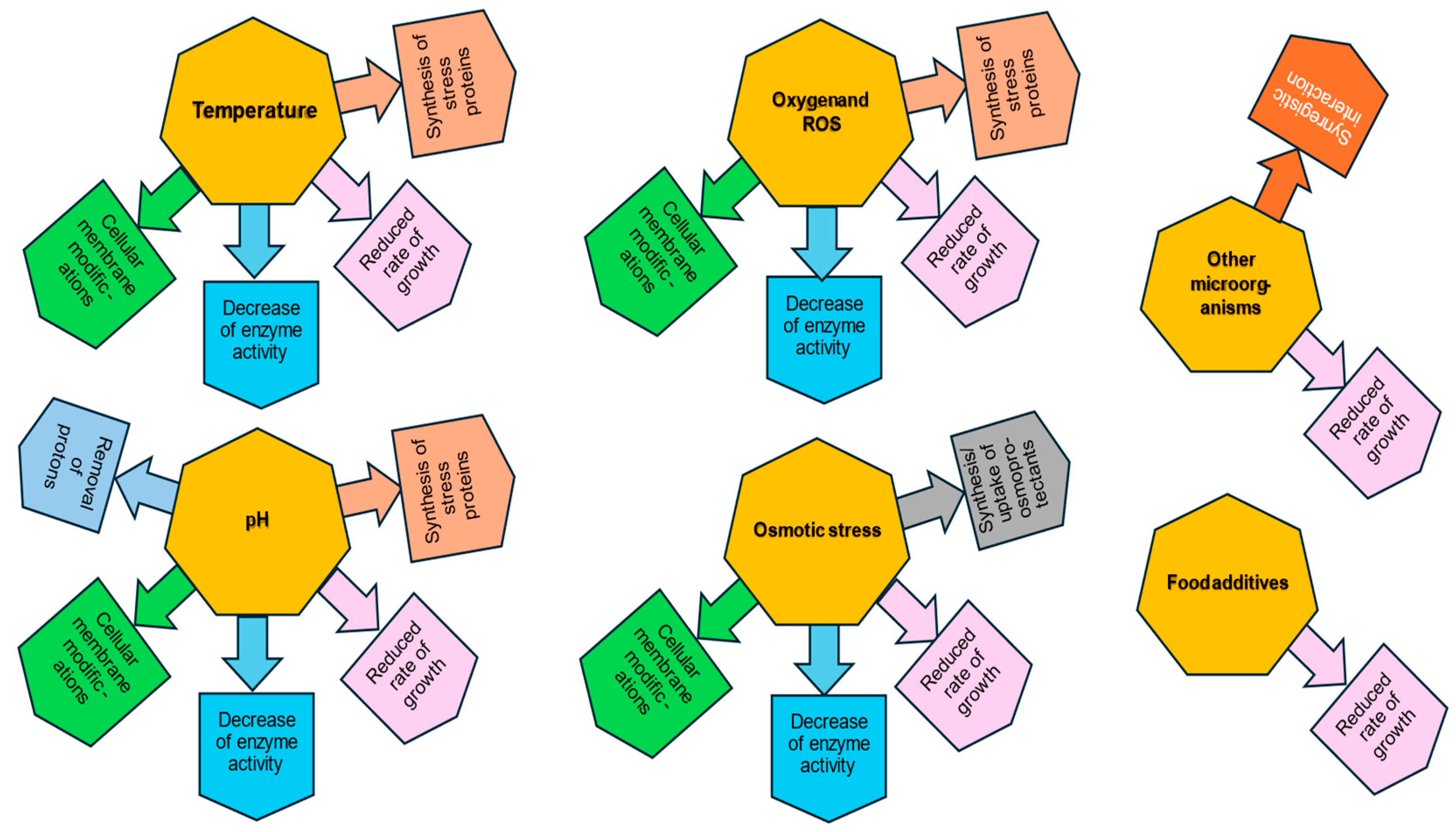
:1. Introduction
2. Effect of Temperature on LAB Survival
3. Influence of pH on LAB and Their Adaptation to Acid Stress
4. Influence of Oxygen
5. Influence of Osmotic Stress and Water Activity
6. Additives
7. Methods to Reduce the Occurrence and Impact of Stress Factors on LAB
8. Conclusions and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Gendy, S.M.; Shaker, M. Fermented foods of Egypt and the Middle East. J. Food Prot. 1983, 46, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P. Diversity of fermented foods. In Fermented Foods and Beverages of the World, 1st ed.; Tamang, J.P., Kailasapathy, K., Eds.; CRC Press: New York, NY, USA, 2010; pp. 41–84. [Google Scholar]
- Sionek, B.; Szydłowska, A.; Küçükgöz, K.; Kołożyn-Krajewska, D. Traditional and New Microorganisms in Lactic Acid Fermentation of Food. Fermentation 2023, 9, 1019. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.; Mattarell, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food. Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Zheng, J.; Ruan, L.; Sun, M.; Gänzle, M.G. A genomic view of Lactobacilli and Pediococci demonstrates that phylogeny matches ecology and physiology. Appl. Environ. Microbiol. 2015, 81, 7233–7243. [Google Scholar] [CrossRef] [PubMed]
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in transition: Evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev. 2017, 41, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Generally Recognized as Safe (GRAS)|FDA. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 20 April 2024).
- Qualified Presumption of Safety (QPS)|EFSA. Available online: https://efsa.europa.eu/en/topic/qualified-presumption-safety-qps (accessed on 20 April 2024).
- Booth, I.R. Stress and the single cell: Intrapopulation diversity is a mechanism to ensure survival upon exposure to stress. Int. J. Food Microbiol. 2002, 78, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Mbye, M.; Baig, M.A.; AbuQamar, S.F.; El-Tarabily, K.A.; Obaid, R.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Turner, M.S.; Shah, N.P.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef]
- Gomand, F.; Borges, F.; Burgain, J.; Guerin, J.; Revol-Junelles, A.M.; Gaiani, C. Food matrix design for effective lactic acid bacteria delivery. Ann. Rev. Food Sci. Technol. 2019, 10, 285–310. [Google Scholar] [CrossRef] [PubMed]
- Kathiriya, M.R.; Vekariya, Y.V.; Hati, S. Understanding the Probiotic Bacterial Responses Against Various Stresses in Food Matrix and Gastrointestinal Tract: A Review. Probiotics Antimicrob. Proteins 2023, 15, 1032–1048. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.; Pinto, C.A.; Saraiva, J.A. The dependence of microbial inactivation by emergent nonthermal processing technologies on pH and water activity. Innov. Food Sci. Emerg. Technol. 2023, 89, 103460. [Google Scholar] [CrossRef]
- Brauer, A.M.; Shi, H.; Levin, P.A.; Huang, K.C. Physiological and regulatory convergence between osmotic and nutrient stress responses in microbes. Curr. Opin. Cell Biol. 2023, 81, 102170. [Google Scholar] [CrossRef] [PubMed]
- Sazykin, I.S.; Sazykina, M.A. The role of oxidative stress in genome destabilization and adaptive evolution of bacteria. Gene 2023, 857, 147170. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.E.D.; Dodd, C.E.R.; Gibson, P.T.; Booth, I.R.; Stewart, G.S.A.B. The significance of bacteria in stationary phase to food microbiology. Int. J. Food Microbiol. 1995, 28, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Davis, C. Enumeration of probiotic strains: Review of culture-dependent and alternative techniques to quantify viable bacteria. J. Microbiol. Method. 2014, 103, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ozogul, F.; Yazgan, H.; Ozogul, Y. Lactic acid bacteria: Lactobacillus acidophilus. In Encyclopedia of Dairy Sciences, 3rd ed.; Mc Sweeney, P.L., McNamara, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 187–197. [Google Scholar]
- Oberg, T.S.; McMahon, D.J.; Culumber, M.D.; McAuliffe, O.; Oberg, C.J. Invited review: Review of taxonomic changes in dairy-related lactobacilli. J. Dairy Sci. 2022, 105, 2750–2770. [Google Scholar] [CrossRef]
- Delcour, J.; Ferain, T.; Hols, P. Advances in the genetics of thermophilic lactic acid bacteria. Curr. Opin. Biotechnol. 2000, 11, 497–504. [Google Scholar] [CrossRef]
- Song, S.; Bae, D.-W.; Lim, K.; Griffiths, M.W.; Oh, S. Cold stress improves the ability of Lactobacillus plantarum L67 to survive freezing. Intern. J. Food Microbiol. 2014, 191, 135–143. [Google Scholar] [CrossRef]
- Neffe-Skocinska, K.; Gierejkiewicz, M.; Kołozyn-Krajewska, D. Optimization of fermentation conditions for dry-aged sirloins with probiotic bacteria added. Food Sci. Technol. Qual. Zywn. Nauk. Technol. Jaskosc 2011, 6, 36–46. [Google Scholar] [CrossRef]
- Wang, A.; Zhong, Q. Drying of probiotics to enhance the viability during preparation, storage, food application, and digestion. A review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13287. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Haq, S.F.; Samant, S.; Sukumaran, S. Adaptation of Lactobacillus acidophilus to thermal stress yields a thermotolerant variant which also exhibits improved survival at pH 2. Probiot. Antimicrob. Proteins 2018, 10, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Ananta, E.; Knorr, D. Evidence on the Role of Protein Biosynthesis in the Induction of Heat Tolerance of Lactobacillus Rhamnosus GG by Pressure Pre-Treatment. Int. J. Food Microbiol. 2004, 96, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, D.; Longo, A.; Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. How probiotics face food stress: They get by with a little help. Crit. Rev. Food Sci. Nutr. 2020, 60, 1552–1580. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, G.; Kiekens, S.; Jokicevic, K.; Byl, E.; Henkens, T.; Vandenheuvel, D.; Claes, I.; Lebeer, S.; Kiekens, F. Heat-pretreated Lactobacillus rhamnosus GG shows enhanced survival capacity after spray drying. Dry. Technol. 2022, 40, 3602–3613. [Google Scholar] [CrossRef]
- de Angelis, M.; Gobbetti, M. Environmental stress responses in Lactobacillus. A review. Proteomics 2004, 4, 106–122. [Google Scholar] [CrossRef]
- Gaucher, F.; Bonnassie, S.; Rabah, H.; Marchand, P.; Blanc, P.; Jeantet, R.; Jan, G. Review: Adaptation of Beneficial Propionibacteria, Lactobacilli, and Bifidobacteria Improves Tolerance Toward Technological and Digestive Stresses. Front. Microbiol. 2019, 10, 841. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004, 14, 835–847. [Google Scholar] [CrossRef]
- Fonseca, F.; Pénicaud, C.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Passot, S. Factors influencing the membrane fluidity and the impact on production of lactic acid bacteria starters. A review. Appl. Microbiol. Biotech. 2019, 103, 6867–6883. [Google Scholar] [CrossRef] [PubMed]
- Barria, C.; Malecki, M.; Arraiano, C.M. Bacterial adaptation to cold. Microbiology 2013, 159, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Haddaji, N.; Mahdhi, A.K.; Ismaiil, M.B.; Bakhrouf, A. Effect of environmental stress on cell surface and membrane fatty acids of Lactobacillus plantarum. Arch. Microbiol. 2017, 199, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Delettre, J.; Guillot, A.; Corrieu, G.; Béal, C. Influence of cooling temperature and duration on cold adaptation of Lactobacillus acidophilus RD758. Cryobiology 2005, 50, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ma, Y.; Zheng, Y.; Zhao, W.; Zhao, X.; Luo, T.; Zhang, J.; Yang, Z. Cold-Stress Response of Probiotic Lactobacillus plantarum K25 by iTRAQ Proteomic Analysis. J. Microbiol. Biotechnol. 2020, 30, 187–195. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Dopson, M. Life in acid: pH homeostasis in acidophiles. Trends Microbiol. 2007, 15, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Fun. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Gao, X.; Kong, J.; Zhu, H.; Mao, B.; Zhao, J. Lactobacillus, Bifidobacterium and Lactococcus response to environmental stress: Mechanisms and application of cross-protection to improve resistance against freeze-drying. J. Appl. Microbiol. 2021, 132, 802–821. [Google Scholar] [CrossRef]
- Sheehan, V.M.; Ross, P.G.; Fitzgerald, F. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 279–284. [Google Scholar] [CrossRef]
- Schumacher, K.; Brameyer, S.; Jung, K. Bacterial acid stress response: From cellular changes to antibiotic tolerance and phenotypic heterogeneity. Curr. Opin. Microbiol. 2023, 75, 102367. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.Z.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C. Surviving the Acid Test: Responses of Gram-Positive Bacteria to Low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. F1F0-ATPase functions under markedly acidic conditions in bacteria. In Regulation of Ca2+-ATPases, V-ATPases and F-ATPases; Chacraborti, S., Dhalla, N.S., Eds.; Adavances in Biochemistry in Health and Desease; Springer: Cham, Switzerland, 2016; pp. 459–468. [Google Scholar]
- Mendonça, A.A.; de Paula Pinto-Neto, W.; da Paixão, G.A.; Santos, D.d.S.; De Morais, M.A., Jr.; De Souza, R.B. Journey of the Probiotic Bacteria: Survival of the Fittest. Microorganisms 2023, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, A.R.; da Mota Silveira, L.P.; da Cruz Filho, I.J.; de Lima, V.F.; da Mota Silveira Filho, V.; Araujo, A.A.; da Silva, T.L.; de Freitas Araújo, K.; da Silva Macedo, L. Metabolism and physiology of Lactobacilli: A review. J. Environ. Anal. Progress 2017, 2, 125–136. [Google Scholar] [CrossRef]
- Wu, C.; He, G.; Zhang, J. Physiological and proteomic analysis of Lactobacillus case in response to acid adaptation. J. Ind. Microbiol. Biotechnol. 2014, 41, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Pan, M.; Wan, C.; Shah, N.P.; Tao, X.; Wei, H. Physiological and transcriptional responses and cross protection of Lactobacillus plantarum ZDY2013 under acid stress. J. Dairy Sci. 2016, 99, 1002–1010. [Google Scholar] [CrossRef]
- Lorca, G.L.; Raya, R.R.; Taranto, M.P.; De Valdez, G.F. Characterization of the protein-synthesis dependent adaptive acid tolerance response in Lactobacillus acidophilus. J. Mol. Microbiol. Biotechnol. 2002, 4, 525–532. [Google Scholar] [PubMed]
- Wu, C.; Zhang, J.; Wang, M.; Du, G.; Chen, J. Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J. Ind. Microbiol. Biotech. 2012, 39, 1031–1039. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kodama, K.; Yasuda, H.; Okamoto-Kainuma, A.; Koizumi, K.; Yamasato, K. Presence of halophilic and alkaliphilic lactic acid bacteria in various cheeses. Lett. Appl Microbiol. 2006, 44, 308–313. [Google Scholar] [CrossRef]
- Sánchez, A.-H.; Rejano, L.; Montaño, A.; de Castro, A. Utilization at high pH of starter cultures of lactobacilli for Spanish-style green olive fermentation. Int. J. Food Microbiol. 2001, 67, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Sawatari, Y.; Sugiyama, H.; Suzuki, Y.; Hanaoka, A.; Saito, K.; Yamauchi, H.; Okada, S.; Yokota, A. Development of fermented instant Chinese noodle using Lactobacillus plantarum. Food Microbiol. 2005, 22, 539–546. [Google Scholar] [CrossRef]
- Sawatari, Y.; Yokota, A. Diversity and mechanisms of alkali tolerance in lactobacilli. Appl. Environ. Microbiol. 2007, 73, 3909–3915. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, X.; Zhang, G.; Sadiq, F.A.; Simal-Gandara, J.; Xiao, J.; Sang, Y. Probiotics in the dairy industry-Advances and opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3937–3982. [Google Scholar] [CrossRef]
- Zotta, T.; Parente, E.; Ricciardi, A. Aerobic metabolism in the genus Lactobacillus: Impact on stress response and potential applications in the food industry. J. App. Microbiol. 2017, 122, 857–869. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Liu, Q.; Hang, X.; Liu, X.; Tan, J.; Li, D.; Yang, H. Cloning and heterologous expression of the manganese superoxide dismutase gene from Lactobacillus casei Lc18. Ann. Microbiol. 2012, 62, 129–137. [Google Scholar] [CrossRef]
- Quatravaux, S.; Remize, F.; Bryckaert, E.; Colavizza, D.; Guzzo, J. Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters. J. Appl. Microbiol. 2006, 101, 903–912. [Google Scholar] [CrossRef]
- Barre, O.; Mourlane, F.; Solioz, M. Copper induction of lactate oxidase of Lactococcus lactis: A novel metal stress response. J. Bacteriol. 2007, 189, 5947–5954. [Google Scholar] [CrossRef]
- Talwalkar, A.; Kailasapathy, K. Oxidative stress adaptation of probiotic bacteria. Milchwissenschaft 2004, 59, 140–143. [Google Scholar]
- Nguyen, A.V.; Yaghoobi, M.; Zhang, S.; Li, P.; Li, Q.; Dogan, B.; Ahnrud, G.P.; Flock, G.; Marek, P.; Simpson, K.W.; et al. Adaptive laboratory evolution of probiotics toward oxidative stress using a microfluidic-based platform. Small 2024. [Google Scholar] [CrossRef]
- Klu, Y.A.K.; Williams, J.H.; Phillips, R.D.; Chen, J. Survival of Lactobacillus rhamnosus GG as influenced by storage conditions and product matrixes. J. Food Sci. 2012, 77, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, A.; Ehsani, M.R.; Azizi, A.; Razavi, S.H.; Yarmand, M.S. Growth and survival of some probiotic strains in simulated ice cream conditions. J. Appl. Sci. 2008, 8, 379–382. [Google Scholar] [CrossRef]
- Liu, B.; Hasrat, Z.; Poolman, B.; Boersma, A.J. Decreased effective macromolecular crowding in Escherichia coli adapted to hyperosmotic stress. J. Bacteriol. 2019, 201, e00708-18. [Google Scholar] [CrossRef]
- Poolman, B. Physicochemical homeostasis in bacteria. FEMS Microbiol Rev. 2023, 47, fuad033. [Google Scholar] [CrossRef] [PubMed]
- Syeda, R.; Qiu, Z.; Dubin, A.E.; Murthy, S.E.; Florendo, M.N.; Mason, D.E.; Mathur, J.; Cahalan, S.M.; Peters, E.C.; Montal, M.; et al. LRRC8 proteins form volume- regulated anion channels that sense ionic strength. Cell 2016, 164, 499. [Google Scholar] [CrossRef] [PubMed]
- Sleator, R.D.; Hill, C. Bacterial osmoadaptation: The role of osmolytes in bacterial stress and virulence. FEMS Microbiol. Rev. 2001, 26, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Wendel, U. Assessing viability and stress tolerance of probiotics. A review. Front. Microbiol. 2022, 12, 818468. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Castro, M.H.; Malcata, F.X.; Kirby, R.M. Survival of Lactobacillus delbrueckii ssp. bulgaricus following spray-drying. J. Dairy Sci. 1995, 78, 1025–1031. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Costa, G.A.; Regenhardt, S.; Reinheimer, J.A. Influence of compounds associated with fermented dairy products on the growth of lactic acid starter and probiotic bacteria. Int. Dairy J. 2002, 12, 579–589. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Boekhorst, J.; Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef]
- Padan, E.; Bibi, E.; Ito, M.; Krulwich, T. Alkaline pH homeostasis in bacteria: New insights. Biochim. Biophys. Acta (BBA)-Biomembr. 2005, 1717, 67–88. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Song, X.; Guo, J.; Wu, J.; Wu, R. iTRAQ-based proteomic analysis of responses of Lactobacillus plantarum FS5-5 to salt tolerance. Ann. Microbiol. 2019, 69, 377–394. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Dolatowski, Z.J. Probiotic meat products and human nutrition. Process Biochem. 2012, 47, 1761–1772. [Google Scholar] [CrossRef]
- Łaszkiewicz, B.; Szymański, P.; Kołożyn-Krajewska, D. The effect of selected lactic acid bacterial strains on the technological and microbiological quality of mechanically separated poultry meat cured with a reduced amount of sodium nitrite. Poult. Sci. 2021, 100, 263–272. [Google Scholar] [CrossRef]
- Gandhi, A.; Shah, N.P. Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microbiol. 2015, 49, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Arihara, K.; Itoh, M. UV-induced Lactobacillus gasseri mutants resisting sodium chloride and sodium nitrite for meat fermentation. Int. J. Food Microbiol. 2000, 56, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; da Cruz, A.G.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods, current opinion. Food Sci. 2018, 22, 160–166. [Google Scholar]
- Ghandi, A.; Powell, I.B.; Broome, M.; Adhikari, B. Survival, fermentation activity and storage stability of spray dried Lactococcus lactis produced via different atomization regimes. J. Food Eng. 2013, 115, 83–90. [Google Scholar] [CrossRef]
- Senadira, S.S.; Prasanna, P.H.P.; Jayawardene, N.W.I.A.; Gunasekara, D.C.S.; Senadira, P.; Chandrasekara, A. Antioxidant, physicochemical, microbiological, and sensory properties of probiotic yoghurt incorporated with various Annona species pulp. Helium 2018, 11, E00955. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, A.G.; Faria, J.D.A.F.; van Dender, A.G.F. Packaging System and Probiotic Dairy Foods. Food Res. Int. 2007, 40, 951–956. [Google Scholar] [CrossRef]
- Asaithambi, N.; Singh, S.K.; Singha, P. Current status of non-thermal processing of probiotic foods: A review. J. Food Eng. 2021, 303, 110567. [Google Scholar] [CrossRef]
- Almada-Érix, C.N.; Almada, C.N.; Souza Pedrosa, G.T.; Dos Santos, P.; Schmiele, M.; Clerici, M.T.P.S.; Martinez, J.; Lollo, P.C.; Magnani, M.; Sant’Ana, A.S. Quantifying the impact of eight unit operations on the survival of eight Bacillus strains with claimed probiotic properties. Food Res. Int. 2021, 142, 110–191. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT-Food Sci. Technol. 2011, 44, 1251–1260. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.-A.; Ren, E.-F.; Xu, F.-Y.; Li, J.; Wang, M.-S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Castell-Perez, M.E.; Moreira, R.G. Irradiation and consumers acceptance. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Elsevier: Cambridge, UK, 2021; pp. 122–135. [Google Scholar]
- Allai, F.M.; Azad, Z.A.; Mir, N.A.; Gul, K. Recent advances in non-thermal processing technologies for enhancing shelf life and improving food safety. Appl. Food Res. 2023, 3, 100258. [Google Scholar] [CrossRef]
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Perez-Masia, R.; Lagaron, J.M. A review on electrospun polymer nanostructures as advanced bioactive platforms. Polym. Eng. Sci. 2016, 56, 500–527. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, C.P.; Ruiz-Capillas, C.; Herrero, A.M.; Jiménez-Colmenero, F.; Menezes, C.R.; Fries, L.L.M. Application of probiotic delivery systems in meat products. Trends Food Sci. Technol. 2015, 46, 120–131. [Google Scholar] [CrossRef]
- Yang, S.; Bai, M.; Kwok, L.Y.; Zhong, Z.; Sun, Z. The intricate symbiotic relationship between lactic acid bacterial starters in the milk fermentation ecosystem. Crit. Rev. Food Sci Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and other cultured dairy products. J Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [PubMed]
- Pette, J.W.; Lolkema, H. Yoghurt. 1. Symbiosis and antibiosis in mixed cultures of Lactobacillus bulgaricus and Streptococcus thermophilus. Nederlandsch Melk-en Zuiveltijdschr. 1950, 4, 197–208. [Google Scholar]
- Jankov, J.; Stoyanov, I.V. Study on the thermophilic lactobacilli in milk for yoghurt production. In Proceedings of the XVII International Dairy Congress, Section F5, Munich, Germany, 25 August 1966; pp. 677–680. [Google Scholar]
- Kneifel, W.; Jaros, D.; Erhard, F. Microflora and acidification properties of yogurt and yogurt-related products fermented with commercially available starter cultures. Internat. J. Food Microbiol. 1993, 18, 179–189. [Google Scholar] [CrossRef]
- Ge, Y.; Yu, X.; Zhao, X.; Liu, C.; Li, T.; Mu, S.; Zhang, L.; Chen, Z.; Zhang, Z.; Song, Z.; et al. Fermentation characteristics and post-acidification of yogurt by Streptococcus thermophilus CICC 6038 and Lactobacillus delbrueckii ssp. Bulgaricus CICC 6047 at optimal inoculum ratio. J. Dairy Sci. 2023, 107, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Taj, R.; Masud, T.; Sohail, A.; Sammi, S.; Naz, R.; Khanal, B.K.S.; Nawaz, M.A. In vitro screening of EPS-producing Streptococcus thermophilus strains for their probiotic potential from Dahi. Food Sci. Nutr. 2022, 10, 2347–2359. [Google Scholar] [CrossRef]
- Ricciardi, A.; Parente, E.; Guidone, A.; Ianniello, R.G.; Zotta, T.; Abu Sayem, S.M.; Varcamonti, M. Genotypic diversity of stress response in Lactobacillus plantarum, Lactobacillus paraplantarum and Lactobacillus pentosus. Int. J. Food Microbiol. 2012, 157, 278–285. [Google Scholar] [CrossRef]
- Chen, M.J.; Tang, H.Y.; Chiang, M.L. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Maus, J.E.; Ingham, S. Employment of stressful conditions during culture production to enhance subsequent cold- and acid-tolerance of bifidobacteria. J. Appl. Microb. 2003, 95, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Xu, C.; Liu, F.; Hou, J.; Shao, H.; Yu, W. Stress adaptation and cross-protection of Lactobacillus plantarum KLDS 1.0628. Cyta-J. Food 2021, 19, 72–80. [Google Scholar] [CrossRef]
- Bolotin, A.; Wincker, P.; Mauger, S.; Jaillon, O.; Malarme, K.; Weissenbach, J.; Ehrlih, S.D.; Sorokin, A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 2001, 11, 731–753. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, H.; Starrenburg, M.J.C.; Molenaar, D.; Kleerebezem, M.; Vlieg, J. Microbial domestication signatures of Lactococcus lactis can be reproduced by experimental evolution. Genome Res. 2012, 22, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hung, S.; Chan, J.; Chen, J.; Solem, C.; Jensen, P.R. Systems Biology—A Guide for Understanding and Developing Improved Strains of Lactic Acid Bacteria. Front. Microbiol. 2019, 10, 876. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. https://doi.org/10.3390/fermentation10060298
Sionek B, Szydłowska A, Trząskowska M, Kołożyn-Krajewska D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation. 2024; 10(6):298. https://doi.org/10.3390/fermentation10060298
Chicago/Turabian StyleSionek, Barbara, Aleksandra Szydłowska, Monika Trząskowska, and Danuta Kołożyn-Krajewska. 2024. "The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products" Fermentation 10, no. 6: 298. https://doi.org/10.3390/fermentation10060298
APA StyleSionek, B., Szydłowska, A., Trząskowska, M., & Kołożyn-Krajewska, D. (2024). The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation, 10(6), 298. https://doi.org/10.3390/fermentation10060298





