Advances and Challenges in Biomanufacturing of Glycosylation of Natural Products
Abstract
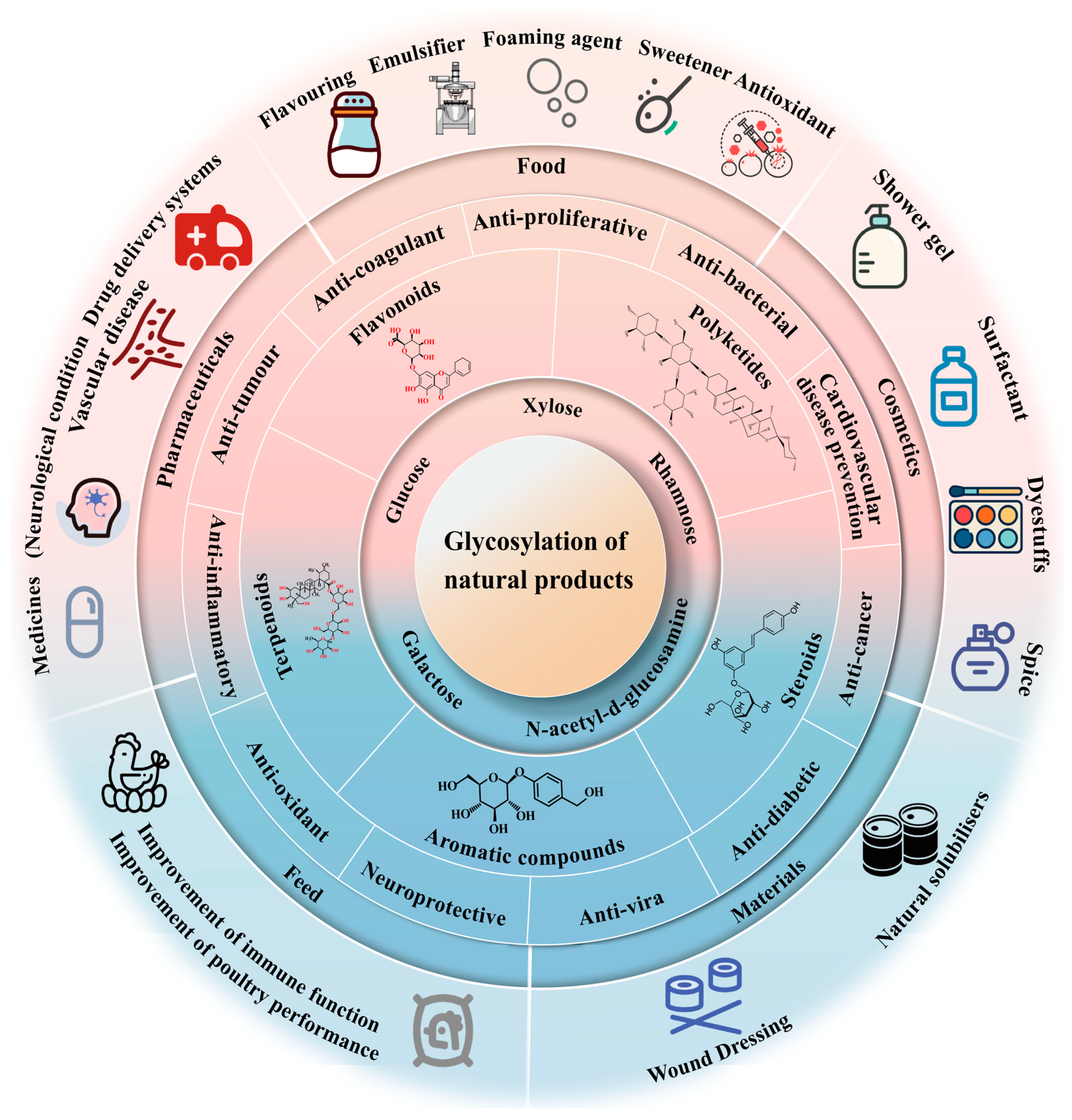
:1. Introduction
| Types of Natural Products | Product | Microbial Sources | GTs | Titer (g/L) | Engineered Strategy | Reference |
|---|---|---|---|---|---|---|
| Terpenoids and its derivatives | Reb M | E. coli | UGT76G1-T284S/M88L/L200A | 0.023 | The UGT76G1-T284S/M88L/L200A variant was obtained by structure-guided evolution | [34] |
| Rh2, PPD | S. cerevisiae | UGTPg45 | 2.25, 11.02 | Modular engineering of the mevalonate pathway was constructed and the expression level of P450 was optimized | [35] | |
| Ro | S. cerevisiae | GT73F3, UGT73P40 | 0.52 | Seven enzymes from five species with high catalytic efficiency and substrate specificity were screened through in vitro and in vivo characterization | [36] | |
| Ginsenoside Rh2 | S. cerevisiae | UGT51 | 0.3 | The semi rational design strategy of UGT51 was conducted, preventing Rh2 degradation and increasing UDP-glucose precursor supply | [37] | |
| CK | S. cerevisiae | UGTPg1 | 5.74 | optimizing the expression of UGTPg1, enhancing the biosynthesis of UDP-glucose, and reducing the consumption of UDP-glucose | [38] | |
| Rg1, R1, R2 | S. cerevisiae | PgUGT71A53, PgUGT94Q13, PgUGT71A54, | 1.95, 1.62, 1.25 | A set of UGT94 family UGTs were identified from ginseng and Panax notoginseng | [39] | |
| Rh2 | Bacillus subtilis | UGT51 | 3.7 | The regioselectivity of Yjic for Rh2 synthesis was successfully improved using a semi rational design | [40] | |
| Rh1, PPT | E. coli | UGTBL1 | 20.48, 18.04 | A structure-directed mutagenesis strategy was proposed to modify the enzyme UGT1 | [41] | |
| Asiaticoside | S. cerevisiae | CaUGT73C7, CaUGT73C8 | 0.00077 | knocking out EGH1, a glycoside hydrolase that degrades asiaticoside, and introducing key pathway enzymes | [42] | |
| Flavonoids and its derivatives | Baicalein-7-O-glucoside, Baicalein-7-O-rhamnoside | E. coli | EbUGT75L25, AtUGT89C1 | 0.57, 0.88 | A whole-cell biocatalytic system that lacks competing genes and incorporates an exogenous UDP-glucose supply pathway, as well as a glucose transferase, rhamnose transferase, and UDP-rhamnose synthesis pathway has been developed | [43] |
| 7-O-glucoside | S. cerevisiae | SbGT34 | 1.20 | Using the endogeneous glucosidase of brewing yeast as a whole-cell biocatalyst | [44] | |
| Fisetin 3-O-glucoside, Fisetin 3-O-rhamnoside | E. coli | UGT78K1 | 1.18, 1.03 | Utilizing a multi-nucleotide synthesis vector to assemble multiple genes of nucleoside diphosphate (NDP)-sugar biosynthetic pathways, a robust genetic circuit for producing valuable flavonoid glycosides in E. coli was constructed | [45] | |
| Astragalosides | E. coli | AtUGT78D2 | 3.6 | An efficient UDP-glucose synthesis pathway was reconstructed in recombinant strains by introducing sucrose permease, sucrose phosphorylase, and uridylic acid transferase | [46] | |
| Populoside, Orientin, Flavopiridoside, Quercetin, Chrysin, Trichoside, | E. coli | TcCGT1, GtfC, PhUGT | 17.2, 36.5, 5.2, 14.1, 6.4, 11.4 | A glycosylation platform strain was established in E. coli through multiple metabolic engineering with UDPG supply | [47] | |
| Hyperoside | E. coli | HmGAT | 0.025 | Four key enzymes were identified by gene screening and functional validation | [48] | |
| Polyketide and its derivatives | Salidroside | E. coli | UGT72B14 | 0.006 | The UGT72B14 from Rhodiola was optimized by codons and expressed in E. coli | [49] |
| Polydatin | S. cerevisiae | PcR3GAT | 0.55 | Key glycosyltransferases for resveratrol production were identified by transcriptome analysis. By combining the resveratrol biosynthetic module, UDP-glucose supply module, and glycosyltransferase expression module, the biosynthesis of glycosylated resveratrol was achieved | [50] | |
| MEB | E. coli | rfbA | 0.048 | Blocking the competing pathway of precursor glucose-1-phosphate | [51] | |
| Quercitrin | E. coli | AtUGT78D1 | 7.7 | Coupling the UDP-rhamnose generating system to Arabidopsis thaliana rhamnosyltransferase (AtUGT78D1) | [52] | |
| Aromatic and its derivatives | Gastrodin | Yarrowia lipolytica | SylUGT, RsUGT, ArUGT | 13.4 | More than fifty genetic modifications were introduced into the yeast genome | [53] |
| Rosavin | E. coli | SlUGT91R1 | 7.54 | Incorporation of SlUGT91R1 and the UDP-arabinose pathway into rosin-generating stains | [31] | |
| Steroid and its derivatives | Steroidal saponins | E. coli | PpUGT6 | - | Molecular docking of the PpUGT6 protein with ligands was performed, and key residues interacting with ligands were predicted | [54] |
| Dioscin | E. coli | DzGT1 | - | The cDNA encoding the trillin rhamnosyltransferase from D. zingiberensis was isolated | [55] | |
| CTS 3-O-glycosides | E. coli | UGT74AN2 | - | Key residues for sugar donor recognition and preference were identified | [56] |
2. Glycosylation of Terpenoids and Its Derivatives
3. Glycosylation of Flavonoid and Its Derivatives
3.1. Glycosylation of Flavonoid Glycosides
3.2. Glycosylation of Flavonols
4. Glycosylation of Polyketide and Its Derivatives
5. Glycosylation of Aromatic and Its Derivatives
6. Glycosylation of Steroid and Its Derivatives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurze, E.; Wüst, M.; Liao, J.; McGraphery, K.; Hoffmann, T.; Song, C.; Schwab, W. Structure–function relationship of terpenoid glycosyltransferases from plants. Nat. Prod. Rep. 2022, 39, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and Its therapeutic applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Grosslight, S.; Miller, S.J.; Sigman, M.S.; Toste, F.D. Site-Selective Acylation of natural products with binol-derived phosphoric acids. ACS Catal. 2019, 9, 9794–9799. [Google Scholar] [CrossRef] [PubMed]
- Majhi, S. Applications of yamaguchi method to esterification and macrolactonization in total synthesis of bioactive natural products. ChemistrySelect 2021, 6, 4178–4206. [Google Scholar] [CrossRef]
- Li, K.; Cai, J.; Su, Z.; Yang, B.; Liu, Y.; Zhou, X.; Huang, J.; Tao, H. Glycosylated natural products from marine microbes. Front. Chem. 2020, 7, 879. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-Y.; Yue, J.-M. Allylic hydroxylation of enones useful for the functionalization of relevant drugs and natural products. Nat. Commun. 2023, 14, 2399. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.-R.; Zhang, K.; Han, T.-Y.; Zhou, Q.; Lin, S.; Hou, X.-F.; Tang, G.-L. Two-enzyme cascade catalyzed trideuteromethylative modification of natural products. Tetrahedron 2022, 129, 133137. [Google Scholar] [CrossRef]
- He, B.; Bai, X.; Tan, Y.; Xie, W.; Feng, Y.; Yang, G.-Y. Glycosyltransferases: Mining, engineering and applications in biosynthesis of glycosylated plant natural products. Synth. Syst. Biotechnol. 2022, 7, 602–620. [Google Scholar] [CrossRef]
- Volchegursky, Y.; Hu, Z.; Katz, L.; McDaniel, R. Biosynthesis of the anti-parasitic agent megalomicin: Transformation of erythromycin to megalomicin in Saccharopolyspora erythraea. Mol. Microbiol. 2000, 37, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Singh, S.; Thorson, J.S.; Phillips, G.N., Jr. Loop dynamics of thymidine diphosphate-rhamnose 3’-o-methyltransferase (cals11), an enzyme in calicheamicin biosynthesis. Struct. Dyn. 2016, 3, 012004. [Google Scholar] [CrossRef]
- Sun, P.; Cai, R.; Chen, L.; Li, Y.; Jia, H.; Yan, M.; Chen, K. Natural Product glycosylation: Biocatalytic synthesis of quercetin-3,4′-o-diglucoside. Appl. Biochem. Biotechnol. 2020, 190, 464–474. [Google Scholar] [CrossRef]
- Trefzer, A.; Hoffmeister, D.; Künzel, E.; Stockert, S.; Weitnauer, G.; Westrich, L.; Rix, U.; Fuchser, J.; Bindseil, K.U.; Rohr, J.; et al. Function of glycosyltransferase genes involved in urdamycin A biosynthesis. Chem. Biol. 2000, 7, 133–142. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Han, T.Y.; Giuliano, A.E.; Hansen, N.; Cabot, M.C. Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. J. Biol. Chem. 2000, 275, 7138–7143. [Google Scholar] [CrossRef]
- Räty, K.; Kunnari, T.; Hakala, J.; Mäntsälä, P.; Ylihonko, K. A gene cluster from Streptomyces galilaeus involved in glycosylation of aclarubicin. Mol. Gen. Genet. MGG 2000, 264, 164–172. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, G.; El-Halfawy, O.; Simon, M.; Brown, E.D.; Pfeifer, B.A. Broadened glycosylation patterning of heterologously produced erythromycin. Biotechnol. Bioeng. 2018, 115, 2771–2777. [Google Scholar] [CrossRef]
- Sato, T.; Takahashi, M.; Kawado, T.; Takayama, E.; Furukawa, K. Effect of staurosporine on n-glycosylation and cell adhesion to fibronectin of SW480 human colorectal adenocarcinoma cells. Eur. J. Pharm. Sci. 2005, 25, 221–227. [Google Scholar] [CrossRef]
- Moore, M.J.; Qu, S.; Tan, C.; Cai, Y.; Mogi, Y.; Jamin Keith, D.; Boger, D.L. Next-generation total synthesis of vancomycin. J. Am. Chem. Soc. 2020, 142, 16039–16050. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Stueckle, T.A.; Tse, W.; Rojanasakul, Y.; Dinu, C.Z. Digitoxin and its analogs as novel cancer therapeutics. Exp. Hematol. Oncol. 2012, 1, 4. [Google Scholar] [CrossRef]
- Kim, H.J.; Kang, S.H.; Choi, S.S.; Kim, E.S. Redesign of antifungal polyene glycosylation: Engineered biosynthesis of disaccharide-modified NPP. Appl. Microbiol. Biotechnol. 2017, 101, 5131–5137. [Google Scholar] [CrossRef]
- Pommerehne, K.; Walisko, J.; Ebersbach, A.; Krull, R. The antitumor antibiotic rebeccamycin—Challenges and advanced approaches in production processes. Appl. Microbiol. Biotechnol. 2019, 103, 3627–3636. [Google Scholar] [CrossRef]
- Huang, G.; Lv, M.; Hu, J.; Huang, K.; Xu, H. Glycosylation and activities of natural products. Mini Rev. Med. Chem. 2016, 16, 1013–1016. [Google Scholar] [CrossRef]
- Teze, D.; Coines, J.; Fredslund, F.; Dubey, K.D.; Bidart, G.N.; Adams, P.D.; Dueber, J.E.; Svensson, B.; Rovira, C.; Welner, D.H. O-/N-/S-Specificity in glycosyltransferase catalysis: From mechanistic understanding to engineering. ACS Catal. 2021, 11, 1810–1815. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, L.; Feng, X.; Liu, X.; Guo, F.; Lv, B.; Li, C. Galactosylation of monosaccharide derivatives of glycyrrhetinic acid by udp-glycosyltransferase gmsgt2 from glycine max. J. Agric. Food Chem. 2020, 68, 8580–8588. [Google Scholar] [CrossRef]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The structure and function of major plant metabolite modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [CrossRef]
- Hellfritsch, C.; Brockhoff, A.; Stähler, F.; Meyerhof, W.; Hofmann, T. Human psychometric and taste receptor responses to steviol glycosides. J. Agric. Food Chem. 2012, 60, 6782–6793. [Google Scholar] [CrossRef]
- Qiu, S.; Blank, L.M. Recent advances in yeast recombinant biosynthesis of the triterpenoid protopanaxadiol and glycosylated derivatives thereof. J. Agric. Food Chem. 2023, 71, 2197–2210. [Google Scholar] [CrossRef]
- Eida, A.A.; Samadi, A.; Tsunoda, T.; Mahmud, T. Modifications of acyl carrier protein-bound glycosylated polyketides in pactamycin biosynthesis. Chem.—A Eur. J. 2023, 29, e202301056. [Google Scholar] [CrossRef]
- Huang, F.-C.; Hinkelmann, J.; Hermenau, A.; Schwab, W. Enhanced production of β-glucosides by in-situ udp-glucose regeneration. J. Biotechnol. 2016, 224, 35–44. [Google Scholar] [CrossRef]
- Chen, A.; Gu, N.; Pei, J.; Su, E.; Duan, X.; Cao, F.; Zhao, L. Synthesis of isorhamnetin-3-o-rhamnoside by a three-enzyme (rhamnosyltransferase, glycine max sucrose synthase, udp-rhamnose synthase) cascade using a udp-rhamnose regeneration system. Molecules 2019, 24, 3042. [Google Scholar] [CrossRef]
- Wen, C.; Wu, H.-C.; Ouyang, W.-H.; Nie, J.-X.; Guo, Y.-P.; Wang, F.; Hu, L.-L.; Yang, J.-H.; Zheng, L.-J.; Wang, J.-L.; et al. Exploring the catalytic flexibility and reversibility of plant glycosyltransferase htugt72as1 for glycodiversification of phenolic compounds. J. Agric. Food Chem. 2023, 71, 8998–9008. [Google Scholar] [CrossRef]
- Li, L.; Liu, M.; Bi, H.; Liu, T. High-level production of Rhodiola rosea characteristic component rosavin from d-glucose and l-arabinose in engineered Escherichia coli. Metab. Eng. 2024, 82, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Dubey, K.K.; Kumar, A.; Baldia, A.; Rajput, D.; Kateriya, S.; Singh, R.; Nikita; Tandon, R.; Mishra, Y.K. Biomanufacturing of glycosylated antibodies: Challenges, solutions, and future prospects. Biotechnol. Adv. 2023, 69, 108267. [Google Scholar] [CrossRef] [PubMed]
- Andreu, A.; Ćorović, M.; Garcia-Sanz, C.; Santos, A.S.; Milivojević, A.; Ortega-Nieto, C.; Mateo, C.; Bezbradica, D.; Palomo, J.M. Enzymatic glycosylation strategies in the production of bioactive compounds. Catalysts 2023, 13, 1359. [Google Scholar] [CrossRef]
- Guo, B.; Deng, Z.; Meng, F.; Wang, Q.; Zhang, Y.; Yuan, Z.; Rao, Y. Enhancement of rebaudioside m production by structure-guided engineering of glycosyltransferase ugt76g1. J. Agric. Food Chem. 2022, 70, 5088–5094. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wei, W.; Ye, W.; Li, X.; Zhao, W.; Yang, C.; Li, C.; Yan, X.; Zhou, Z. Synthesizing ginsenoside rh2 in Saccharomyces cerevisiae cell factory at high-efficiency. Cell Discov. 2019, 5, 5. [Google Scholar] [CrossRef]
- Ren, S.; Sun, Q.; Zhang, L.; Sun, W.; Li, Y.; Feng, X.; Li, C. Sustainable production of rare oleanane-type ginsenoside ro with an artificial glycosylation pathway in Saccharomyces cerevisiae. Green Chem. 2022, 24, 8302–8313. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yang, G.-Y.; Chen, X.; Liu, Q.; Zhang, X.; Deng, Z.; Feng, Y. Biosynthesis of plant-derived ginsenoside rh2 in yeast via repurposing a key promiscuous microbial enzyme. Metab. Eng. 2017, 42, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Zhao, G.; Yan, X.; Zhou, Z. Systematic optimization of the yeast cell factory for sustainable and high efficiency production of bioactive ginsenoside compound k. Synth. Syst. Biotechnol. 2021, 6, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Fan, Z.; Wang, Y.; Wang, P.; Yan, X.; Zhou, Z. High-level sustainable production of the characteristic protopanaxatriol-type saponins from Panax species in engineered Saccharomyces cerevisiae. Metab. Eng. 2021, 66, 87–97. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Ma, Y.; Li, Y.; Qin, S.; He, B. Oriented efficient biosynthesis of rare ginsenoside rh2 from ppd by compiling UGT-Yjic mutant with sucrose synthase. Int. J. Biol. Macromol. 2020, 146, 853–859. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, L.; Xu, X.; Li, Y.; Wu, B.; Qin, S.; He, B. Evolving the 3-o/6-o regiospecificity of a microbial glycosyltransferase for efficient production of ginsenoside rh1 and unnatural ginsenoside. Int. J. Biol. Macromol. 2024, 261, 129678. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, W.; Li, S.; Gao, S.; Liu, S.; Zeng, W.; Zhou, J. Elucidation of the biosynthesis of asiaticoside and its reconstitution in yeast. ACS Sustain. Chem. Eng. 2024, 12, 4028–4040. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Cao, S.; Zhang, H.; Pei, J.; Bu, S.; Zhao, L. Efficient production of the glycosylated derivatives of baicalein in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2023, 107, 2831–2842. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Lin, L.; Zhou, W.; Liu, M.; Cheng, K.; Wang, W. Engineering Saccharomyces cerevisiae with the deletion of endogenous glucosidases for the production of flavonoid glucosides. Microb. Cell Factories 2016, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.; Pandey, R.P.; Trang, N.T.H.; Chaudhary, A.K.; Sohng, J.K. Synthetic sugar cassettes for the efficient production of flavonol glycosides in Escherichia coli. Microb. Cell Factories 2015, 14, 76. [Google Scholar] [CrossRef]
- Pei, J.; Dong, P.; Wu, T.; Zhao, L.; Fang, X.; Cao, F.; Tang, F.; Yue, Y. Metabolic engineering of Escherichia coli for astragalin biosynthesis. J. Agric. Food Chem. 2016, 64, 7966–7972. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Qin, Z.; Zeng, W.; Zhou, J. Enhancing glycosylation of flavonoids by engineering the uridine diphosphate glucose supply in Escherichia coli. J. Agric. Food Chem. 2023, 71, 17842–17851. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Z.; Li, Q.; Zhang, S.; Li, Y.; Li, X.; Kong, L.; Luo, J. The parallel biosynthesis routes of hyperoside from naringenin in Hypericum monogynum. Hortic. Res. 2023, 10, uhad166. [Google Scholar] [CrossRef]
- Xue, F.; Guo, H.; Hu, Y.; Liu, R.; Huang, L.; Lv, H.; Liu, C.; Yang, M.; Ma, L. Expression of codon-optimized plant glycosyltransferase ugt72b14 in Escherichia coli enhances salidroside production. BioMed Res. Int. 2016, 2016, 9845927. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Li, L.; Liu, X.; Guo, Z.; Cheng, J.; Zhu, X.; Lu, L.; Zhang, J.; Fan, G.; et al. De novo biosynthesis of polydatin in Saccharomyces cerevisiae. J. Agric. Food Chem. 2021, 69, 5917–5925. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Feng, Z.; Wang, Y. Multi-strategy engineering unusual sugar tdp-l-mycarose biosynthesis to improve the production of 3-o-α-mycarosylerythronolide b in Escherichia coli. Synth. Syst. Biotechnol. 2022, 7, 756–764. [Google Scholar] [CrossRef]
- Gu, N.; Qiu, C.; Zhao, L.; Zhang, L.; Pei, J. Enhancing udp-rhamnose supply for rhamnosylation of flavonoids in Escherichia coli by regulating the modular pathway and improving nadph availability. J. Agric. Food Chem. 2020, 68, 9513–9523. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Jiang, Y.; Li, C.; Zhu, J.; Lu, X.; Ge, J.; Hu, M.; Deng, J.; Ma, J.; Yang, Z.; et al. High titer production of gastrodin enabled by systematic refactoring of yeast genome and an antisense-transcriptional regulation toolkit. Metab. Eng. 2024, 82, 250–261. [Google Scholar] [CrossRef]
- He, M.; Guo, S.; Yin, Y.; Zhang, C.; Zhang, X. A novel sterol glycosyltransferase catalyses steroidal sapogenin 3-o glucosylation from Paris polyphylla var. yunnanensis. Mol. Biol. Rep. 2023, 50, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mosongo, I.; Li, H.; Wu, Y.; Li, C.; Yang, S.; Zhang, Y. Identification and characterization of a trillin rhamnosyltransferase from Dioscorea zingiberensis. Front. Plant Sci. 2021, 12, 713036. [Google Scholar] [CrossRef]
- Huang, W.; He, Y.; Jiang, R.; Deng, Z.; Long, F. Functional and structural dissection of a plant steroid 3-o-glycosyltransferase facilitated the engineering enhancement of sugar donor promiscuity. ACS Catal. 2022, 12, 2927–2937. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.-l.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 3555. [Google Scholar] [CrossRef]
- Osbourn, A.; Goss, R.J.; Field, R.A. The saponins: Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef]
- Pu, Z.; Ge, F.; Wang, Y.; Jiang, Z.; Zhu, S.; Qin, S.; Dai, Q.; Liu, H.; Hua, H. Ginsenoside-rg3 inhibits the proliferation and invasion of hepatoma carcinoma cells via regulating long non-coding rna hox antisense intergenic. Bioengineered 2021, 12, 2398–2409. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.M.; Lu, T.C.; Sun, M.L.; Jia, W.Y.; Ji, X.; Luo, Y.G. Ginsenoside Rd inhibits glioblastoma cell proliferation by up-regulating the expression of mir-144-5p. Biol. Pharm. Bull. 2020, 43, 1534–1541. [Google Scholar] [CrossRef]
- Li, C.T.; Wang, H.B.; Xu, B.J. A comparative study on anticoagulant activities of three Chinese herbal medicines from the genus Panax and anticoagulant activities of ginsenosides rg1 and rg2. Pharm. Biol. 2013, 51, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Bhattamisra, S.K.; Chellappan, D.K.; Candasamy, M. Actions and therapeutic potential of madecassoside and other major constituents of Centella asiatica: A Review. Appl. Sci. 2021, 11, 8475. [Google Scholar] [CrossRef]
- Wong, J.H.; Barron, A.M.; Abdullah, J.M. Mitoprotective effects of Centella asiatica (L.) Urb.: Anti-inflammatory and neuroprotective opportunities in neurodegenerative disease. Front. Pharmacol. 2021, 12, 687935. [Google Scholar] [CrossRef]
- Yao, M.; Wang, J.; Zhang, J.; Guo, Y.; Ni, Z.; Jia, X.; Feng, H. Asiaticoside attenuates oxygen-glucose deprivation/reoxygenation-caused injury of cardiomyocytes by inhibiting autophagy. J. Appl. Toxicol. JAT 2023, 43, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Philippaert, K.; Pironet, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Segal, A.; Antoine, N.; Gysemans, C.; et al. Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of trpm5 channel activity. Nat. Commun. 2017, 8, 14733. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Carlsen, S.; Semmler, A.; Simón, E.; Mikkelsen, M.D.; Møller, B.L. Microbial production of next-generation stevia sweeteners. Microb. Cell Fact. 2016, 15, 207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, M.; Chen, Y.; Ke, D.; Zhou, J.; Xu, X.; Yang, W.; He, J.; Dong, H.; Wei, Y.; et al. Catalytic flexibility of rice glycosyltransferase osugt91c1 for the production of palatable steviol glycosides. Nat. Commun. 2021, 12, 7030. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, J.; Sun, Y.; Zhang, P.; Wang, Y. Structural insights into the catalytic mechanism of a plant diterpene glycosyltransferase srugt76g1. Plant Commun. 2020, 1, 100004. [Google Scholar] [CrossRef]
- Jeon, B.M.; Baek, J.I.; Kim, M.S.; Kim, S.C.; Cui, C.H. Characterization of a novel ginsenoside mt1 produced by an enzymatic transrhamnosylation of protopanaxatriol-type ginsenosides re. Biomolecules 2020, 10, 525. [Google Scholar] [CrossRef]
- Wei, W.; Wang, P.; Wei, Y.; Liu, Q.; Yang, C.; Zhao, G.; Yue, J.; Yan, X.; Zhou, Z. Characterization of panax ginseng udp-glycosyltransferases catalyzing protopanaxatriol and biosyntheses of bioactive ginsenosides f1 and rh1 in metabolically engineered yeasts. Mol. Plant 2015, 8, 1412–1424. [Google Scholar] [CrossRef]
- Rachpirom, M.; Pichayakorn, W.; Puttarak, P. Preparation, development, and scale-up of standardized pentacyclic triterpenoid-rich extract from Centella asiatica (L.) urb. and study of its wound healing activity. Heliyon 2023, 9, e17807. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.; Jin, M.L.; Lee, D.Y.; Jetter, R. Characterization of the asiatic acid glucosyltransferase, ugt73ah1, involved in asiaticoside biosynthesis in Centella asiatica (L.) urban. Int. J. Mol. Sci. 2017, 18, 2630. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, J.; Chang, X.; Li, Q.; Deng, Z.; Yu, Y. Revisiting the transcriptome data of Centella asiatica identified an ester-forming triterpenoid: Udp-glucose 28-o-glucosyltransferase. Tetrahedron 2022, 129, 133136. [Google Scholar] [CrossRef]
- de Costa, F.; Barber, C.J.S.; Kim, Y.B.; Reed, D.W.; Zhang, H.; Fett-Neto, A.G.; Covello, P.S. Molecular cloning of an ester-forming triterpenoid: UDP-glucose 28-o-glucosyltransferase involved in saponin biosynthesis from the medicinal plant Centella asiatica. Plant Sci. Int. J. Exp. Plant Biol. 2017, 262, 9–17. [Google Scholar] [CrossRef]
- Hanson, J.R.; Nichols, T.; Mukhrish, Y.; Bagley, M.C. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2019, 36, 1499–1512. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, Y.; Guo, C.; Yang, Y.; Han, J.; Yu, B.; Yin, W.; Liu, H. Reconstitution of biosynthetic pathway for mushroom-derived cyathane diterpenes in yeast and generation of new “non-natural” analogues. Acta Pharm. Sinica B 2021, 11, 2945–2956. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Diao, M.; Peng, L.; Huang, S.; Xie, N. Characterization of a group of udp-glycosyltransferases involved in the biosynthesis of triterpenoid saponins of Panax notoginseng. ACS Synth. Biol. 2022, 11, 770–779. [Google Scholar] [CrossRef]
- Sun, Q.; Guo, F.; Ren, S.; Zhang, L.; Liu, X.; Li, C.; Feng, X. Construction of a udp-arabinose regeneration system for efficient arabinosylation of pentacyclic triterpenoids. ACS Synth. Biol. 2023, 12, 2463–2474. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, B.; Wang, D.E.; Yao, T.; Pang, L.; Tu, Q.; Ahmed, S.M.; Liu, J.J.; Wang, J. Nitrogen-containing apigenin analogs: Preparation and biological activity. Molecules 2012, 17, 14748–14764. [Google Scholar] [CrossRef]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, R.; Wishart, A.D.; Rupasinghe, H.P.; Arcellana-Panlilio, M.; Nelson, C.M.; Mayne, M.; Robertson, G.S. Quercetin 3-glucoside protects neuroblastoma (sh-sy5y) cells in vitro against oxidative damage by inducing sterol regulatory element-binding protein-2-mediated cholesterol biosynthesis. J. Biol. Chem. 2008, 283, 2231–2245. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2011, 28, 1626–1695. [Google Scholar] [CrossRef]
- Juergenliemk, G.; Boje, K.; Huewel, S.; Lohmann, C.; Galla, H.J.; Nahrstedt, A. In vitro studies indicate that miquelianin (quercetin 3-o-beta-d-glucuronopyranoside) is able to reach the cns from the small intestine. Planta Medica 2003, 69, 1013–1017. [Google Scholar] [CrossRef]
- Ionkova, I.; Shkondrov, A.; Krasteva, I.; Ionkov, T. Recent progress in phytochemistry, pharmacology and biotechnology of Astragalus saponins. Phytochem. Rev. 2014, 13, 343–374. [Google Scholar] [CrossRef]
- Mahmud, A.R.; Ema, T.I.; Siddiquee, M.F.; Shahriar, A.; Ahmed, H.; Mosfeq-Ul-Hasan, M.; Rahman, N.; Islam, R.; Uddin, M.R.; Mizan, M.F.R. Natural flavonols: Actions, mechanisms, and potential therapeutic utility for various diseases. Beni-Suef Univ. J. Basic. Appl. Sci. 2023, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, G.; Zhang, H.; Peng, Z.; Ding, W.; Wen, H.; Zhou, H.; Zeng, J.; Chen, J.; Xu, J. Four novel cit7glcts functional in flavonoid 7-o-glucoside biosynthesis are vital to flavonoid biosynthesis shunting in citrus. Hortic. Res. 2024, 11, uhae098. [Google Scholar] [CrossRef]
- Xu, Z.S.; Ma, J.; Wang, F.; Ma, H.Y.; Wang, Q.X.; Xiong, A.S. Identification and characterization of dcucgalt1, a galactosyltransferase responsible for anthocyanin galactosylation in purple carrot (Daucus carota L.) taproots. Sci. Rep. 2016, 6, 27356. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Huang, R.; Liu, L.; Li, Y.; Wang, W.; Xu, Q.; Yu, Y.; Zhou, T. Csugt78a15 catalyzes the anthocyanidin 3-o-galactoside biosynthesis in tea plants. Plant Physiol. Biochem. PPB 2021, 166, 738–749. [Google Scholar] [CrossRef]
- Zhao, S.; Fu, S.; Cao, Z.; Liu, H.; Huang, S.; Li, C.; Zhang, Z.; Yang, H.; Wang, S.; Luo, J.; et al. Osugt88c3 encodes a udp-glycosyltransferase responsible for biosynthesis of malvidin 3-o-galactoside in rice. Plants 2024, 13, 697. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, M.; Gao, B.; Hasan, A.; Li, J.; Bao, Y.; Fan, J.; Yu, R.; Yi, Y.; Ågren, H.; et al. Characterization and protein engineering of glycosyltransferases for the biosynthesis of diverse hepatoprotective cycloartane-type saponins in Astragalus membranaceus. Plant Biotechnol. J. 2023, 21, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.-o.; Zhang, M.; Li, H.; Wang, Z.; Zhou, J.; Yi, Y.; Li, F.; Ye, L.; Li, H.; Jin, H.; et al. Functional characterization and protein engineering of a glycosyltransferase gccgt to produce flavone 6,8-di-c- and 6-c-4′-o-glycosides. ACS Catal. 2024, 14, 1075–1082. [Google Scholar] [CrossRef]
- Xue, Q.; Su, X.; Yu, W.; Liu, J.; Hou, K.; Wang, C. Efficient production of neohesperidin enabled by protein engineering of rhamnosyltransferase cm1,2rhat. ACS Sustain. Chem. Eng. 2024, 12, 1960–1972. [Google Scholar] [CrossRef]
- He, J.B.; Zhao, P.; Hu, Z.M.; Liu, S.; Kuang, Y.; Zhang, M.; Li, B.; Yun, C.H.; Qiao, X.; Ye, M. Molecular and structural characterization of a promiscuous c-glycosyltransferase from Trollius chinensis. Angew. Chem. 2019, 58, 11513–11520. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, Y.; Usui, S.; Ito, T.; Kato, A.; Shimosaka, M.; Taguchi, G. Purification, molecular cloning and functional characterization of flavonoid c-glucosyltransferases from Fagopyrum esculentum M. (buckwheat) cotyledon. Plant J. 2014, 80, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, Y.; Liu, J.; Zhang, S.; Tan, X.; Zhao, Y.; Mao, J.; Jiang, N.; Zhou, J.; Shan, Y. Systematic engineering of saccharomyces cerevisiae chassis for efficient flavonoid-7-o-disaccharide biosynthesis. ACS Synth. Biol. 2023, 12, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Fei, S.; Liu, X.; Li, J.; Gao, Y.; Yang, X.; Wang, X.; Shen, Y. Crystal structures of rhamnosyltransferase ugt89c1 from Arabidopsis thaliana reveal the molecular basis of sugar donor specificity for udp-β-l-rhamnose and rhamnosylation mechanism. Plant J. 2019, 99, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Thoma, J.; Grabherr, R.; Staudacher, E. Determination, expression and characterization of an udp-n-acetylglucosamine:A-1,3-d-mannoside β-1,2-n-acetylglucosaminyltransferase i (gnt-i) from the Pacific oyster, Crassostrea gigas. Glycoconj. J. 2024, 41, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.J.; Kim, K.-T.; Paik, H.-D. Microbial bioconversion of ginsenosides in Panax ginseng and their improved bioactivities. Food Rev. Int. 2018, 34, 698–712. [Google Scholar] [CrossRef]
- Yin, Q.; Han, X.; Chen, J.; Han, Z.; Shen, L.; Sun, W.; Chen, S. Identification of specific glycosyltransferases involved in flavonol glucoside biosynthesis in ginseng using integrative metabolite profiles, dia proteomics, and phylogenetic analysis. J. Agric. Food Chem. 2021, 69, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, R.; Wang, R.; Li, J.; Xie, K.; Bian, C.; Sun, L.; Zhang, X.; Liu, J.; Yang, L.; et al. Probing the catalytic promiscuity of a regio- and stereospecific c-glycosyltransferase from Mangifera indica. Angew. Chem. 2015, 54, 12678–12682. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, Z.-M.; Zhong, L.; Fan, J.; Li, M.; Ma, Y.; Zhou, Y.; Zhang, W.; Guo, B.; Chen, B.; et al. Directed evolution of a plant glycosyltransferase for chemo- and regioselective glycosylation of pharmaceutically significant flavonoids. ACS Catal. 2021, 11, 14781–14790. [Google Scholar] [CrossRef]
- Li, D.; Chen, G.; Ma, B.; Zhong, C.; He, N. Metabolic profiling and transcriptome analysis of mulberry leaves provide insights into flavonoid biosynthesis. J. Agric. Food Chem. 2020, 68, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Mrudulakumari Vasudevan, U.; Lee, E.Y. Flavonoids, terpenoids, and polyketide antibiotics: Role of glycosylation and biocatalytic tactics in engineering glycosylation. Biotechnol. Adv. 2020, 41, 107550. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chen, H.C.; Wu, C.S.; Wu, P.Y.; Wen, K.C. Rhodiola plants: Chemistry and biological activity. J. Food Drug Anal. 2015, 23, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Giner, R.M.; Máñez, S. Immunmodulatory and antiproliferative properties of Rhodiola species. Planta Medica 2016, 82, 952–960. [Google Scholar] [CrossRef]
- Wang, C.; Luo, Y.; Lu, J.; Wang, Y.; Sheng, G. Polydatin induces apoptosis and inhibits growth of acute monocytic leukemia cells. J. Biochem. Mol. Toxicol. 2016, 30, 200–205. [Google Scholar] [CrossRef]
- Wang, H.L.; Gao, J.P.; Han, Y.L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.H. Comparative studies of polydatin and resveratrol on mutual transformation and antioxidative effect in vivo. Phytomedicine Int. J. Phytother. Phytopharm. 2015, 22, 553–559. [Google Scholar] [CrossRef]
- Lanzilli, G.; Cottarelli, A.; Nicotera, G.; Guida, S.; Ravagnan, G.; Fuggetta, M.P. Anti-inflammatory effect of resveratrol and polydatin by in vitro IL-17 modulation. Inflammation 2012, 35, 240–248. [Google Scholar] [CrossRef]
- Ito, T.; Roongsawang, N.; Shirasaka, N.; Lu, W.; Flatt, P.M.; Kasanah, N.; Miranda, C.; Mahmud, T. Deciphering pactamycin biosynthesis and engineered production of new pactamycin analogues. Chembiochem 2009, 10, 2253–2265. [Google Scholar] [CrossRef] [PubMed]
- Eida, A.A.; Mahmud, T. The secondary metabolite pactamycin with potential for pharmaceutical applications: Biosynthesis and regulation. Appl. Microbiol. Biotechnol. 2019, 103, 4337–4345. [Google Scholar] [CrossRef]
- Eida, A.A.; Abugrain, M.E.; Brumsted, C.J.; Mahmud, T. Glycosylation of acyl carrier protein-bound polyketides during pactamycin biosynthesis. Nat. Chem. Biol. 2019, 15, 795–802. [Google Scholar] [CrossRef]
- Kudo, F.; Zhang, J.; Sato, S.; Hirayama, A.; Eguchi, T. Functional characterization of 3-aminobenzoic acid adenylation enzyme pctu and udp-n-acetyl-d-glucosamine: 3-aminobenzoyl-acp glycosyltransferase pctl in pactamycin biosynthesis. ChemBioChem 2019, 20, 2458–2462. [Google Scholar] [CrossRef]
- Zeng, Y.-Q.; Gu, J.-H.; Chen, L.; Zhang, T.-T.; Zhou, X.-F. Gastrodin as a multi-target protective compound reverses learning memory deficits and ad-like pathology in app/ps1 transgenic mice. J. Funct. Foods 2021, 77, 104324. [Google Scholar] [CrossRef]
- Yang, C.Y.; Lee, S.Y.; Seo, M.H.; Yoon, J.B. Cinnamyl alcohol: An attractant of the flower thrips Frankliniella intonsa. J. Asia-Pac. Entomol. 2022, 25, 101925. [Google Scholar] [CrossRef]
- Gottardi, M.; Grün, P.; Bode, H.B.; Hoffmann, T.; Schwab, W.; Oreb, M.; Boles, E. Optimisation of trans-cinnamic acid and hydrocinnamyl alcohol production with recombinant Saccharomyces cerevisiae and identification of cinnamyl methyl ketone as a by-product. FEMS Yeast Res. 2017, 17, fox091. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.D.; Li, K.; Wang, Z.L.; Wang, Y.X.; He, J.B.; Su, H.F.; Zhang, Z.Y.; Chi, C.B.; Shi, X.M.; et al. Functional characterization and structural basis of an efficient di-c-glycosyltransferase from Glycyrrhiza glabra. J. Am. Chem. Soc. 2020, 142, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Korvorapun, K.; De Sarkar, S.; Rogge, T.; Burns, D.J.; Warratz, S.; Ackermann, L. Ruthenium(II)-catalysed remote C-H alkylations as a versatile platform to meta-decorated arenes. Nat. Commun. 2017, 8, 15430. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Q.; Ji, Y.; Bai, X.; Li, D.; Mu, R.; Guo, K.; Yang, M.; Tao, Y.; Gershenzon, J.; et al. Unraveling the serial glycosylation in the biosynthesis of steroidal saponins in the medicinal plant Paris polyphylla and their antifungal action. Acta Pharm. Sinica. B 2023, 13, 4638–4654. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, C.; Wu, J.; Qi, J.; Hua, X.; Kang, L.; Yuan, Q.; Yuan, J.; Xue, Z. Characterization of three Paris polyphylla glycosyltransferases from different ugt families for steroid functionalization. ACS Synth. Biol. 2022, 11, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, X.; Li, J.; Lv, J.; Wang, Y.; He, Y.; Song, J.; Ågren, H.; Jiang, R.; Deng, Z.; et al. Substrate promiscuity, crystal structure, and application of a plant udp-glycosyltransferase ugt74an3. ACS Catal. 2024, 14, 475–488. [Google Scholar] [CrossRef]
- Wen, C.; Huang, W.; Zhu, X.L.; Li, X.S.; Zhang, F.; Jiang, R.W. UGT74AN1, a permissive glycosyltransferase from Asclepias curassavica for the regiospecific steroid 3-o-glycosylation. Org. Lett. 2018, 20, 534–537. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Wang, B.; Pei, L.; Wang, J.; Gan, Y.; Jiang, L.; Liu, B.; Cheng, J.; Li, W. Advances and Challenges in Biomanufacturing of Glycosylation of Natural Products. Fermentation 2024, 10, 349. https://doi.org/10.3390/fermentation10070349
Hu S, Wang B, Pei L, Wang J, Gan Y, Jiang L, Liu B, Cheng J, Li W. Advances and Challenges in Biomanufacturing of Glycosylation of Natural Products. Fermentation. 2024; 10(7):349. https://doi.org/10.3390/fermentation10070349
Chicago/Turabian StyleHu, Shunyang, Bangxu Wang, Liang Pei, Jisheng Wang, Ya Gan, Liangzhen Jiang, Bingliang Liu, Jie Cheng, and Wei Li. 2024. "Advances and Challenges in Biomanufacturing of Glycosylation of Natural Products" Fermentation 10, no. 7: 349. https://doi.org/10.3390/fermentation10070349
APA StyleHu, S., Wang, B., Pei, L., Wang, J., Gan, Y., Jiang, L., Liu, B., Cheng, J., & Li, W. (2024). Advances and Challenges in Biomanufacturing of Glycosylation of Natural Products. Fermentation, 10(7), 349. https://doi.org/10.3390/fermentation10070349






