Phenotypic Characterization of Fermentation Performance and Stress Tolerance in Commercial Ale Yeast Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Media
2.3. Microscopic Imaging of Yeast Morphology
2.4. Fermentation Parameters Measurement
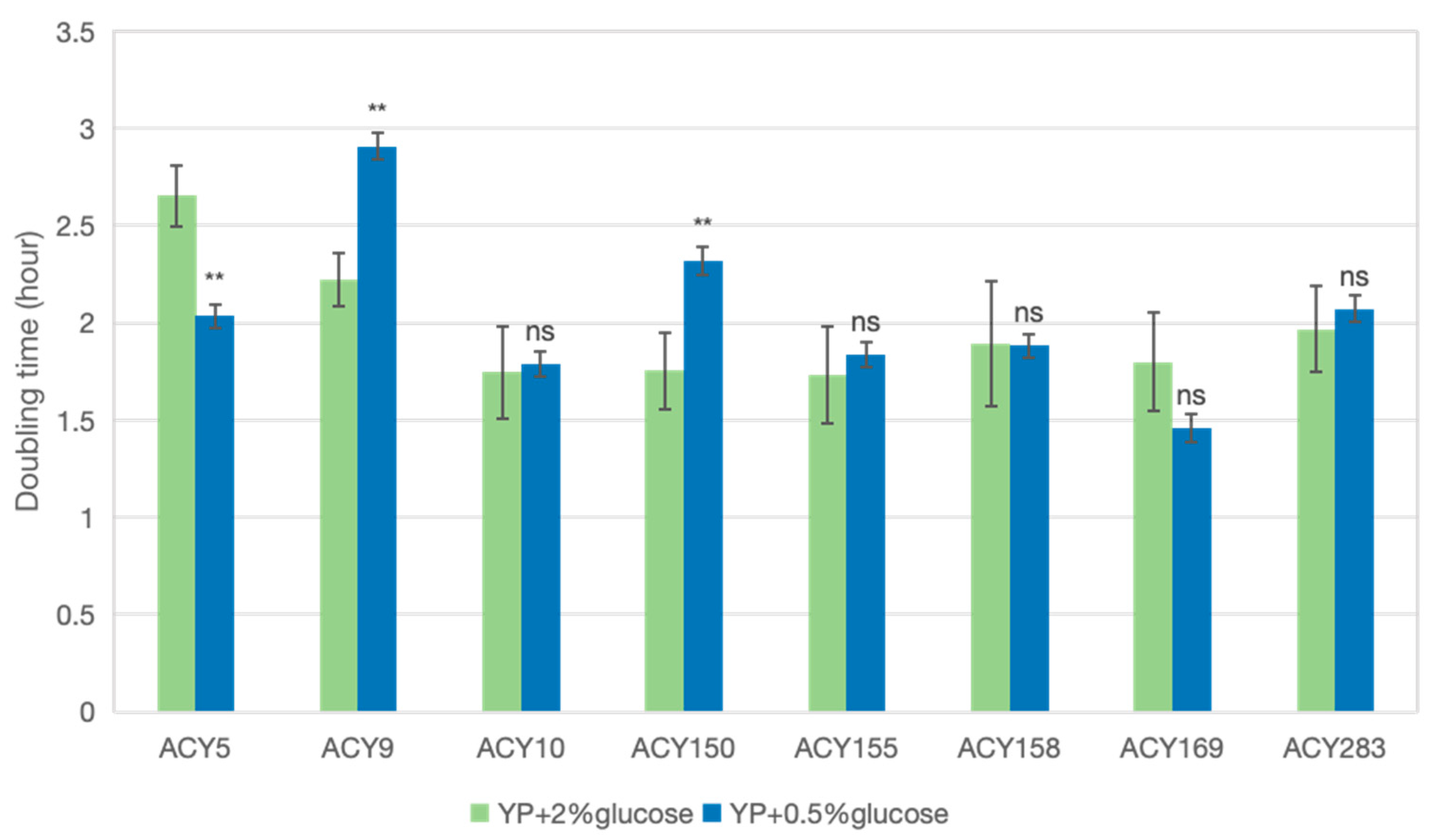
2.5. Cell Density Measurement and Doubling Time Calculation
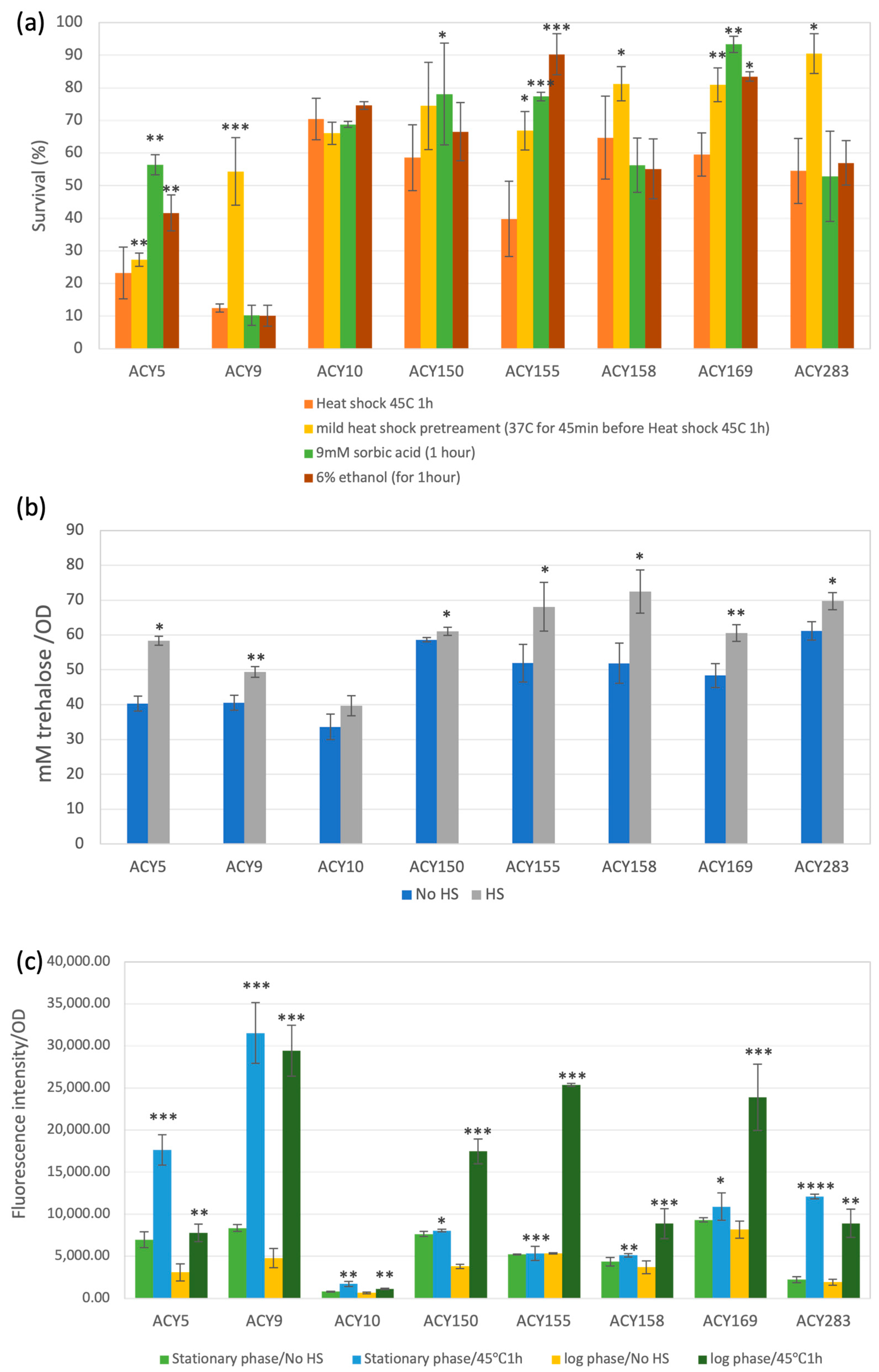
2.6. Assessment of Thermotolerance
2.7. Measurement of Trehalose
2.8. Measurement of Reactive Oxygen Species (ROS) Levels
2.9. Stress Tolerance Assessment
2.10. Statistical Analysis
3. Results
3.1. Fermentation Profile
3.2. Carbon Source Utilization
3.3. Stress Tolerance
3.3.1. Glucose Limitation
3.3.2. Osmotic Stress Tolerance
3.3.3. Cold Shock Tolerance
3.3.4. Acid Tolerance
3.3.5. Ethanol Tolerance
3.3.6. Heat Tolerance
3.3.7. Overall Stress Tolerance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and Its Industrial Applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Maicas, S. Yeast Fermentation and the Make of Biotechnological Products. Microorganisms 2023, 11, 1463. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; He, X.; Weng, P.; Liu, Y.; Wu, Z. A Review of Yeast: High Cell-Density Culture, Molecular Mechanisms of Stress Response and Tolerance during Fermentation. FEMS Yeast Res. 2022, 22, foac050. [Google Scholar] [CrossRef] [PubMed]
- Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A.I. Cellular Stress Impact on Yeast Activity in Biotechnological Processes—A Short Overview. Microorganisms 2023, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, K.; Tuszyński, T. The Effect of Temperature on Fermentation and Beer Volatiles at an Industrial Scale. J. Inst. Brew. 2018, 124, 230–235. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast Fermentation at Low Temperatures: Adaptation to Changing Environmental Conditions and Formation of Volatile Compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef] [PubMed]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. A Review of Recent Advances in High Gravity Ethanol Fermentation. Renew. Energy 2019, 133, 1366–1379. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. Recent Developments in High Gravity Beer-Brewing. Innov. Food Sci. Emerg. Technol. 2020, 64, 102399. [Google Scholar] [CrossRef]
- Sipiczki, M. Yeast Two- and Three-Species Hybrids and High-Sugar Fermentation. Microb. Biotechnol. 2019, 12, 1101–1108. [Google Scholar] [CrossRef]
- Yoshida, M.; Furutani, N.; Imai, F.; Miki, T.; Izawa, S. Wine Yeast Cells Acquire Resistance to Severe Ethanol Stress and Suppress Insoluble Protein Accumulation during Alcoholic Fermentation. Microbiol. Spectr. 2022, 10, e0090122. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Li, L.; Tian, J.; Li, X.; Pan, Y. Screening and Transcriptomic Analysis of the Ethanol-Tolerant Mutant Saccharomyces cerevisiae YN81 for High-Gravity Brewing. Front. Microbiol. 2022, 13, 976321. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Gao, Z.; Chen, K.; Ouyang, Q.; Luo, C. Yeast Cells under Glucose-Limitation Environment Need Increased Response Cost for Osmostress Defense. bioRxiv 2021. [Google Scholar] [CrossRef]
- Lane, S.; Xu, H.; Oh, E.J.; Kim, H.; Lesmana, A.; Jeong, D.; Zhang, G.; Tsai, C.S.; Jin, Y.S.; Kim, S.R. Glucose Repression Can Be Alleviated by Reducing Glucose Phosphorylation Rate in Saccharomyces cerevisiae. Sci. Rep. 2018, 8, 2613. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, B.; Liang, X.B.; Robert, F.; Soontorngun, N. Transcriptional Regulation of Nonfermentable Carbon Utilization in Budding Yeast. FEMS Yeast Res. 2010, 10, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Coppola, F.; Letizia, F.; Testa, B.; Sorrentino, E. Role of Yeasts in the Brewing Process: Tradition and Innovation. Processes 2021, 9, 839. [Google Scholar] [CrossRef]
- Hickman, M.J.; Winston, F. Heme Levels Switch the Function of Hap1 of Saccharomyces cerevisiae between Transcriptional Activator and Transcriptional Repressor. Mol. Cell. Biol. 2007, 27, 7414–7424. [Google Scholar] [CrossRef]
- Botstein, D.; Fink, G.R. Yeast: An Experimental Organism for 21st Century Biology. Genetics 2011, 189, 695–704. [Google Scholar] [CrossRef]
- Jordan, M.A. Bioprocess Engineering Principles; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Principles of Fermentation Technology, 3rd ed.; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Korzyńska, A.; Zychowicz, M. A Method of Estimation of the Cell Doubling Time on Basis of the Cell Culture Monitoring Data. Biocybern. Biomed. Eng. 2008, 28, 75–82. [Google Scholar]
- Henderi, H. Comparison of Min-Max Normalization and Z-Score Normalization in the K-Nearest Neighbor (KNN) Algorithm to Test the Accuracy of Types of Breast Cancer. IJIIS Int. J. Inform. Inf. Syst. 2021, 4, 13–20. [Google Scholar] [CrossRef]
- Dattalo, P. Analysis of Multiple Dependent Variables; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Shulman, R.G.; Rothman, D.L. Homeostasis and the Glycogen Shunt Explains Aerobic Ethanol Production in Yeast. Proc. Natl. Acad. Sci. USA 2015, 112, 10902–10907. [Google Scholar] [CrossRef] [PubMed]
- Boer, V.M.; Crutchfield, C.A.; Bradley, P.H.; Botstein, D.; Rabinowitz, J.D. Growth-Limiting Intracellular Metabolites in Yeast Growing under Diverse Nutrient Limitations. Mol. Biol. Cell 2010, 21, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Winderickx, J.; Holsbeeks, I.; Lagatie, O.; Giots, F.; Thevelein, J.; de Winde, H. From Feast to Famine; Adaptation to Nutrient Availability in Yeast. In Yeast Stress Response; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Jung, H.M.; Im, D.K.; Lim, J.H.; Jung, G.Y.; Oh, M.K. Metabolic Perturbations in Mutants of Glucose Transporters and Their Applications in Metabolite Production in Escherichia coli. Microb. Cell Factor. 2019, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Piddocke, M.P.; Kreisz, S.; Heldt-Hansen, H.P.; Nielsen, K.F.; Olsson, L. Physiological Characterization of Brewer’s Yeast in High-Gravity Beer Fermentations with Glucose or Maltose Syrups as Adjuncts. Appl. Microbiol. Biotechnol. 2009, 84, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Dziugan, P.; Balcerek, M.; Pielech-Przybylska, K.; Patelski, P. Evaluation of the Fermentation of High Gravity Thick Sugar Beet Juice Worts for Efficient Bioethanol Production. Biotechnol. Biofuels 2013, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Miedl, M.; Bamforth, C.W. The Relative Importance of Temperature and Time in the Cold Conditioning of Beer. J. Am. Soc. Brew. Chem. 2004, 62, 75–78. [Google Scholar] [CrossRef]
- Dysvik, A.; La Rosa, S.L.; De Rouck, G.; Rukke, E.O.; Westereng, B.; Wicklund, T. Microbial Dynamics in Traditional and Modern Sour Beer Production. Appl. Environ. Microbiol. 2020, 86, e00566-20. [Google Scholar] [CrossRef] [PubMed]
- Doğan, A.; Demirci, S.; Aytekin, A.Ö.; Şahin, F. Improvements of Tolerance to Stress Conditions by Genetic Engineering in Saccharomyces cerevisiae during Ethanol Production. Appl. Biochem. Biotechnol. 2014, 174, 28–42. [Google Scholar] [CrossRef]
- Choudhary, J.; Singh, S.; Nain, L. Thermotolerant Fermenting Yeasts for Simultaneous Saccharification Fermentation of Lignocellulosic Biomass. Electron. J. Biotechnol. 2016, 21, 82–92. [Google Scholar] [CrossRef]
- Harvey, H.J.; Hendry, A.C.; Chirico, M.; Archer, D.B.; Avery, S.V. Adaptation to Sorbic Acid in Low Sugar Promotes Resistance of Yeast to the Preservative. Heliyon 2023, 9, e22057. [Google Scholar] [CrossRef]
- Yoshida, M.; Kato, S.; Fukuda, S.; Izawa, S. Acquired Resistance to Severe Ethanol Stress in Saccharomyces cerevisiae Protein Quality Control. Appl. Environ. Microbiol. 2021, 87, e02353-20. [Google Scholar] [CrossRef] [PubMed]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The Response to Heat Shock and Oxidative Stress in Saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef] [PubMed]
- Caspeta, L.; Chen, Y.; Nielsen, J. Thermotolerant Yeasts Selected by Adaptive Evolution Express Heat Stress Response at 30 °C. Sci. Rep. 2016, 6, 27003. [Google Scholar] [CrossRef] [PubMed]
- Stratford, M.; Vallières, C.; Geoghegan, I.A.; Archer, D.B.; Avery, S.V. The Preservative Sorbic Acid Targets Respiration, Explaining the Resistance of Fermentative Spoilage Yeast Species. mSphere 2020, 5, e00273-20. [Google Scholar] [CrossRef] [PubMed]
- Gibney, P.A.; Schieler, A.; Chen, J.C.; Rabinowitz, J.D.; Botstein, D. Characterizing the In Vivo Role of Trehalose in Saccharomyces cerevisiae Using the AGT1 Transporter. Proc. Natl. Acad. Sci. USA 2015, 112, 6116–6121. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Gibney, P.A. Intracellular Trehalose Accumulation via the Agt1 Transporter Promotes Freeze–Thaw Tolerance in Saccharomyces cerevisiae. J. Appl. Microbiol. 2022, 133, 2390–2402. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Barajas, J.A.; Montoya-Pérez, R.; Salgado-Garciglia, R.; Aguilera-Aguirre, L.; Cortés-Rojo, C.; Mejía-Zepeda, R.; Arellano-Plaza, M.; Saavedra-Molina, A. Oxidative Stress and Antioxidant Response in a Thermotolerant Yeast. Braz. J. Microbiol. 2017, 48, 326–332. [Google Scholar] [CrossRef]
- Carrasco, P.; Querol, A.; Del Olmo, M. Analysis of the Stress Resistance of Commercial Wine Yeast Strains. Arch. Microbiol. 2001, 175, 450–457. [Google Scholar] [CrossRef]
- Zuzuarregui, A.; del Olmo, M. lí Analyses of Stress Resistance under Laboratory Conditions Constitute a Suitable Criterion for Wine Yeast Selection. Antonie Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2004, 85, 271–280. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, W.; Lu, Y.; Shen, C.; Yao, H.; Yang, X.; Yao, X.; Lu, T.; Hu, B. PEP4-Allele Modification Provides an Industrial Brewing Yeast with Malate Stress Tolerance. Fermentation 2023, 9, 378. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Guo, Y.; Wu, F.; Zhang, Y.; Qi, X.; Wang, Z.; Wang, Q. Stress Tolerance Enhancement via SPT15 Base Editing in Saccharomyces cerevisiae. Biotechnol. Biofuels 2021, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, T.; Maeda, T. Adaptive Laboratory Evolution of Microorganisms: Methodology and Application for Bioproduction. Microorganisms 2023, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, Y.L.; Fang, Y.; Zhao, H. Adaptive Evolution and Selection of Stress-Resistant Saccharomyces cerevisiae for Very High-Gravity Bioethanol Fermentation. Electron. J. Biotechnol. 2019, 41, 88–94. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Pan, Y.; Li, X.; Liu, G.; Sun, H.; Zhang, R.; Zhang, C. Breeding of High-Tolerance Yeast by Adaptive Evolution and High-Gravity Brewing of Mutant. J. Sci. Food Agric. 2024, 104, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.R.; Marques, L.F.; de Almeida, L.F.; Lázari, L.C.; de Moraes, L.N.; Cardoso, L.H.; de Oliveira Alves, C.C.; Nakajima, R.T.; Schnepper, A.P.; Golim, M.d.A.; et al. Integrative Analysis of the Ethanol Tolerance of Saccharomyces cerevisiae. Int. J. Mol. Sci. 2023, 24, 5646. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, J.; Niu, C.; Liu, C.; Zheng, F.; Li, Q. Transcriptomic and Metabolomic Analysis Reveals Genes Related to Stress Tolerance in High Gravity Brewing. World J. Microbiol. Biotechnol. 2022, 38, 59. [Google Scholar] [CrossRef]
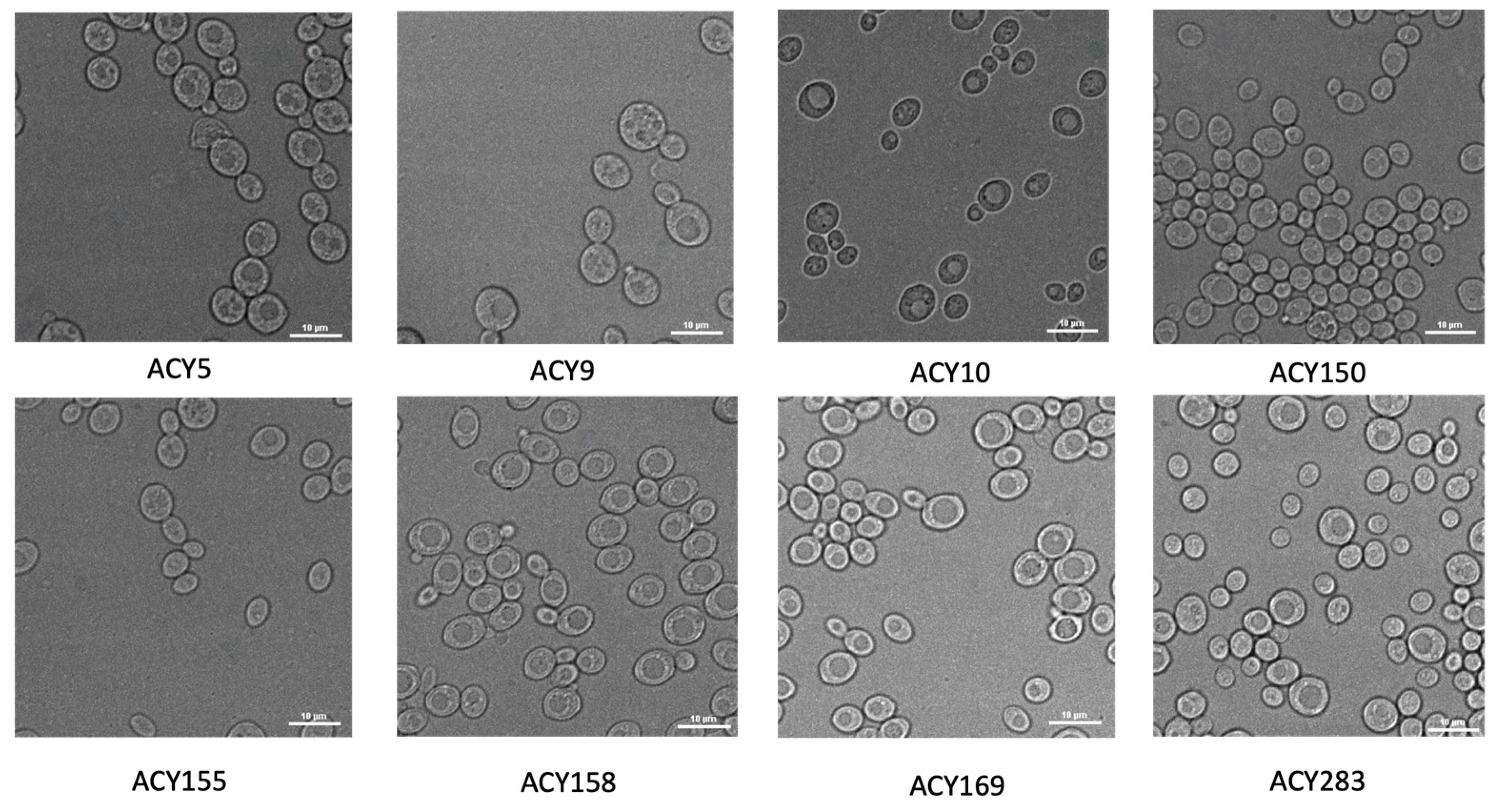
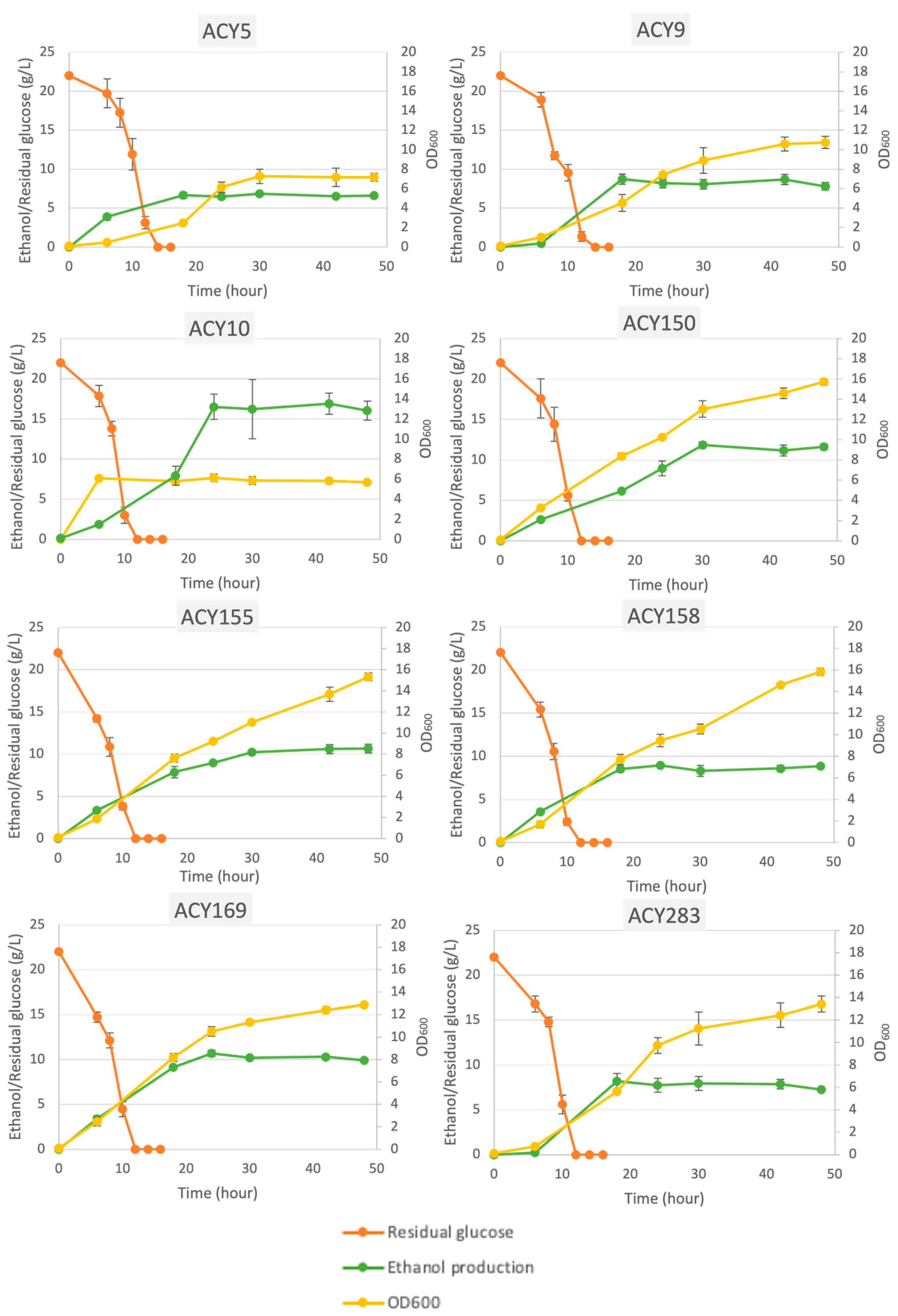
| Strain | Description | Fermentation Profile | ||
|---|---|---|---|---|
| Average Glucose Consumption Rate (g/L/h) | Average Ethanol Production Rate (g/L/h) | pH Change (pHinitial − pHfinal) | ||
| ACY5 | WLP001 California Ale (White Labs) | 0.662 ± 0.004 | 0.185 ± 0.000 | 0.483 ± 0.080 |
| ACY9 | WLP004 Irish Ale Yeast (White Labs) | 0.661 ± 0.004 | 0.236 ± 0.010 | 1.947 ± 0.238 |
| ACY10 | WLP300 Hefeweizen Ale Yeast (White Labs) | 0.996 ± 0.004 | 0.197 ± 0.005 | −1.487 ± 0.050 |
| ACY150 | Lalvin 71B (Lallemand) | 0.990 ± 0.010 | 0.255 ± 0.023 | −1.253 ± 0.110 |
| ACY155 | Lalvin BM45 (Lallemand) | 0.976 ± 0.002 | 0.228 ± 0.019 | −1.327 ± 0.117 |
| ACY158 | Lalvin ICV D254 (Lallemand) | 0.981 ± 0.004 | 0.173 ± 0.009 | −1.397 ± 0.106 |
| ACY169 | Lalvin V1116 (Lallemand) | 0.978 ± 0.002 | 0.195 ± 0.007 | −1.633 ± 0.144 |
| ACY283 | Lab strain S288C derivative | 0.658 ± 0.002 | 0.023 ± 0.007 | −0.353 ± 0.097 |
| Strain | Doubling Time (h) | |||||||
|---|---|---|---|---|---|---|---|---|
| YP + 2% Glucose | YP + 2% Fructose | YP + 2% Galactose | YP + 2% Maltose | YP + 2% Trehalose | YP + 2% Sucrose | YP + 2% Raffinose | AVERAGE (Normalized) | |
| ACY5 | 2.70 ± 0.17 | 3.04 ± 0.34 | 3.75 ± 0.27 | 3.90 ± 0.32 | 9.85 ± 0.09 | 2.86 ± 0.30 | 4.29 ± 0.19 | 0.275 |
| ACY9 | 2.22 ± 0.15 | 3.91 ± 0.36 | 5.66 ± 0.30 | 4.63 ± 0.34 | 4.09 ± 0.24 | 3.83 ± 0.22 | 3.11 ± 0.26 | 0.261 |
| ACY10 | 2.22 ± 0.26 | 2.09 ± 0.24 | 2.76 ± 0.35 | 3.15 ± 0.12 | 10.12 ± 0.18 | 2.06 ± 0.48 | 2.69 ± 0.20 | 0.722 |
| ACY150 | 1.34 ± 0.22 | 2.59 ± 0.25 | 3.35 ± 0.31 | 3.14 ± 0.09 | 6.37 ± 0.18 | 3.35 ± 0.44 | 2.91 ± 0.38 | 0.630 |
| ACY155 | 1.32 ± 0.27 | 2.70 ± 0.24 | 2.32 ± 0.23 | 2.83 ± 0.11 | 5.80 ± 0.24 | 2.61 ± 0.10 | 3.32 ± 0.29 | 0.735 |
| ACY158 | 1.73 ± 0.36 | 2.72 ± 0.33 | 2.43 ± 0.24 | 2.87 ± 0.06 | 5.48 ± 0.40 | 2.64 ± 0.08 | 3.10 ± 0.37 | 0.718 |
| ACY169 | 1.07 ± 0.28 | 2.55 ± 0.09 | 2.56 ± 0.26 | 2.56 ± 0.14 | 3.46 ± 0.06 | 2.74 ± 0.19 | 1.67 ± 0.18 | 0.875 |
| ACY283 | 1.80 ± 0.24 | 2.02 ± 0.24 | 2.89 ± 0.31 | 2.51 ± 0.21 | 5.75 ± 0.24 | 1.92 ± 0.14 | 2.66 ± 0.16 | 0.836 |
| Strain | Doubling Time (h) | Maximum OD600 | Time before Enters Log Phase (h) | Scoring (Normalized) | ||||
|---|---|---|---|---|---|---|---|---|
| Osmotic Stress (1 M Sorbitol) | Cold Stress (4 °C) | Osmotic Stress (1 M Sorbitol) | Cold Stress (4 °C) | Osmotic Stress (1 M Sorbitol) | Cold Stress (4 °C) | Osmotic Stress (1 M Sorbitol) | Cold Stress (4 °C) | |
| ACY5 | 2.63 ± 0.23 | 2.15 ± 0.03 | 1.74 ± 0.06 | 2.46 ± 0.13 | 13.0 ± 0.30 | 27.0 ± 0.14 | 0.110 | 0.861 |
| ACY9 | 2.07 ± 0.25 | 2.09 ± 0.03 | 1.47 ± 0.09 | 1.34 ± 0.07 | 8.0 ± 0.27 | 34.0 ± 0.14 | 0.480 | 0.650 |
| ACY10 | 2.24 ± 0.26 | 5.63 ± 0.03 | 1.89 ± 0.01 | 0.26 ± 0.03 | 13.0 ± 0.28 | 47.0 ± 0.13 | 0.334 | 0.309 |
| ACY150 | 1.83 ± 0.27 | 2.00 ± 0.03 | 2.20 ± 0.08 | 2.23 ± 0.07 | 8.0 ± 0.29 | 21.0 ± 0.12 | 0.880 | 0.882 |
| ACY155 | 1.93 ± 0.27 | 4.13 ± 0.03 | 1.56 ± 0.08 | 2.33 ± 0.07 | 9.0 ± 0.30 | 14.0 ± 0.12 | 0.526 | 0.902 |
| ACY158 | 1.66 ± 0.29 | 18.31 ± 0.02 | 2.00 ± 0.11 | 0.29 ± 0.03 | 8.0 ± 0.26 | 47.0 ± 0.13 | 0.866 | 0.004 |
| ACY169 | 1.95 ± 0.31 | 1.89 ± 0.02 | 2.21 ± 0.07 | 1.64 ± 0.02 | 7.0 ± 0.34 | 11.0 ± 0.13 | 0.879 | 0.887 |
| ACY283 | 2.38 ± 0.31 | 2.18 ± 0.02 | 1.89 ± 0.02 | 2.13 ± 0.12 | 7.5 ± 0.32 | 31.0 ± 0.11 | 0.551 | 0.782 |
| Strain | Doubling Time (h) | Maximum OD600 | Time before Enters Log Phase (h) | Scoring (Normalized) | ||||
|---|---|---|---|---|---|---|---|---|
| Acid Stress (pH = 2.2) | 10% Ethanol Stress | Acid Stress (pH = 2.2) | 10% Ethanol Stress | Acid Stress (pH = 2.2) | 10% Ethanol Stress | Acid Stress (pH = 2.2) | 10% Ethanol Stress | |
| ACY5 | 5.25 ± 0.11 | 24.30 ± 0.07 | 1.63 ± 0.16 | 0.39 ± 0.03 | 23.0 ± 0.59 | 47.0 ± 0.25 | 0.076 | 0.003 |
| ACY9 | 2.48 ± 0.11 | 11.38 ± 0.08 | 1.16 ± 0.07 | 0.38 ± 0.03 | 13.0 ± 0.62 | 47.0 ± 0.24 | 0.537 | 0.277 |
| ACY10 | 2.18 ± 0.11 | 9.41 ± 0.10 | 3.01 ± 0.11 | 1.09 ± 0.11 | 7.0 ± 0.64 | 26.0 ± 0.33 | 0.982 | 0.645 |
| ACY150 | 2.29 ± 0.11 | 5.62 ± 0.13 | 2.01 ± 0.12 | 1.92 ± 0.12 | 7.0 ± 0.61 | 22.0 ± 0.30 | 0.806 | 0.915 |
| ACY155 | 2.43 ± 0.10 | 8.22 ± 0.12 | 2.30 ± 0.18 | 1.57 ± 0.14 | 7.0 ± 0.57 | 18.0 ± 0.15 | 0.836 | 0.833 |
| ACY158 | 2.61 ± 0.11 | 6.87 ± 0.12 | 2.44 ± 0.02 | 1.57 ± 0.02 | 7.0 ± 0.61 | 26.0 ± 0.31 | 0.834 | 0.786 |
| ACY169 | 3.35 ± 0.11 | 6.59 ± 0.12 | 2.30 ± 0.17 | 2.03 ± 0.24 | 8.0 ± 0.57 | 15.0 ± 0.19 | 0.698 | 0.979 |
| ACY283 | 3.26 ± 0.11 | 8.31 ± 0.12 | 2.59 ± 0.19 | 1.28 ± 0.05 | 6.0 ± 0.58 | 26.0 ± 0.26 | 0.791 | 0.703 |
| Strain | Overall Scoring |
|---|---|
| ACY5 | 0.296 |
| ACY9 | 0.315 |
| ACY10 | 0.622 |
| ACY150 | 0.773 |
| ACY155 | 0.695 |
| ACY158 | 0.671 |
| ACY169 | 0.864 |
| ACY283 | 0.726 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, A.; Si, Q.; Xu, Q.; Pan, C.; Cheng, Y.; Chen, J. Phenotypic Characterization of Fermentation Performance and Stress Tolerance in Commercial Ale Yeast Strains. Fermentation 2024, 10, 364. https://doi.org/10.3390/fermentation10070364
Chen A, Si Q, Xu Q, Pan C, Cheng Y, Chen J. Phenotypic Characterization of Fermentation Performance and Stress Tolerance in Commercial Ale Yeast Strains. Fermentation. 2024; 10(7):364. https://doi.org/10.3390/fermentation10070364
Chicago/Turabian StyleChen, Anqi, Qiqi Si, Qingyun Xu, Chenwei Pan, Yuhan Cheng, and Jian Chen. 2024. "Phenotypic Characterization of Fermentation Performance and Stress Tolerance in Commercial Ale Yeast Strains" Fermentation 10, no. 7: 364. https://doi.org/10.3390/fermentation10070364
APA StyleChen, A., Si, Q., Xu, Q., Pan, C., Cheng, Y., & Chen, J. (2024). Phenotypic Characterization of Fermentation Performance and Stress Tolerance in Commercial Ale Yeast Strains. Fermentation, 10(7), 364. https://doi.org/10.3390/fermentation10070364







