Impact of Lactic Acid Fermentation on the Organic Acids and Sugars of Developed Oat and Buckwheat Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of the Potential Probiotic Strains
2.2. Development of Potential Probiotic Plant-Based Drinks
2.3. pH Changes
2.4. Microbiological Viability Analysis
2.5. Organic Acids and Sugar Detection
2.6. Statistical Analysis
3. Results and Discussion
3.1. Viability in L. rhamnosus K3 and L. johnsonii K4 during Storage
3.2. pH Changes during Storage
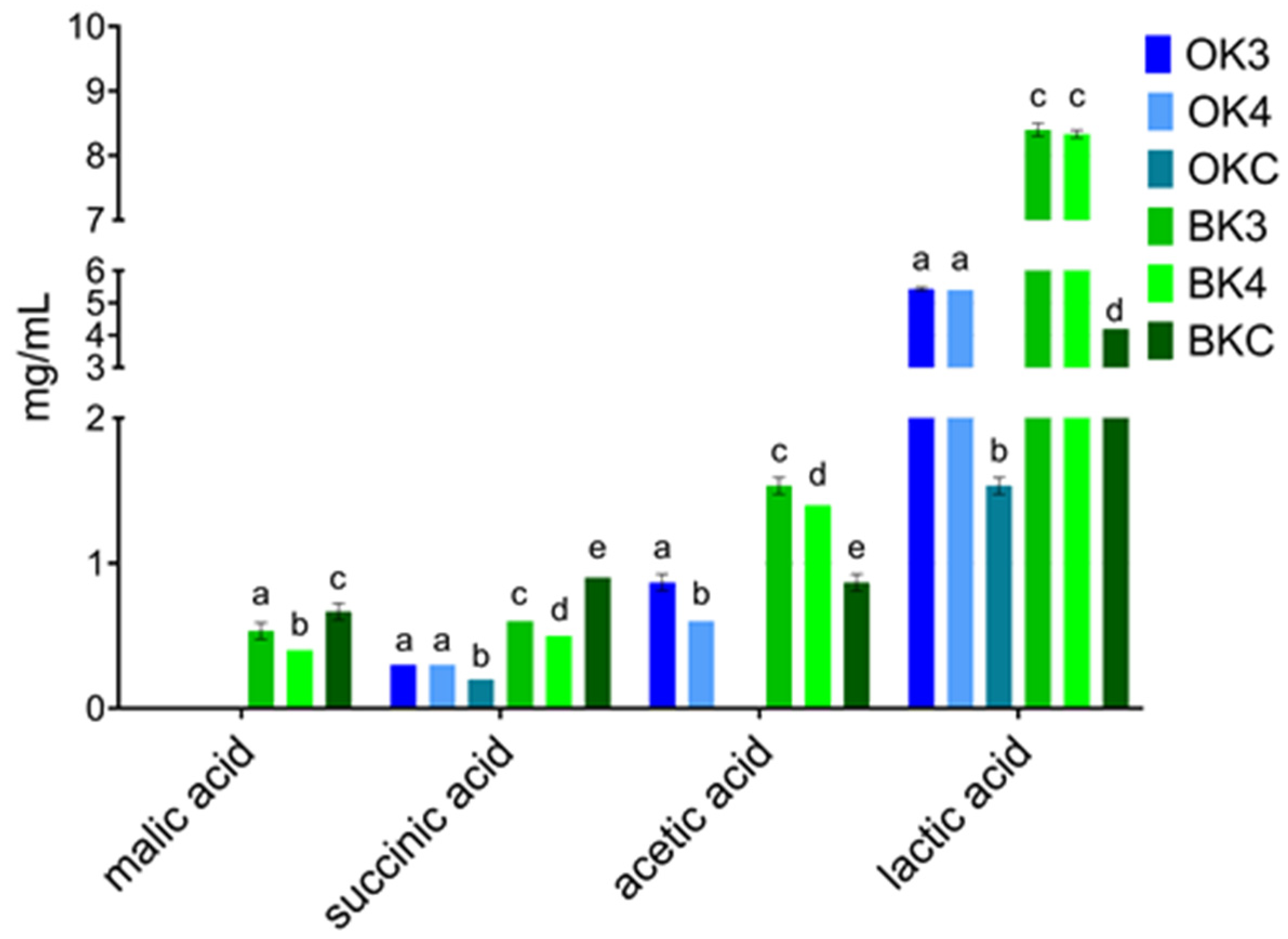
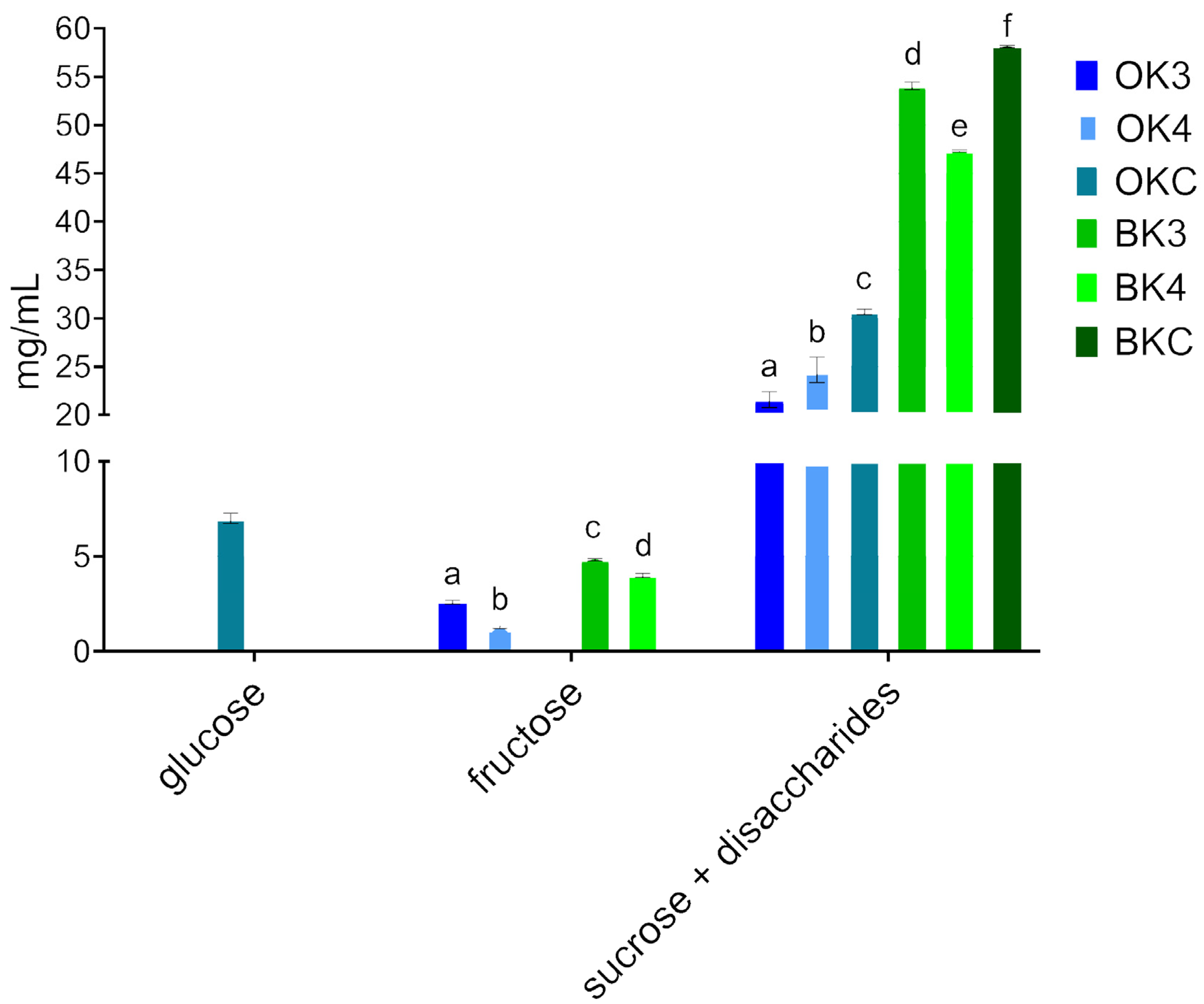
3.3. Organic Acids and Sugar Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Fuentes, B.; de Jesús-José, E.; Cabrera-Hidalgo, A.d.J.; Sandoval-Castilla, O.; Espinosa-Solares, T.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Liceaga, A.M.; Aguilar-Toalá, J.E. Plant-Based Fermented Beverages: Nutritional Composition, Sensory Properties, and Health Benefits. Foods 2024, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Silventoinen-Veijalainen, P.; Vanhatalo, S.; Rosa-Sibakov, N.; Sozer, N. The Nutritional Quality of Animal-Alternative Processed Foods Based on Plant or Microbial Proteins and the Role of the Food Matrix. Trends Food Sci. Technol. 2022, 129, 144–154. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Harper, A.R.; Dobson, R.C.J.; Morris, V.K.; Moggré, G. Fermentation of Plant-based Dairy Alternatives by Lactic Acid Bacteria. Microb. Biotechnol. 2022, 15, 1404–1421. [Google Scholar] [CrossRef] [PubMed]
- Angelov, A.; Yaneva-Marinova, T.; Gotcheva, V. Oats as a Matrix of Choice for Developing Fermented Functional Beverages. J. Food Sci. Technol. 2018, 55, 2351–2360. [Google Scholar] [CrossRef]
- Ziarno, M.; Cichońska, P. Lactic Acid Bacteria-Fermentable Cereal- and Pseudocereal-Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef]
- Cardinali, F.; Osimani, A.; Milanović, V.; Garofalo, C.; Aquilanti, L. Innovative Fermented Beverages Made with Red Rice, Barley, and Buckwheat. Foods 2021, 10, 613. [Google Scholar] [CrossRef]
- Kowalska, E.; Ziarno, M. Characterization of Buckwheat Beverages Fermented with Lactic Acid Bacterial Cultures and Bifidobacteria. Foods 2020, 9, 1771. [Google Scholar] [CrossRef]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Buckwheat and Amaranth as Raw Materials for Brewing, a Review. Plants 2022, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Kwarteng, J.; Agyei, D.; Akabanda, F.; Atuna, R.A.; Amagloh, F.K. Plant-Based Alkaline Fermented Foods as Sustainable Sources of Nutrients and Health-Promoting Bioactive Compounds. Front. Sustain. Food Syst. 2022, 6, 885328. [Google Scholar] [CrossRef]
- Kim, M.H.; Han, S.Y.; Ko, J.M.; Kim, Y.S. Degradation Characteristics of Proteins in Cheonggukjang (Fermented Unsalted Soybean Paste) Prepared with Various Soybean Cultivars. Food Sci. Biotechnol. 2012, 21, 9–18. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on Fermented Foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; He, Y.; Tang, Y.; Hu, R.; Lv, Y.; Zhang, Q.; Tardy, B.L.; Richardson, J.J.; He, Q.; Guo, J.; et al. Biofilms in Plant-Based Fermented Foods: Formation Mechanisms, Benefits and Drawbacks on Quality and Safety, and Functionalization Strategies. Trends Food Sci. Technol. 2021, 116, 940–953. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Manzoor, M.; Singh, D.; Aseri, G.K.; Sohal, J.S.; Vij, S.; Sharma, D. Role of Lacto-Fermentation in Reduction of Antinutrients in Plant-Based Foods. J. Appl. Biol. Biotech. 2021, 9, 7–16. [Google Scholar] [CrossRef]
- Gustaw, K.; Niedźwiedź, I.; Rachwał, K.; Polak-Berecka, M. New Insight into Bacterial Interaction with the Matrix of Plant-Based Fermented Foods. Foods 2021, 10, 1603. [Google Scholar] [CrossRef]
- Nazir, Y.; Hussain, S.A.; Abdul Hamid, A.; Song, Y. Probiotics and Their Potential Preventive and Therapeutic Role for Cancer, High Serum Cholesterol, and Allergic and HIV Diseases. BioMed Res. Int. 2018, 2018, e3428437. [Google Scholar] [CrossRef]
- Christensen, L.F.; García-Béjar, B.; Bang-Berthelsen, C.H.; Hansen, E.B. Extracellular Microbial Proteases with Specificity for Plant Proteins in Food Fermentation. Int. J. Food Microbiol. 2022, 381, 109889. [Google Scholar] [CrossRef] [PubMed]
- Küçükgöz, K.; Trząskowska, M. Nondairy Probiotic Products: Functional Foods That Require More Attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.M.F.; Ramos, A.M.; Vanzela, E.S.L.; Stringheta, P.C.; de Oliveira Pinto, C.L.; Martins, J.M. Products of Vegetable Origin: A New Alternative for the Consumption of Probiotic Bacteria. Food Res. Int. 2013, 51, 764–770. [Google Scholar] [CrossRef]
- Rasika, D.M.D.; Vidanarachchi, J.K.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ranadheera, C.S. Probiotic Delivery through Non-Dairy Plant-Based Food Matrices. Agriculture 2021, 11, 599. [Google Scholar] [CrossRef]
- Zielińska, D.; Rzepkowska, A.; Radawska, A.; Zieliński, K. In Vitro Screening of Selected Probiotic Properties of Lactobacillus Strains Isolated from Traditional Fermented Cabbage and Cucumber. Curr. Microbiol. 2014, 70, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Ziarno, M. The Possibility of Obtaining Buckwheat Beverages Fermented with Lactic Acid Bacteria and Bifidobacteria. In Milk Substitutes-Selected Aspects; IntechOpen: London, UK, 2020; ISBN 978-1-78985-637-8. [Google Scholar]
- Aida, V.; Daniela, P.; Ștefan, D.; Gabriela, B. Growth and Cell Viability Improve of the Probiotic Strain Lactobacillus Casei Ssp. Paracasei in the Presence of Oat Bran and Buckwheat Flour. Innov. Rom. Food Biotechnol. 2011, 9, 52–59. [Google Scholar]
- Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861. [Google Scholar] [CrossRef] [PubMed]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Vasile, A.; Bahrim, G.E.; Orpianesi, C.; Cresci, A. Effect of Buckwheat Flour and Oat Bran on Growth and Cell Viability of the Probiotic Strains Lactobacillus Rhamnosus IMC 501®, Lactobacillus Paracasei IMC 502® and Their Combination SYNBIO®, in Synbiotic Fermented Milk. Int. J. Food Microbiol. 2013, 167, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Klajn, V.M.; Ames, C.W.; da Cunha, K.F.; Lorini, A.; dos Santos Hackbart, H.C.; Filho, P.J.S.; dos Santos Cruxen, C.E.; Fiorentini, Â.M. Probiotic Fermented Oat Dairy Beverage: Viability of Lactobacillus Casei, Fatty Acid Profile, Phenolic Compound Content and Acceptability. J. Food Sci. Technol. 2021, 58, 3444–3452. [Google Scholar] [CrossRef]
- Rosburg, V.; Boylston, T.; White, P. Viability of Bifidobacteria Strains in Yogurt with Added Oat Beta-Glucan and Corn Starch during Cold Storage. J. Food Sci. 2010, 75, C439–C444. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Liptáková, D.; Valík, Ľ. Functional Probiotic Products Based on Fermented Buckwheat with Lactobacillus Rhamnosus. LWT Food Sci. Technol. 2017, 81, 35–41. [Google Scholar] [CrossRef]
- Mousavi, M.-H.; Gharekhani, M.; Alirezalu, K.; Roufegarinejad, L.; Azadmard-Damirchi, S. Production and Characterization of Nondairy Gluten-Free Fermented Beverage Based on Buckwheat and Lentil. Food Sci. Nutr. 2023, 11, 2197–2210. [Google Scholar] [CrossRef]
- Passos, F.V.; Fleming, H.P.; Hassan, H.M.; McFeeters, R.F. Effect of Malic Acid on the Growth Kinetics of Lactobacillus Plantarum. Appl. Microbiol. Biotechnol. 2003, 63, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Okada, S.; Hols, P.; Satoh, E. Metabolic Engineering of Lactobacillus Plantarum for Succinic Acid Production through Activation of the Reductive Branch of the Tricarboxylic Acid Cycle. Enzym. Microb. Technol. 2013, 53, 97–103. [Google Scholar] [CrossRef]
- Aparicio-García, N.; Martínez-Villaluenga, C.; Frias, J.; Peñas, E. Production and Characterization of a Novel Gluten-Free Fermented Beverage Based on Sprouted Oat Flour. Foods 2021, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of Potentially Probiotic Lactic Acid Bacteria on the Physicochemical Composition and Acceptance of Fermented Cereal Beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Sigüenza-Andrés, T.; Mateo, J.; Rodríguez-Nogales, J.M.; Gómez, M.; Caro, I. Characterization of a Fermented Beverage from Discarded Bread Flour Using Two Commercial Probiotics Starters. Foods 2024, 13, 951. [Google Scholar] [CrossRef]
- Kawai, M.; Harada, R.; Yoda, N.; Yamasaki-Yashiki, S.; Fukusaki, E.; Katakura, Y. Suppression of Lactate Production by Using Sucrose as a Carbon Source in Lactic Acid Bacteria. J. Biosci. Bioeng. 2020, 129, 47–51. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Nedić, N.; Sredojević, M.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Distribution of Polyphenolic and Sugar Compounds in Different Buckwheat Plant Parts. RSC Adv. 2021, 11, 25816. [Google Scholar] [CrossRef]

| Sample | Oats [g] | Buckwheat [g] | Water [mL] | Sugar [g] | Bacteria Strain |
|---|---|---|---|---|---|
| OKC | 30 | - | 500 | 10 | - |
| BKC | - | 30 | 500 | 10 | - |
| OK4 | 30 | - | 500 | 10 | L. johnsonii K4 |
| OK3 | 30 | - | 500 | 10 | L. rhamnosus K3 |
| BK4 | - | 30 | 500 | 10 | L. johnsonii K4 |
| BK3 | - | 30 | 500 | 10 | L. rhamnosus K3 |
| Sample | 0 Day | 7 Day | 15 Day |
|---|---|---|---|
| OK3 | 9.39 aB | 9.92 bA | 9.48 aAB |
| OK4 | 9.94 aA | 9.58 bB | 9.67 aABC |
| BK3 | 9.18 aBC | 9.98 bA | 9.88 bC |
| BK4 | 9.66 aAB | 9.97 bA | 8.67 cB |
| Sample | 0 Days | 7 Days | 15 Days |
|---|---|---|---|
| OKC | 6.53 ± 0.04 aA | 6.49 ± 0.08 aA | 6.45 ± 0.02 aA |
| BKC | 6.74 ± 0.11 aA | 6.77 ± 0.16 aB | 6.67 ± 0.09 aA |
| OK4 | 4.86 ± 0.04 aB | 4.68 ± 0.08 bC | 3.88 ± 0.02 cBC |
| OK3 | 4.67 ± 0.04 aB | 4.07 ± 0.03 bD | 3.95 ± 0.03 cB |
| BK4 | 4.75 ± 0.02 aB | 3.76 ± 0.02 bE | 3.66 ± 0.17 bCD |
| BK3 | 4.70 ± 0.35 aB | 3.83 ± 0.03 bE | 3.63 ± 0.08 bD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Küçükgöz, K.; Franczak, A.; Borysewicz, W.; Kamińska, K.; Salman, M.; Mosiej, W.; Kruk, M.; Kołożyn-Krajewska, D.; Trząskowska, M. Impact of Lactic Acid Fermentation on the Organic Acids and Sugars of Developed Oat and Buckwheat Beverages. Fermentation 2024, 10, 373. https://doi.org/10.3390/fermentation10070373
Küçükgöz K, Franczak A, Borysewicz W, Kamińska K, Salman M, Mosiej W, Kruk M, Kołożyn-Krajewska D, Trząskowska M. Impact of Lactic Acid Fermentation on the Organic Acids and Sugars of Developed Oat and Buckwheat Beverages. Fermentation. 2024; 10(7):373. https://doi.org/10.3390/fermentation10070373
Chicago/Turabian StyleKüçükgöz, Kübra, Anna Franczak, Wiszko Borysewicz, Klaudia Kamińska, Muhammad Salman, Wioletta Mosiej, Marcin Kruk, Danuta Kołożyn-Krajewska, and Monika Trząskowska. 2024. "Impact of Lactic Acid Fermentation on the Organic Acids and Sugars of Developed Oat and Buckwheat Beverages" Fermentation 10, no. 7: 373. https://doi.org/10.3390/fermentation10070373






