Amylolytic Capability and Performance of Probiotic Strains in a Controlled Sorghum Fermentation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
2.2. Amylolytic Activity of LAB Strains
2.3. DNA Amplification of Amylase Gene of Probiotic Strains
2.4. Sorghum Fermentation with LAB Strains
2.4.1. Fermentation Medium
2.4.2. Population Levels of Probiotic Strains during Fermentation
2.4.3. pH
2.4.4. Titratable Acidity
- -
- N is the normality of the sodium hydroxide: 0.1 N;
- -
- V is the volume of sodium hydroxide (mL) used to reach the titration endpoint;
- -
- ME (milli-equivalent of Lactic acid): 0.09008.
2.5. Antimicrobial Activity of LAB Strains Co-Cultured with Pathogenic Strains
2.6. Statistical Analysis
3. Results
3.1. Amylase Assay of Probiotic Strains
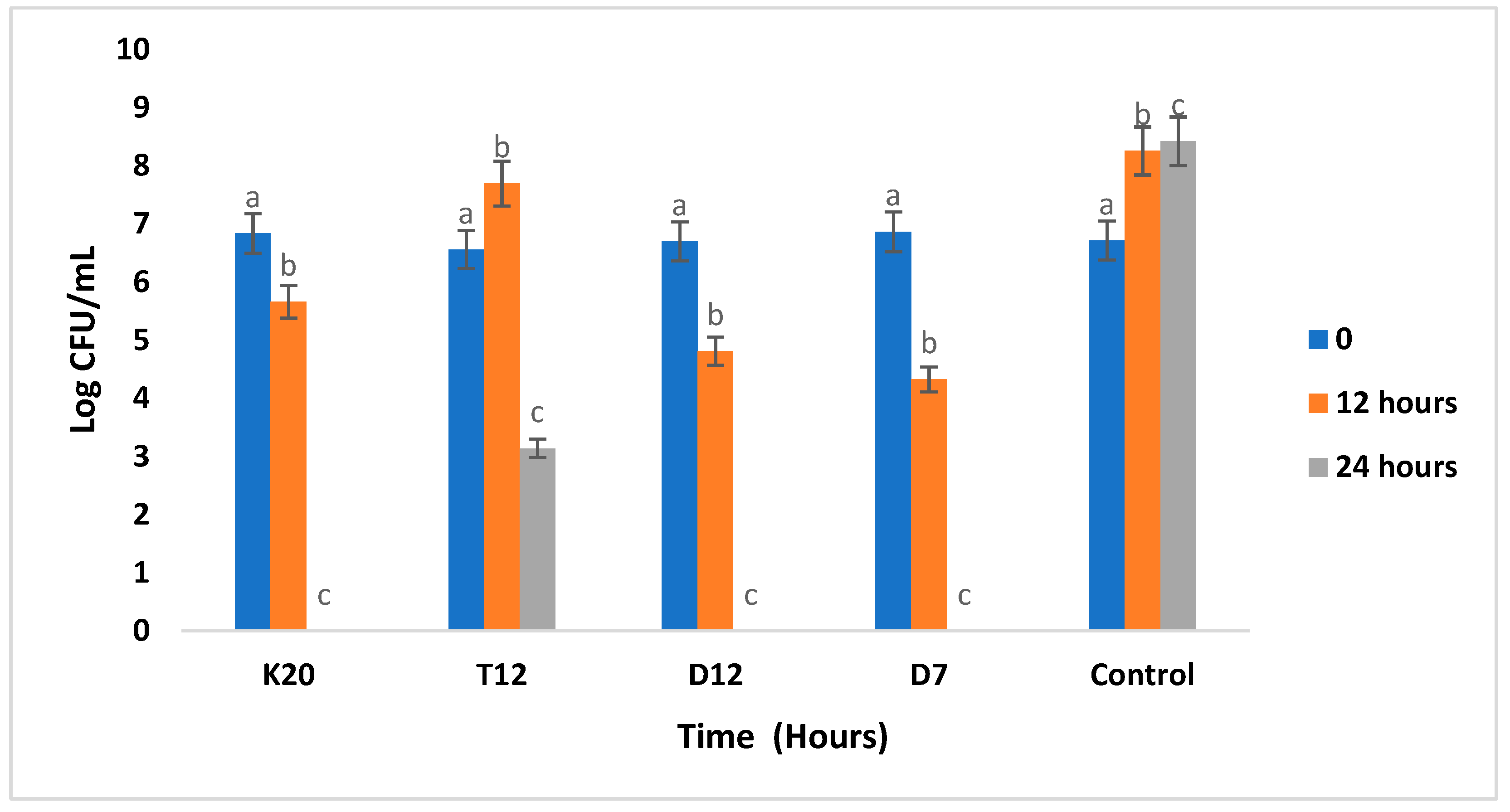
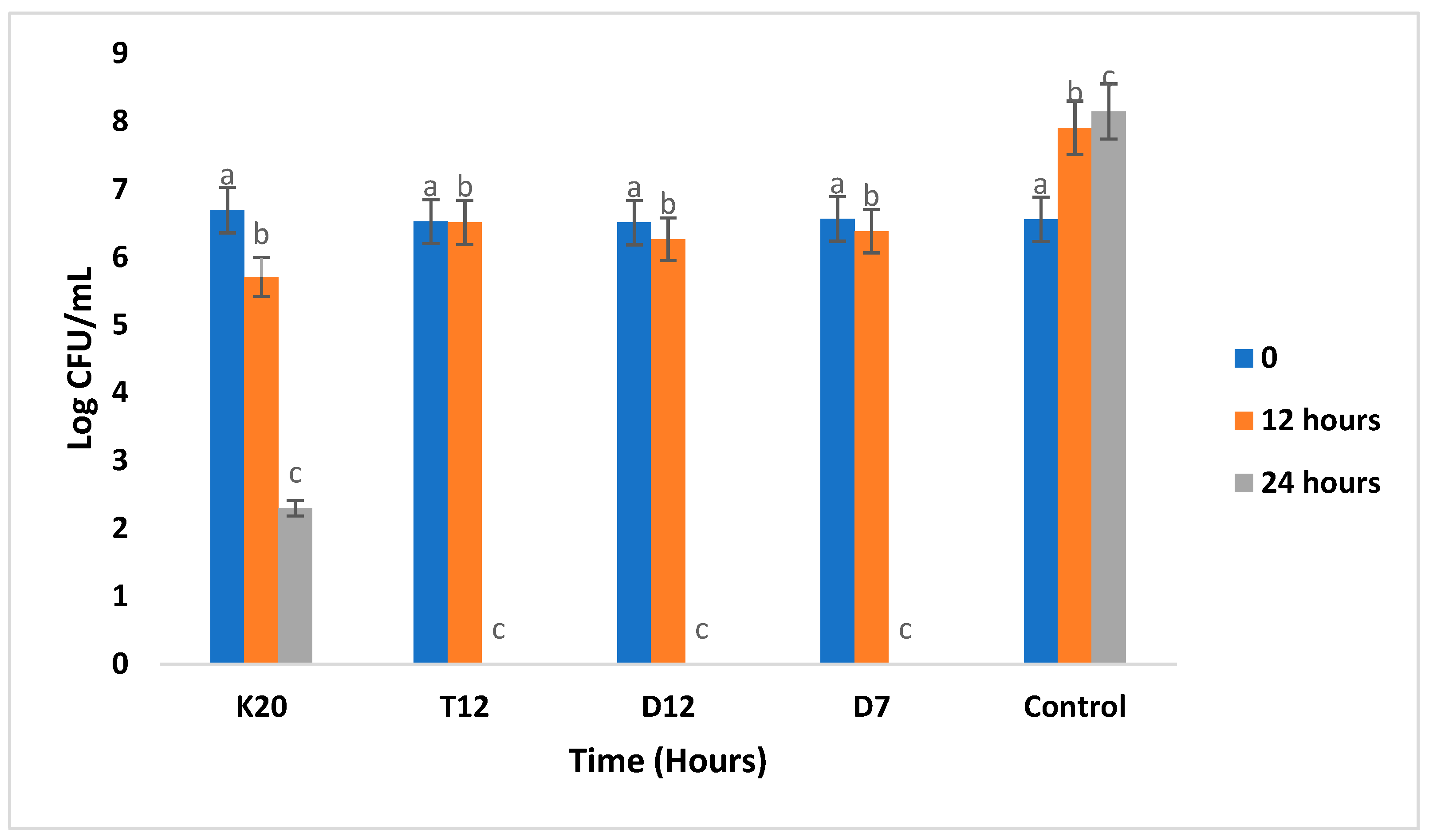
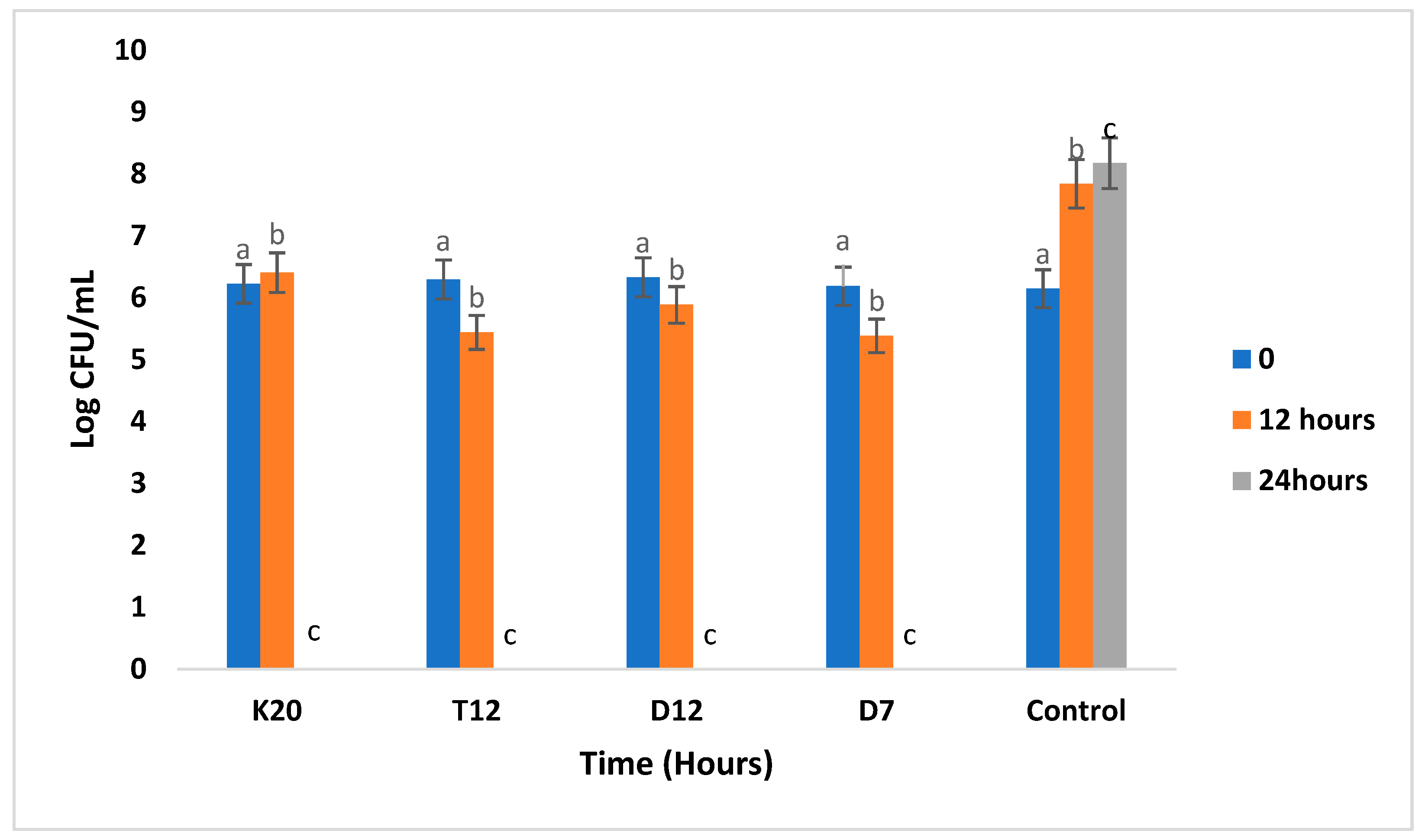
3.2. Population Levels of Lactobacillus Strains during Fermentation in the Absence and Presence of Glucose
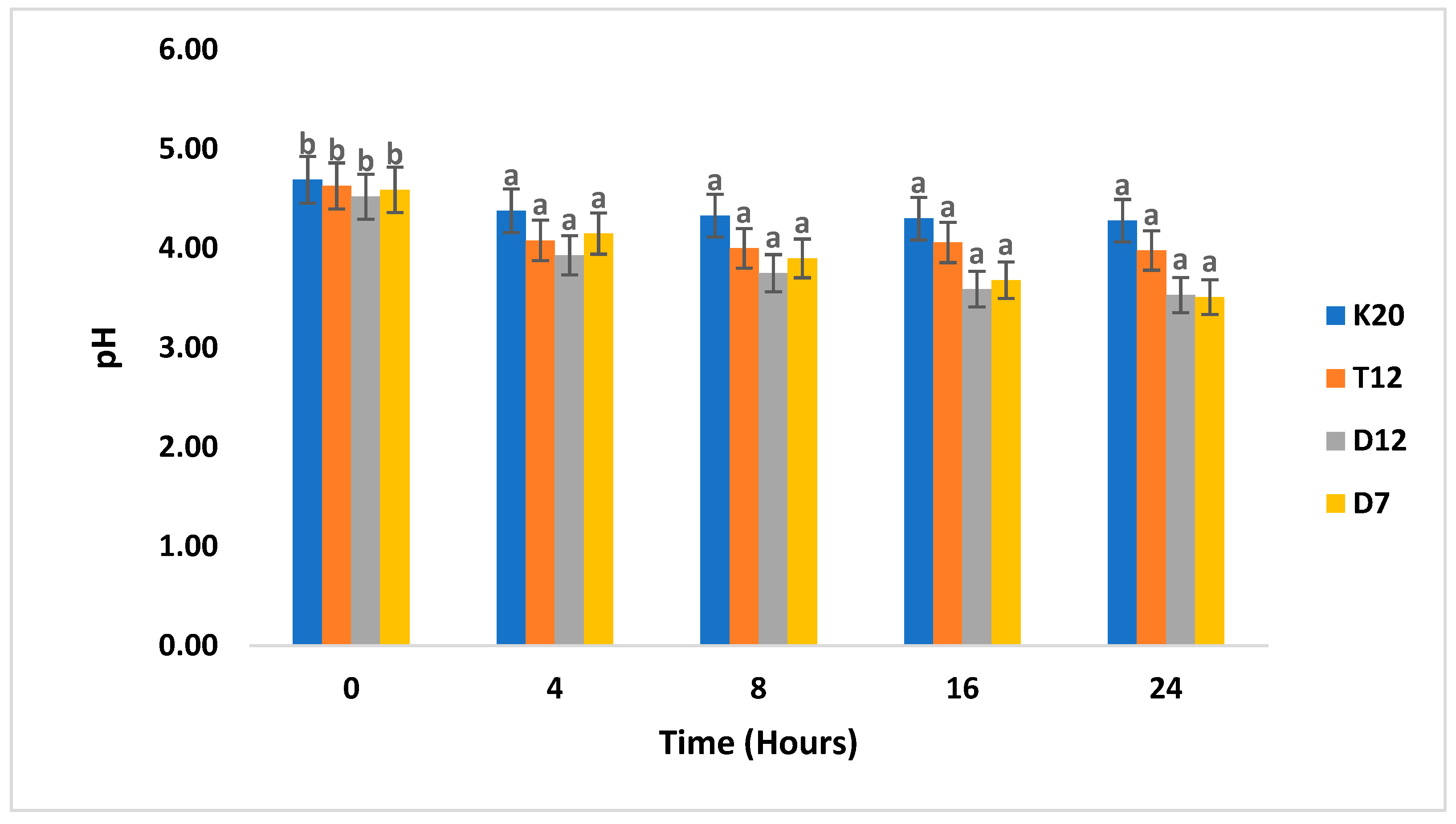
3.3. pH
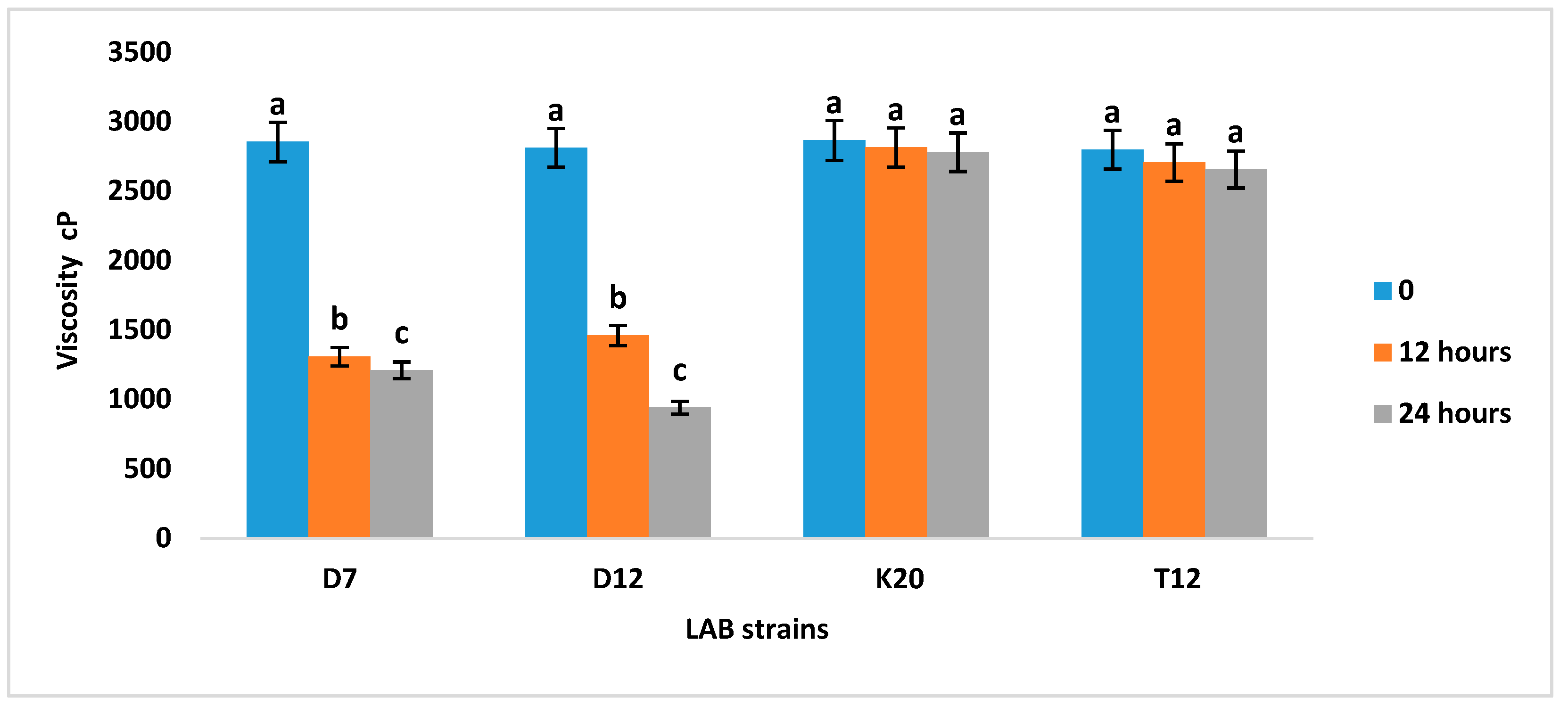
3.4. Titratable Acidity
3.5. Antibacterial Activity of LAB Strains against Pathogenic Strains in Sorghum Gruel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Setta, M.C.; Matemu, A.; Mbega, E.R. Potential of probiotics from fermented cereal-based beverages in improving health of poor people in Africa. J. Food Sci. Technol. 2020, 57, 3935–3946. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Lee, J.E.; Lee, C.H. Importance of lactic acid bacteria in Asian fermented foods. Microb. Cell Fact. 2011, 10 (Suppl. S1), S5. [Google Scholar] [CrossRef] [PubMed]
- Nyanzi, R.; Awouafack, M.D.; Steenkamp, P.; Jooste, P.J.; Eloff, J.N. Anticandidal activity of cell extracts from 13 probiotic Lactobacillus strains and characterisation of lactic acid and a novel fatty acid derivative from one strain. Food Chem. 2014, 164, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Jhamb, V.; Swaminathan, P. Role and importance of lactic acid bacteria in different Indian fermented foods. Biologia 2023, 78, 3609–3623. [Google Scholar] [CrossRef]
- Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Advances in probiotic incorporation into cereal-based baked foods: Strategies, viability, and effects–A review. Appl. Food Res. 2023, 3, 100330. [Google Scholar] [CrossRef]
- Tang, H.; Huang, W.; Yao, Y.F. The metabolites of lactic acid bacteria: Classification, biosynthesis and modulation of gut microbiota. Microb. Cell 2023, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lunelli, B.H.; Andrade, R.R.; Atala, D.I.P.; MacIel, M.R.W.; Filho, F.M.; Filho, R.M.I. Production of lactic acid from sucrose: Strain selection, fermentation, and kinetic modeling. Appl. Biochem. Biotechnol. 2010, 161, 227–237. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef]
- Wee, Y.J.; Kim, J.N.; Ryu, H.W. Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol. 2006, 44, 163–172. [Google Scholar]
- Madoroba, E.; Steenkamp, E.T.; Theron, J.; Scheirlinck, I.; Cloete, T.E.; Huys, G. Diversity and dynamics of bacterial populations during spontaneous sorghum fermentations used to produce ting, a South African food. Syst. Appl. Microbiol. 2011, 34, 227–234. [Google Scholar] [CrossRef]
- Szala, B.; Paluszak, Z.; Motyl, I. Antagonistic Effect of Lactic Acid Bacteria on Salmonella Senftenberg in Mixed Cultures. Pol. J. Environ. Stud. 2012, 21, 1399–1403. [Google Scholar]
- Mugula, J.K.; Nnko, S.A.M.; Narvhus, J.A.; Sørhaug, T. Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 2003, 80, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A. African sorghum-based fermented foods: Past, current and future prospects. Nutrients 2020, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–Nomad and Ideal Probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef] [PubMed]
- Rapoo, S.M.; Budeli, P.; Thaoge, M.L. Recovery of Potential Starter Cultures and Probiotics from Fermented Sorghum (Ting) Slurries. Microorganisms 2023, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Ekpe, O.D.; Lee, H.; Oh, J. Perfluoroalkyl substances and pharmaceuticals removal in full-scale drinking water treatment plants. J. Hazard. Mater. 2020, 400, 123235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Esmail, G.A.; Alzeer, A.F.; Arasu, M.V.; Vijayaraghavan, P.; Choi, K.C.; Al-Dhabi, N.A. Saudi Journal of Biological Sciences Probiotic characteristics of Lactobacillus strains isolated from cheese and their antibacterial properties against gastrointestinal tract pathogens. Saudi J. Biol. Sci. 2020, 27, 3505–3513. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, Y.; Xia, Y.; Song, X.; Ai, L. Characteristics of Probiotic Preparations and Their Applications. Foods 2022, 11, 2472. [Google Scholar] [CrossRef] [PubMed]
- Nyanzi, R.; Jooste, P.J.; Buys, E.M. Invited review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. J. Dairy Sci. 2021, 104, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, T.; Bhargavi, R.; Priyanka, P.R.; Niranjan, N.R.; Pavitra, P.V. Screening of potential probiotic lactic acid bacteria and production of amylase and its partial purification. J. Genet. Eng. Biotechnol. 2018, 16, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Altaf, M.; Naveena, B.J.; Venkateshwar, M.; Kumar, E.V. Amylolytic bacterial lactic acid fermentation—A review. Biotechnol. Adv. 2008, 26, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.S.; Babiker, E.E.; Yousif, N.E.; El Tinay, A.H. Effect of fermentation on biochemical and sensory characteristics of sorghum flour supplemented with whey protein. Food Chem. 2005, 92, 285–292. [Google Scholar] [CrossRef]
- Ferry, A.L.; Hort, J.; Mitchell, J.R.; Lagarrigue, S.; Pamies, B.V. Effect of amylase activity on starch paste viscosity and its implications for flavor perception. J. Texture Stud. 2004, 35, 511–524. [Google Scholar] [CrossRef]
- Songré-Ouattara, L.T.; Mouquet-Rivier, C.; Icard-Vernière, C.; Humblot, C.; Diawara, B.; Guyot, J.P. Enzyme activities of lactic acid bacteria from a pearl millet fermented gruel (ben-saalga) of functional interest in nutrition. Int. J. Food Microbiol. 2008, 128, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Yousif, N.M.; Huch, M.; Schuster, T.; Cho, G.S.; Dirar, H.A.; Holzapfel, W.H.; Franz, C.M. Diversity of lactic acid bacteria from Hussuwa, a traditional African fermented sorghum food. Food Microbiol. 2010, 27, 757–768. [Google Scholar] [CrossRef]
- Molelekoa, T.B.J.; Regnier, T.; Da Silva, L.S.; Augustyn, W.A. Potential of marula (Sclerocarya birrea subsp. caffra) waste for the production of vinegar through surface and submerged fermentation. S. Afr. J. Sci. 2018, 114, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Zarnkow, M.; Becker, T. Optimisation of fermentation conditions for probiotication of sorghum wort by Lactobacillus acidophilus LA5. Int. J. Food Sci. Technol. 2015, 50, 2271–2279. [Google Scholar] [CrossRef]
- Nwachukwu, E.; Achi, O.K.; Ijeoma, I.O. Lactic acid bacteria in fermentation of cereals for the production of indigenous Nigerian foods. Afr. J. Food Sci. Technol. 2010, 1, 21–26. [Google Scholar]
- Salari, M.; Razavi, S.H.; Gharibzahedi, S.M.T. Characterising the synbiotic beverages based on barley and malt flours fermented by Lactobacillus delbrueckii and paracasei strains. Qual. Assur. Saf. Crops Foods 2015, 7, 355–361. [Google Scholar] [CrossRef]
- Fossi, B.T.; Tavea, F. Application of Amylolytic Lactobacillus fermentum 04BBA19 in Fermentation for Simultaneous Production of Thermostable a-Amylase and Lactic Acid. In Lactic Acid Bacteria—R & D for Food, Health and Livestock Purposes; IntechOpen: London, UK, 2013; pp. 633–658. [Google Scholar]
- Dejene, F.; Dadi, B.R.; Tadesse, D. In Vitro Antagonistic Effect of Lactic Acid Bacteria Isolated from Fermented Beverage and Finfish on Pathogenic and Foodborne Pathogenic Microorganism in Ethiopia. Int. J. Microbiol. 2021, 2021, 5370556. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; González, M.P.; Murado, M.A. Effects of lactic acid bacteria cultures on pathogenic microbiota from fish. Aquaculture 2005, 245, 149–161. [Google Scholar] [CrossRef]
- Akhondali, Z.; Dianat, M.; Radan, M. The antimicrobial activity of probiotic bacteria Escherichia coli isolated from different natural sources against hemorrhagic E. coli O157:H7. Electron Physician 2015, 7, 971–976. [Google Scholar] [PubMed]
- De Mello Santos, A.C.; Santos, F.F.; Silva, R.M.; Gomes, T.A.T. Diversity of Hybrid- and Hetero-Pathogenic Escherichia coli and Their Potential Implication in More Severe Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Mante, E.S.; Sakyi-Dawson, E.; Amoa-Awua, W.K. Antimicrobial interactions of microbial species involved in the fermentation of cassava dough into agbelima with particular reference to the inhibitory effect of lactic acid bacteria on enteric pathogens. Int. J. Food Microbiol. 2003, 89, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Technol. 2016, 54, 17–25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapoo, S.M.; Thaoge-Zwane, M.-L. Amylolytic Capability and Performance of Probiotic Strains in a Controlled Sorghum Fermentation System. Fermentation 2024, 10, 308. https://doi.org/10.3390/fermentation10060308
Rapoo SM, Thaoge-Zwane M-L. Amylolytic Capability and Performance of Probiotic Strains in a Controlled Sorghum Fermentation System. Fermentation. 2024; 10(6):308. https://doi.org/10.3390/fermentation10060308
Chicago/Turabian StyleRapoo, Seth Molamu, and Mathoto-Lydia Thaoge-Zwane. 2024. "Amylolytic Capability and Performance of Probiotic Strains in a Controlled Sorghum Fermentation System" Fermentation 10, no. 6: 308. https://doi.org/10.3390/fermentation10060308
APA StyleRapoo, S. M., & Thaoge-Zwane, M.-L. (2024). Amylolytic Capability and Performance of Probiotic Strains in a Controlled Sorghum Fermentation System. Fermentation, 10(6), 308. https://doi.org/10.3390/fermentation10060308





