Exopolysaccharide Production in Submerged Fermentation of Pleurotus ostreatus under Red and Green Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Microorganism
2.3. Media and Growth Conditions
2.4. Biomass Determination
2.5. Protein Estimation
2.6. Polysaccharide Estimation and Analysis
2.7. Antioxidant Activity Tests
2.8. Determination of Total Phenolic Content
2.9. Cultivation in Different Light Wavelengths
2.10. Cultivation Using Green LEDs
2.11. Cultivation Using Red LEDs
2.12. Scale up in a 3.5 L Stirred Tank Bioreactor Using Red LEDs
3. Results
3.1. Screening of Different Light Wavelengths on Submerged Cultivation of P. ostreatus LGAM 1123
3.2. Cultivation in Baffled Flasks Using Green Light
3.3. Cultivation in Baffled Flasks Using Red Light
3.4. Cultivation in a 3.5 L Stirred Tank Bioreactor Using Red Light
3.5. Analysis of the Produced Polysaccharides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tisch, D.; Schmoll, M. Light Regulation of Metabolic Pathways in Fungi. Appl. Microbiol. Biotechnol. 2010, 85, 1259–1277. [Google Scholar] [CrossRef]
- Smith, H. Light Quality, Photoperception, and Plant Strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, H.; Sun, Y.; Xia, R.; Hou, Z.; Li, Y.; Wang, Y.; Pan, S.; Li, L.; Zhao, C.; et al. Effect of Light on Quality of Preharvest and Postharvest Edible Mushrooms and Its Action Mechanism: A Review. Trends Food Sci. Technol. 2023, 139, 104119. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent Trends in Submerged Cultivation of Mushrooms and Their Application as a Source of Nutraceuticals and Food Additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom Polysaccharides as Potential Prebiotics with Their Antitumor and Immunomodulating Properties: A Review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, M.; Zhao, L.; Chen, L.; Ding, Z. Novel Insights into the Mechanism Underlying High Polysaccharide Yield in Submerged Culture of Ganoderma lucidum Revealed by Transcriptome and Proteome Analyses. Microorganisms 2023, 11, 772. [Google Scholar] [CrossRef]
- Manzi, P.; Pizzoferrato, L. Beta-Glucans in Edible Mushrooms. Food Chem. 2000, 68, 315–318. [Google Scholar] [CrossRef]
- Castro-Alves, V.C.; Gomes, D.; Menolli, N.; Sforça, M.L.; do Nascimento, J.R.O. Characterization and Immunomodulatory Effects of Glucans from Pleurotus albidus, a Promising Species of Mushroom for Farming and Biomass Production. Int. J. Biol. Macromol. 2017, 95, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.A.R.; Coimbra, M.A. The Antioxidant Activity of Polysaccharides: A Structure-Function Relationship Overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridi, L.M.; Zerva, A.; Topakas, E. Biocatalytic Synthesis of Fungal β-Glucans. Catalysts 2018, 8, 274. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Jablonský, I.; Sluková, M.; Čopíková, J. Mushrooms of Genus Pleurotus as a Source of Dietary Fibres and Glucans for Food Supplements. Czech J. Food Sci. 2008, 26, 441–446. [Google Scholar] [CrossRef]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus Mushrooms Content in Glucans and Ergosterol Assessed by ATR-FTIR Spectroscopy and Multivariate Analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- Papaspyridi, L.M.; Katapodis, P.; Gonou-Zagou, Z.; Kapsanaki-Gotsi, E.; Christakopoulos, P. Optimization of Biomass Production with Enhanced Glucan and Dietary Fibres Content by Pleurotus ostreatus ATHUM 4438 under Submerged Culture. Biochem. Eng. J. 2010, 50, 131–138. [Google Scholar] [CrossRef]
- Zerva, A.; Papaspyridi, L.M.; Christakopoulos, P.; Topakas, E. Valorization of Olive Mill Wastewater for the Production of β-Glucans from Selected Basidiomycetes. Waste Biomass Valorization 2017, 8, 1721–1731. [Google Scholar] [CrossRef]
- Vamanu, E. Antioxidant Properties of Polysaccharides Obtained by Batch Cultivation of Pleurotus ostreatus Mycelium. Nat. Prod. Res. 2013, 27, 1115–1118. [Google Scholar] [CrossRef]
- Pilafidis, S.; Tsouko, E.; Sougleri, G.; Diamantopoulou, P. Submerged Cultivation of Selected Macro-Fungi to Produce Mycelia Rich in β-Glucans and Other Bioactive Compounds, Valorizing Side Streams of the Food Industry. Carbon. Resour. Convers. 2024, 7, 100198. [Google Scholar] [CrossRef]
- Kho, C.H.; Kan, S.C.; Chang, C.Y.; Cheng, H.Y.; Lin, C.C.; Chiou, P.C.; Shieh, C.J.; Liu, Y.C. Analysis of Exopolysaccharide Production Patterns of Cordyceps militaris under Various Light-Emitting Diodes. Biochem. Eng. J. 2016, 112, 226–232. [Google Scholar] [CrossRef]
- Ha, S.Y.; Jung, J.Y.; Yang, J.K. Effect of Light-Emitting Diodes on Cordycepin Production in Submerged Culture of Paecilomyces Japonica. J. Korean Wood Sci. Technol. 2020, 48, 548–561. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Chen, M.; Kan, S.; Shieh, C. Quantitative Analysis of LED Effects on Exopolysaccharide and Mycelial Growth Productions in Pleurotus Erygii Cultures. Soc. Chem. Ind. 2012, 88, 1841–1846. [Google Scholar] [CrossRef]
- Qi, Y.; Sun, X.; Ma, L.; Wen, Q.; Qiu, L.; Shen, J. Identification of Two Pleurotus ostreatus Blue Light Receptor Genes (PoWC-1 and PoWC-2) and in Vivo Confirmation of Complex PoWC-12 Formation through Yeast Two Hybrid System. Fungal Biol. 2020, 124, 8–14. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Tian, F.; Jia, C.; Li, C.; Li, Y. Transcriptomic Profiling Sheds Light on the Blue-Light and Red-Light Response of Oyster Mushroom (Pleurotus ostreatus). AMB Express 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Barbosa, J.; dos Santos Freitas, M.M.; da Silva Martins, L.H.; de Carvalho, R.N. Polysaccharides of Mushroom Pleurotus spp.: New Extraction Techniques, Biological Activities and Development of New Technologies. Carbohydr. Polym. 2020, 229, 115550. [Google Scholar] [CrossRef] [PubMed]
- Gutirrrez, A.; Prieto, A.; Martlnez, A.T. Structural Characterization of Extracellular Polysaccharides Produced by Fungi from the Genus Pleurotus. Carbohydr. Res. 1996, 281, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Gern, R.M.M.; Wisbeck, E.; Rampinelli, J.R.; Ninow, J.L.; Furlan, S.A. Alternative Medium for Production of Pleurotus ostreatus Biomass and Potential Antitumor Polysaccharides. Bioresour. Technol. 2008, 99, 76–82. [Google Scholar] [CrossRef] [PubMed]
- El-Enshasy, H.; Daba, A.; El-Demellawy, M.; Ibrahim, A.; El Sayed, S.; El-Badry, I. Bioprocess Development for Large Scale Production of Anticancer Exo-Polysaccharide by Pleurotus ostreatus in Submerged Culture. J. Appl. Sci. 2010, 10, 2523–2529. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Κossatz, L.; Xiros, C.; Katapodis, P.; Stamatis, H. Single-Cell Protein Production by Pleurotus ostreatus in Submerged Fermentation. Sustain. Food Technol. 2023, 1, 377–389. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Nilson, O.; Chatzikonstantinou, A.V.; Xiros, C.; Katapodis, P.; Stamatis, H. Mycoprotein Production by Submerged Fermentation of the Edible Mushroom Pleurotus ostreatus in a Batch Stirred Tank Bioreactor Using Agro-Industrial Hydrolysate. Foods 2023, 12, 2295. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The Bradford Method for Protein Quantitation. In The Protein Protocols Handbook; Springer Protocols Handbooks: Totowa, NJ, USA, 1996; Volume 32, pp. 15–20. [Google Scholar]
- Fan, X.; Yao, F.; Yin, C.; Shi, D.; Gao, H. Mycelial Biomass and Intracellular Polysaccharides Production, Characterization, and Activities in Auricularia Auricula-Judae Cultured with Different Carbon Sources. Int. J. Biol. Macromol. 2023, 244, 125426. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Giraldo, L.R.D.; Baez, P.X.V.; Forero, C.J.Z.; Arango, W.M. Production, Extraction, and Solubilization of Exopolysaccharides Using Submerged Cultures of Agaricomycetes. Bio-Protocol 2023, 13, e4841. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, D.; Sainis, I.; Touka, A.; Vareli, K.; Stamatis, H.; Katapodis, P. Biomass and β-Glucosidase Production by the Cyanobacterium Pseudanabaena sp. under Heterotrophic Conditions. Biomass 2022, 2, 299–315. [Google Scholar] [CrossRef]
- Athanasiou, P.E.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Stamatis, H. Valorization of Wine Lees: Assessment of Antioxidant, Antimicrobial and Enzyme Inhibitory Activity of Wine Lees Extract and Incorporation in Chitosan Films. Waste Biomass Valor 2024. [Google Scholar] [CrossRef]
- Fotiadou, R.; Lefas, D.; Vougiouklaki, D.; Tsakni, A.; Houhoula, D.; Stamatis, H. Enzymatic Modification of Pomace Olive Oil with Natural Antioxidants: Effect on Oxidative Stability. Biomolecules 2023, 13, 1034. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, P.E.; Gkountela, C.I.; Patila, M.; Fotiadou, R.; Chatzikonstantinou, A.V.; Vouyiouka, S.N.; Stamatis, H. Laccase-Mediated Oxidation of Phenolic Compounds from Wine Lees Extract towards the Synthesis of Polymers with Potential Applications in Food Packaging. Biomolecules 2024, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Bakratsas, G.; Antoniadis, K.; Athanasiou, P.E.; Katapodis, P.; Stamatis, H. Laccase and Biomass Production via Submerged Cultivation of Pleurotus ostreatus Using Wine Lees. Biomass 2024, 4, 1–22. [Google Scholar] [CrossRef]
- Araújo, N.L.; Avelino, K.V.; Halabura, M.I.W.; Marim, R.A.; Kassem, A.S.S.; Linde, G.A.; Colauto, N.B.; do Valle, J.S. Use of Green Light to Improve the Production of Lignocellulose-Decay Enzymes by Pleurotus spp. in Liquid Cultivation. Enzym. Microb. Technol. 2021, 149, 109860. [Google Scholar] [CrossRef]
- Zhu, L.; Su, Y.; Ma, S.; Guo, L.; Yang, S.; Yu, H. Comparative Proteomic Analysis Reveals Candidate Pathways Related to the Effect of Different Light Qualities on the Development of Mycelium and Fruiting Body of Pleurotus ostreatus. J. Agric. Food Chem. 2023, 72, 1361–1375. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Muñoz, S.V.; Atehortua, L.; Michel, F.C. Effects of Different Wavelengths of Light on Lignin Peroxidase Production by the White-Rot Fungi Phanerochaete Chrysosporium Grown in Submerged Cultures. Bioresour. Technol. 2010, 101, 9213–9220. [Google Scholar] [CrossRef]
- Halabura, M.I.W.; Avelino, K.V.; Araújo, N.L.; Kassem, A.S.S.; Seixas, F.A.V.; Barros, L.; Fernandes, Â.; Liberal, Â.; Ivanov, M.; Soković, M.; et al. Light Conditions Affect the Growth, Chemical Composition, Antioxidant and Antimicrobial Activities of the White-Rot Fungus Lentinus crinitus Mycelial Biomass. Photochem. Photobiol. Sci. 2023, 22, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Maftoun, P.; Malek, R.; Abdel-Sadek, M.; Aziz, R.; El Enshasy, H. Bioprocess for Semi-Industrial Production of Immunomodulator Polysaccharide Pleuran by Pleurotus ostreatus in Submerged Culture. J. Sci. Ind. Res. (India) 2013, 72, 655–662. [Google Scholar]
- Alsaheb, R.A.; Zjeh, K.Z.; Malek, R.A.; Abdullah, J.K.; El Baz, A.; El Deeb, N.; Dailin, D.; Hanapi, S.Z.; Sukmawati, D.; El Enshasy, H. Bioprocess Optimization for Exopolysaccharides Production by Ganoderma Lucidum in Semi-Industrial Scale. Recent. Pat. Food Nutr. Agric. 2020, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, E. Biological Activities of the Polysaccharides Produced in Submerged Culture of Two Edible Pleurotus ostreatus Mushrooms. J. Biomed. Biotechnol. 2012, 2012, 565974. [Google Scholar] [CrossRef] [PubMed]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from Basidiocarps of Cultivating Mushroom Pleurotus ostreatus: Isolation and Structural Characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novak, M. Structural Analysis of Glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→3)-β-d-Glucans, Their Preparation, Physico-Chemical Characterization and Immunomodulatory Activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]



| Dark | Red | Yellow | Green | Blue | White | |
|---|---|---|---|---|---|---|
| μ (d−1) | 0.75 ± 0.02 b | 0.82 ± 0.02 a | 0.78 ± 0.02 ab | 0.80 ± 0.01 a | 0.80 ± 0.01 a | 0.80 ± 0.01 a |
| Biomass (g/L) | 20.6 ± 0.4 a | 21.3 ± 0.4 a | 19.5 ± 0.4 b | 19.7 ± 0.4 b | 17.8 ± 0.4 c | 18.0 ± 0.4 c |
| Protein content (% dw) | 37.7 ± 4.1 b | 45.3 ± 3.3 a | 46.0 ± 1.7 a | 45.2 ± 2.0 a | 45.6 ± 4.3 a | 47.0 ± 2.5 a |
| EPS (g/L) | 1.6 ± 0.4 bc | 1.8 ± 0.2 ab | 1.5 ± 0.2 bc | 2.1 ± 0.3 a | 1.6 ± 0.1 bc | 1.7 ± 0.2 bc |
| IPS (% dw) | 8.9 ± 3.6 c | 27.2 ± 0.2 a | 15.3 ± 0.2 bc | 18.0 ± 3.2 b | 29.7 ± 6.5 a | 22.4 ± 1.0 ab |
| IPS (g/L) | 1.8 ± 0.0 f | 5.8 ± 0.1 a | 3.0 ± 0.1 e | 3.5 ± 0.1 d | 5.3 ± 0.1 b | 4.0 ± 0.1 c |
| Biomass production (g/L) | 12.6 ± 0.6 |
| EPS production (g/L) | 3.7 ± 0.1 |
| EPS productivity (g/L/d) | 0.48 ± 0.02 |
| IPS content (% dw) | 28.8 ± 0.1 |
| Protein content (% dw) | 50.3 ± 2.5 |
| Polysaccharide Analysis | Red Light EPS | Green Light EPS |
|---|---|---|
| Production (g/L) | 4.16 ± 0.03 ns | 4.14 ± 0.06 ns |
| Sugar content (% dw) | 50.0 ± 5.0 *** | 42.5 ± 4.0 *** |
| Protein content (% dw) | 19.5 ± 1.1 * | 14.7 ± 0.9 * |
| Lipid content (% dw) | 24.0 ± 2.3 * | 19.2 ± 0.1 * |
| Antioxidant activity—ABTS (%) (0.5 mg/mL) | 78.3 ± 2.5 **** | 27.7 ± 0.1 **** |
| Antioxidant activity—ABTS (μg Trolox/mg EPS) | 18.7 ± 0.2 **** | 7.1 ± 1.0 **** |
| Antioxidant activity—CUPRAC (μg Trolox/mg EPS) | 16.8 ± 0.5 **** | 8.1 ± 0.4 **** |
| Phenolic content (mg GAE/mg polysaccharide) | 4.9 ± 0.2 ns | 4.32 ± 0.07 ns |
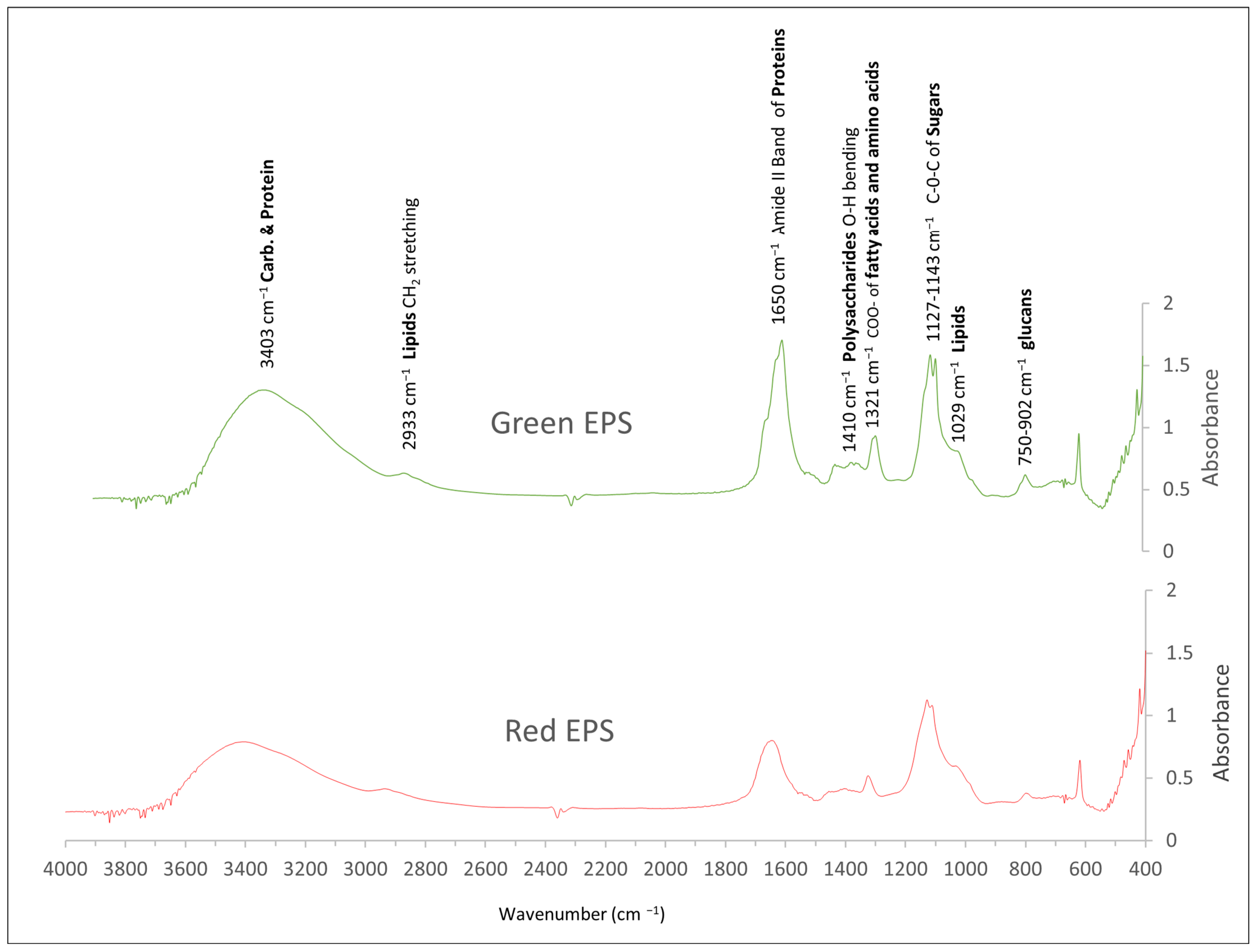
| β-glucan peaks (cm−1) | Baeva et al. (2019) [46] | Zerva et al. (2017) [23] | Synytsya et al. (2014) [20] | Synytsya et al. (2009) [20] | Sandula et al. (1999) [48] | Red EPS | Green EPS |
| 1374 | - | - | 1376 | - | 1382 | 1388 | |
| 1318 | - | - | 1317 | - | 1316 | 1313 | |
| 1158–1160 | 1127–1150 | 1160 | 1162 | 1160 | 1110 | 1126 | |
| 1080 | - | 1078 | 1100 | 1078 | 1106 | - | |
| 1038–1040 | - | 1044 | 1080 | 1041 | 1020 | 1024 | |
| 890–894 | 881, 893 | 890 | 1040 | 889 | 869 | 886 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakratsas, G.; Tsoumanis, C.; Stamatis, H.; Katapodis, P. Exopolysaccharide Production in Submerged Fermentation of Pleurotus ostreatus under Red and Green Light. Fermentation 2024, 10, 313. https://doi.org/10.3390/fermentation10060313
Bakratsas G, Tsoumanis C, Stamatis H, Katapodis P. Exopolysaccharide Production in Submerged Fermentation of Pleurotus ostreatus under Red and Green Light. Fermentation. 2024; 10(6):313. https://doi.org/10.3390/fermentation10060313
Chicago/Turabian StyleBakratsas, Georgios, Christoforos Tsoumanis, Haralambos Stamatis, and Petros Katapodis. 2024. "Exopolysaccharide Production in Submerged Fermentation of Pleurotus ostreatus under Red and Green Light" Fermentation 10, no. 6: 313. https://doi.org/10.3390/fermentation10060313
APA StyleBakratsas, G., Tsoumanis, C., Stamatis, H., & Katapodis, P. (2024). Exopolysaccharide Production in Submerged Fermentation of Pleurotus ostreatus under Red and Green Light. Fermentation, 10(6), 313. https://doi.org/10.3390/fermentation10060313






_Stamatis.png)


