Biohydrogen Production from Methane-Derived Biomass of Methanotroph and Microalgae by Clostridium
Abstract
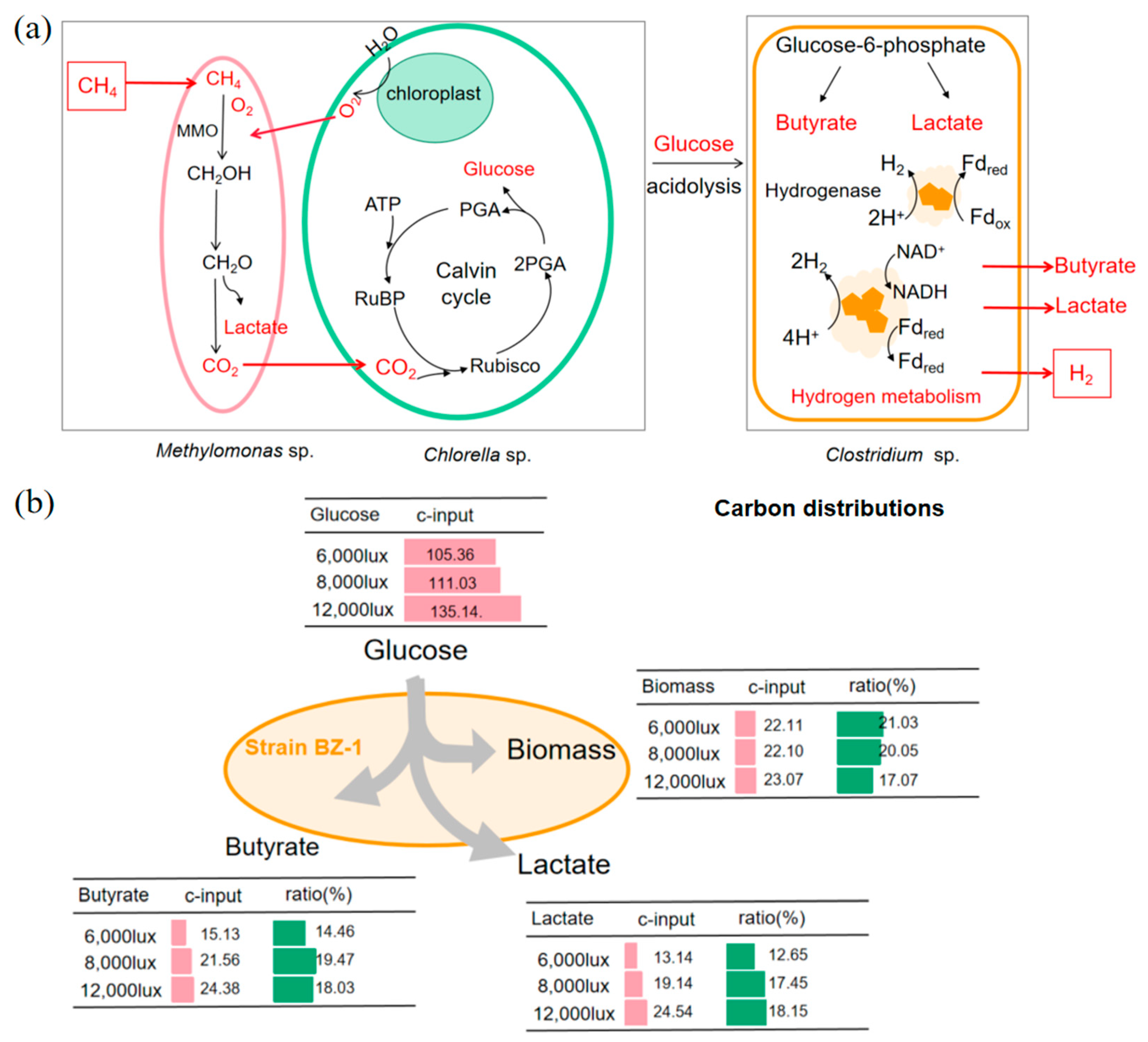
:1. Introduction
2. Materials and Methods
2.1. Precultures of the Microorganism
2.2. Co-Culture Experiments of Microalgae and Methanotroph
2.3. Hydrolysis of the Biomass of Co-Cultures
2.4. Fermentation Experiments
2.5. Analysis of Soluble Metabolites
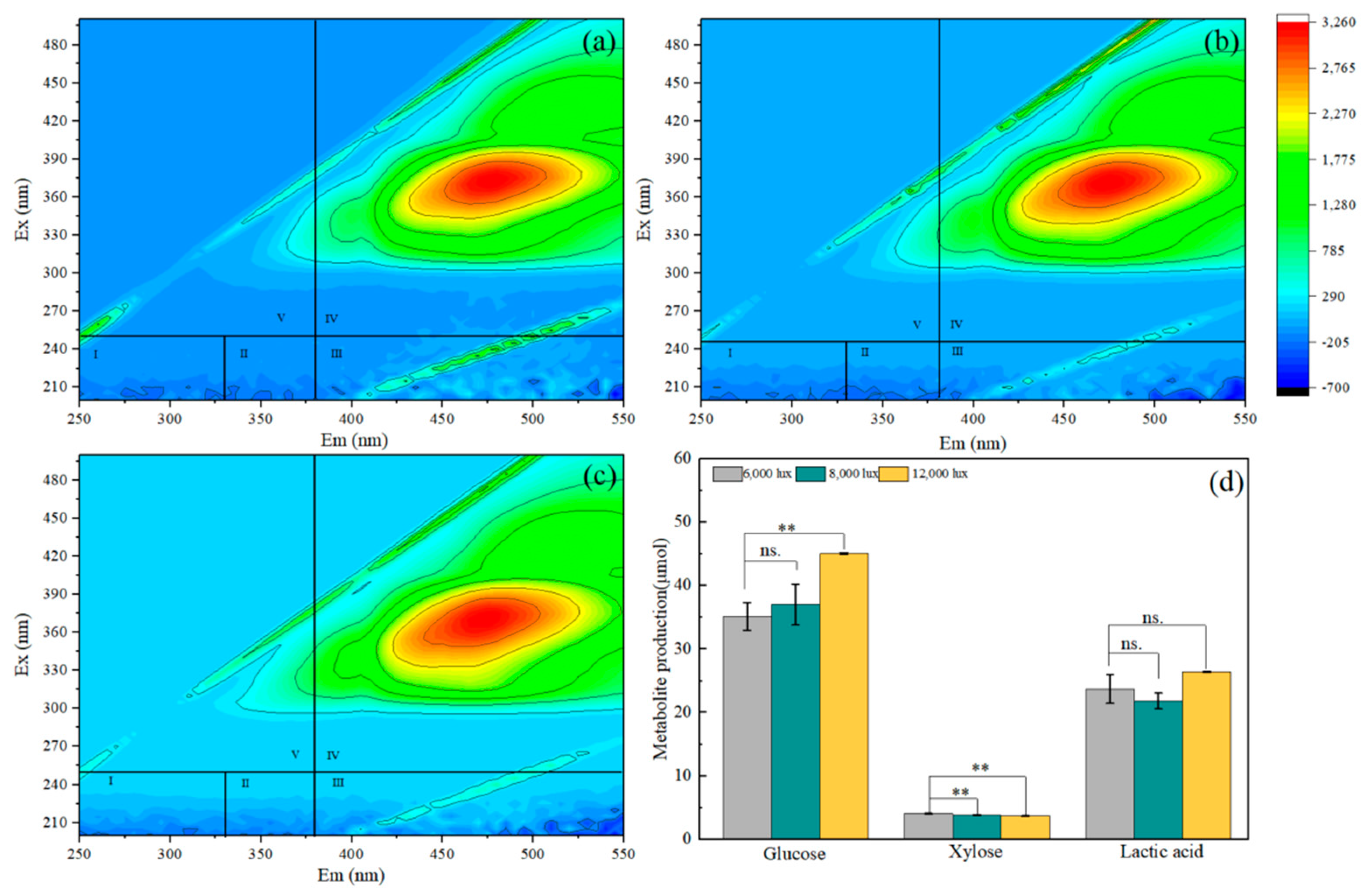
2.6. Three-Dimensional Fluorescence Spectrum
2.7. Statistical Analysis
3. Results and Discussion
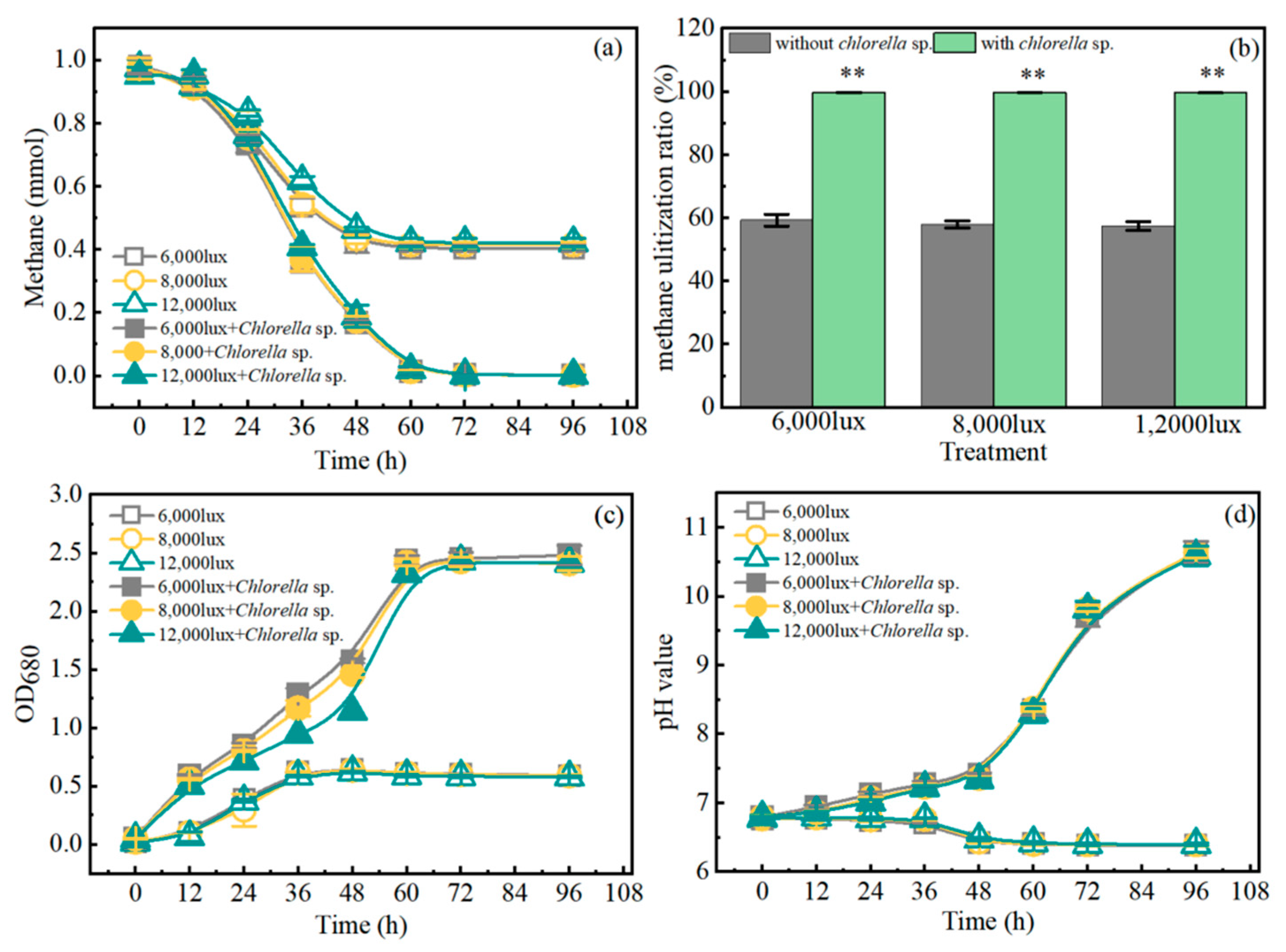
3.1. Methane Consumption of Co-Cultures
3.2. Metabolite Production in Co-Cultures
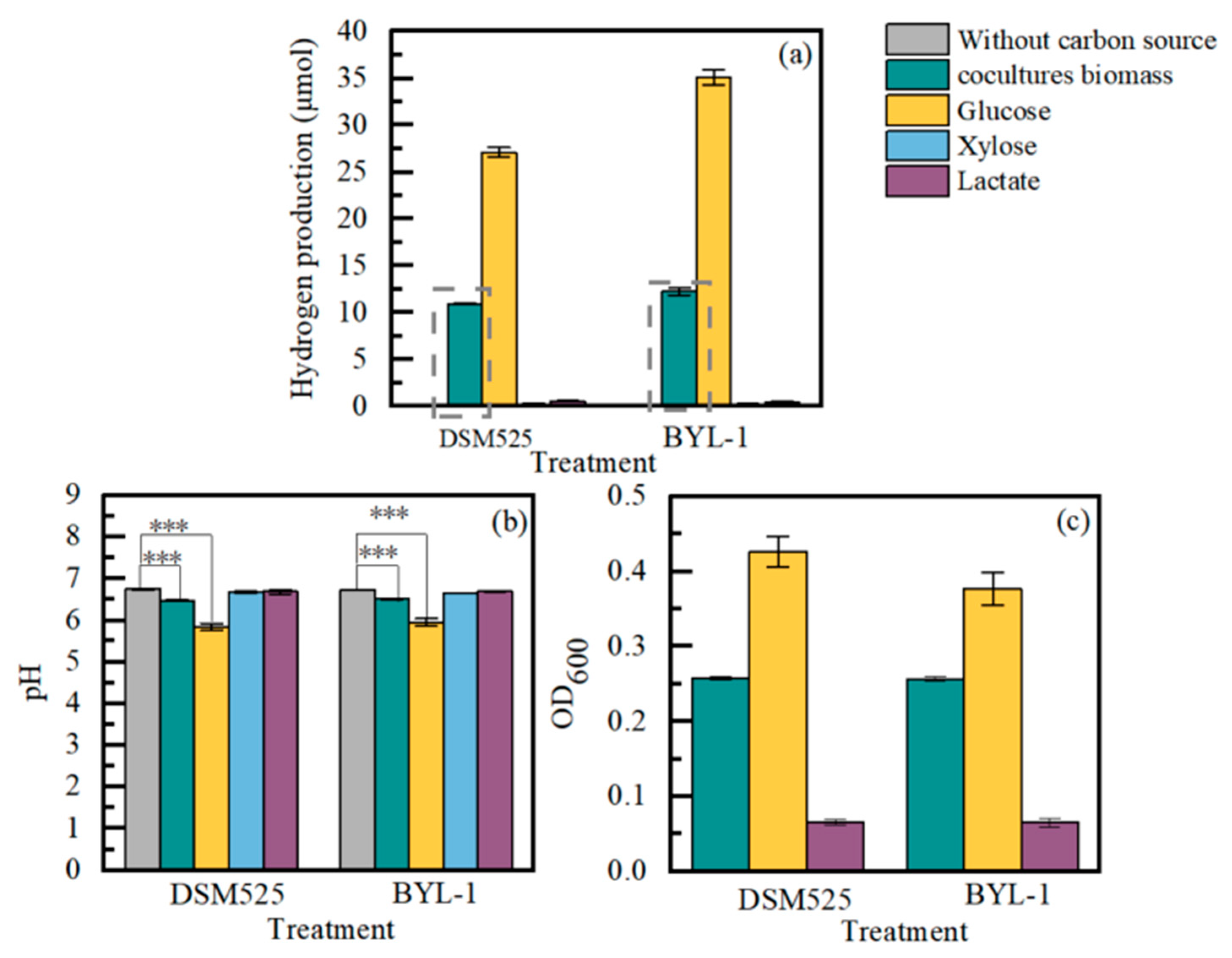
3.3. Biohydrogen of Clostridium sp. from Co-Culture Biomass Hydrolysate
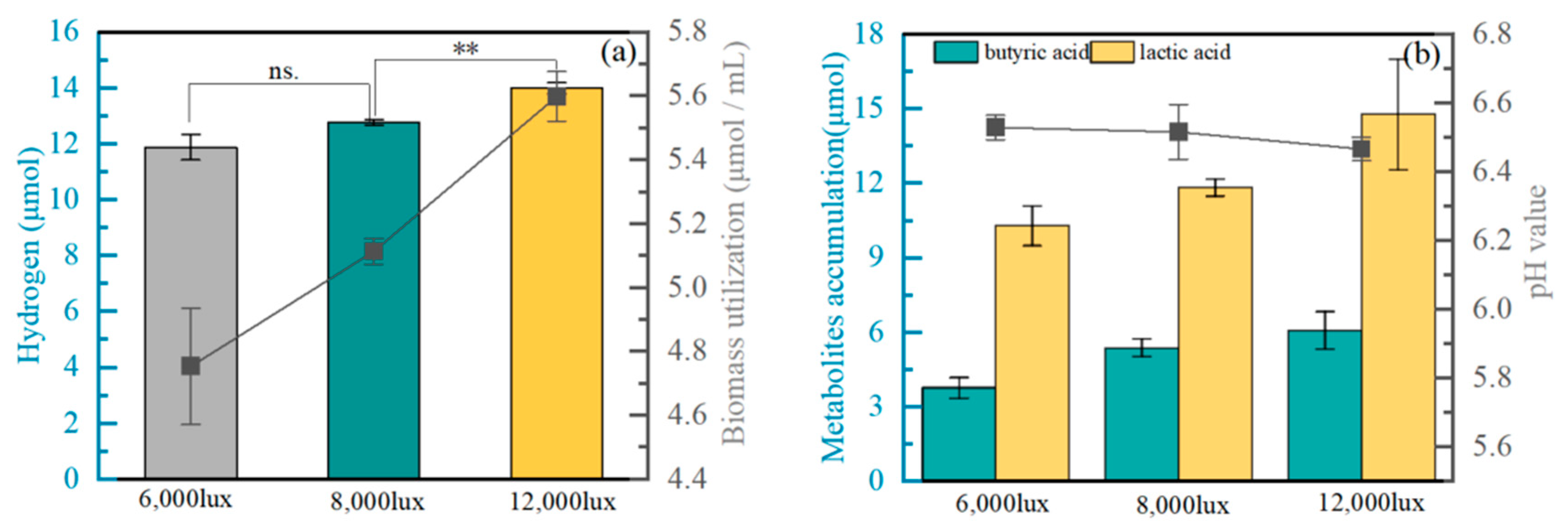
3.4. Hydrogen Production from Co-Cultured Biomass under Different Light Intensities by Strain BZ-1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Zheng, Y.; Bodelier, P.L.E.; Conrad, R.; Jia, Z. Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat. Commun. 2016, 7, 11728. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wik, M.; Varner, R.K.; Anthony, K.W.; MacIntyre, S.; Bastviken, D. Climate-sensitive northern lakes and ponds are critical components of methane release. Nat. Geosci. 2016, 9, 99–105. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, S.; Ma, C.; Xia, Y.; Qiao, H.; Zhao, J.; Gao, W.; Tu, Q.; Zhang, Y.; Rui, Y.; et al. Type I dominated methane oxidation and assimilation in rice paddy fields by the consequence of niche differentiation. Biol. Fert. Soils 2024, 60, 153–165. [Google Scholar] [CrossRef]
- Burlacot, A.; Peltier, G. Energy crosstalk between photosynthesis and the algal CO2-concentrating mechanisms. Trends Plant Sci. 2023, 28, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Roberts, N.; Hilliard, M.; He, Q.P.; Wang, J. A Microalgae-Methanotroph Coculture is a Promising Platform for Fuels and Chemical Production From Wastewater. Front. Energy Res. 2020, 8, 563352. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, P.; Gómez-Borraz, T.L.; Revah, S.; Morales, M. Methanotroph-microalgae co-culture for greenhouse gas mitigation: Effect of initial biomass ratio and methane concentration. Chemosphere 2020, 259, 127418. [Google Scholar] [CrossRef] [PubMed]
- Van der Ha, D.; Bundervoet, B.; Verstraete, W.; Boon, N. A sustainable, carbon neutral methane oxidation by a partnership of methane oxidizing communities and microalgae. Water Res. 2011, 45, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Van der Ha, D.; Nachtergaele, L.; Kerckhof, F.M.; Rameiyanti, D.; Bossier, P.; Verstraete, W.; Boon, N. Conversion of Biogas to Bioproducts by Algae and Methane Oxidizing Bacteria. Environ. Sci. Technol. 2012, 46, 13425–13431. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Li, N.; Wang, Y.; Yu, R.; Zhu, G.; Zeng, R.J. Mixotrophic Cultivation of Microalgae Using Biogas as the Substrate. Environ. Sci. Technol. 2022, 56, 3669–3677. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, P.; Gómez-Borraz, T.L.; Saldivar, A.; Hernández, S.; Morales-Ibarría, M.; Revah, S. Diluted methane mitigation by a co-culture of alkaliphilic methanotrophs and the microalgae Scenedesmus obtusiusculus towards carbon neutrality. Biochem. Eng. J. 2024, 203, 109211. [Google Scholar] [CrossRef]
- Yun, J.H.; Lee, H.; Nam, J.W.; Ko, M.; Park, J.; Lee, D.H.; Lee, S.G.; Kim, H.S. Unlocking synergies: Harnessing the potential of biological methane sequestration through metabolic coupling between Methylomicrobium alcaliphilum 20Z and Chlorella sp. HS2. Bioresour. Technol. 2024, 399, 130607. [Google Scholar] [CrossRef]
- Argun, H.; Kargi, F. Bio-hydrogen production by different operational modes of dark and photo-fermentation: An overview. Int. J. Hydrogen Energy 2011, 36, 7443–7459. [Google Scholar] [CrossRef]
- Saray, J.A.; Gharehghani, A.; Hosseinzadeh, D. Towards sustainable energy Carriers: A solar and Wind-Based systems for green liquid hydrogen and ammonia production. Energy Convers. Manag. 2024, 304, 118215. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, C.Y.; Cheng, C.L.; Lee, D.J.; Chang, J.S. Fermentative hydrogen production by Clostridium butyricum CGS5 using carbohydrate-rich microalgal biomass as feedstock. Int. J. Hydrogen Energy 2012, 37, 15458–15464. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Lin, R.; Lu, H.; Zhou, J.; Cen, K. Comparison in dark hydrogen fermentation followed by photo hydrogen fermentation and methanogenesis between protein and carbohydrate compositions in Nannochloropsis oceanica biomass. Bioresour. Technol. 2013, 138, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chang, H.Y.; Chang, J.S. Producing carbohydrate-rich microalgal biomass grown under mixotrophic conditions as feedstock for biohydrogen production. Int. J. Hydrogen Energy 2016, 41, 4413–4420. [Google Scholar] [CrossRef]
- Cheng, J.; Xia, A.; Liu, Y.; Lin, R.; Zhou, J.; Cen, K. Combination of dark- and photo-fermentation to improve hydrogen production from Arthrospira platensis wet biomass with ammonium removal by zeolite. Int. J. Hydrogen Energy 2012, 37, 13330–13337. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Elshobary, M.; Abdullah, E.; Abdel-Basset, R.; Metwally, M. Application of a novel biological-nanoparticle pretreatment to Oscillatoria acuminata biomass and coculture dark fermentation for improving hydrogen production. Microb. Cell Factories 2023, 22, 34. [Google Scholar] [CrossRef]
- But, S.Y.; Rozova, O.N.; Khmelenina, V.N.; Reshetnikov, A.S.; Trotsenko, Y.A. Properties of Recombinant ATP-Dependent Fructokinase from the Halotolerant Methanotroph Methylomicrobium alcaliphilum 20Z. Biochemistry 2012, 77, 372–377. [Google Scholar] [CrossRef]
- Rozova, O.N.; Khmelenina, V.N.; Vuilleumier, S.; Trotsenko, Y.A. Characterization of recombinant pyrophosphate-dependent 6-phosphofructokinase from halotolerant methanotroph Methylomicrobium alcaliphilum 20Z. Res. Microbiol. 2010, 161, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, Z.; Valverde-Pérez, B.; D’Este, M.; De Francisci, D.; Angelidaki, I. Nutrient recovery from industrial wastewater as single cell protein by a co-culture of green microalgae and methanotrophs. Biochem. Eng. J. 2018, 134, 129–135. [Google Scholar] [CrossRef]
- Hao, Q.; Liu, F.; Zhang, Y.; Wang, O.; Xiao, L. Methylobacter accounts for strong aerobic methane oxidation in the Yellow River Delta with characteristics of a methane sink during the dry season. Sci. Total. Environ. 2020, 704, 135383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, L.; Wang, S.; Liu, F. Stimulation of ferrihydrite nanorods on fermentative hydrogen production by Clostridium pasteurianum. Bioresour. Technol. 2019, 283, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Zhu, M.Y.; Lian, S.J.; Zou, H.; Fu, S.F.; Guo, R.B. Conversion of Renewable Biogas into Single-Cell Protein Using a Combined Microalga- and Methane-Oxidizing Bacterial System. ACS EST Eng. 2022, 2, 2317–2325. [Google Scholar] [CrossRef]
- Onay, M. Enhancing carbohydrate productivity from Nannochloropsis gaditana for bio-butanol production. Energy Rep. 2020, 6, 63–67. [Google Scholar] [CrossRef]
- Sriram, S.; Seenivasan, R. Biophotonic perception on Desmodesmus sp. VIT growth, lipid and carbohydrate content. Bioresour. Technol. 2015, 198, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, S.; Ueda, R. Production of optically pure D-Lactic acid by Nannochlorum sp. 26A4. Appl. Biochem. and Biotech. 2004, 119, 71–77. [Google Scholar] [CrossRef]
- Henard, C.A.; Franklin, T.G.; Youhenna, B.; But, S.; Alexander, D.; Kalyuzhnaya, M.G.; Guarneri, M.T. Biogas Biocatalysis: Methanotrophic Bacterial Cultivation, Metabolite Profiling, and Bioconversion to Lactic Acid. Front. Microbiol. 2018, 9, 2610. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, S.; Kim, W.; Kim, S.; Cha, S.; Moon, H.; Hur, D.H.; Kim, S.Y.; Na, J.G.; Lee, J.W.; et al. Efficient production of d-lactate from methane in a lactate-tolerant strain of Methylomonas sp. DH-1 generated by adaptive laboratory evolution. Biotechnol. Biofuels 2019, 12, 234. [Google Scholar] [CrossRef]
- Radu, E.; Oprescu, E.E.; Enascuta, C.E.; Calin, C.; Stoica, R.; Scaeteanu, G.V.; Vasilievici, G.; Capra, L.; Ivan, G.; Ion, A.C. Kinetic Adsorption of Humic Acids Mixture Obtained from Microalgae on Exfoliated Graphite Nanoplatelets. Rev. Chim. 2018, 69, 191–195. [Google Scholar] [CrossRef]
- Dos Santos Vieira, C.F.; Codogno, M.C.; Maugeri Filho, F.; Maciel Filho, R.; Mariano, A.P. Sugarcane bagasse hydrolysates as feedstock to produce the isopropanol-butanol-ethanol fuel mixture: Effect of lactic acid derived from microbial contamination on Clostridium beijerinckii DSM 6423. Bioresour. Technol. 2021, 319, 124140. [Google Scholar]
- Kim, S.H.; Yi, Y.D.; Kim, H.J.; Bhatia, S.K.; Gurav, R.; Jeon, J.M.; Yoon, J.J.; Kim, S.H.; Park, J.H.; Yang, Y.H. Hyper biohydrogen production from xylose and xylose-based hemicellulose biomass by the novel strain Clostridium sp. YD09. Biochem. Eng. J. 2022, 187, 108624. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, L.; Hao, Q.; Li, X.; Liu, F. Ferrihydrite Reduction Exclusively Stimulated Hydrogen Production by Clostridium with Community Metabolic Pathway Bifurcation. ACS Sustain. Chem. Eng. 2020, 8, 7574–7580. [Google Scholar] [CrossRef]
- Chng, L.M.; Lee, K.T.; Chan, D.J.C. Synergistic effect of pretreatment and fermentation process on carbohydrate-rich Scenedesmus dimorphus for bioethanol production. Energy Convers. Manag. 2017, 141, 410–419. [Google Scholar] [CrossRef]
- Park, J.H.; Cheon, H.C.; Yoon, J.J.; Park, H.D.; Kim, S.H. Optimization of batch dilute-acid hydrolysis for biohydrogen production from red algal biomass. Int. J. Hydrogen Energy 2013, 38, 6130–6136. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Yang, S.T. Construction and characterization of ack deleted mutant of Clostridium tyrobutyricum for enhanced butyric acid and hydrogen production. Biotechnol. Progress. 2006, 22, 1265–1275. [Google Scholar] [CrossRef]
- Liu, S.; Bischoff, K.M.; Leathers, T.D.; Qureshi, N.; Rich, J.O.; Hughes, S.R. Butyric acid from anaerobic fermentation of lignocellulosic biomass hydrolysates by Clostridium tyrobutyricum strain RPT-4213. Bioresour. Technol. 2013, 143, 322–329. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.Y.; Lee, K.M.; Youn, S.H.; Lee, S.-M.; Woo, H.M.; Oh, M.-K.; Um, Y. Butyric acid production from softwood hydrolysate by acetate-consuming Clostridium sp. S1 with high butyric acid yield and selectivity. Bioresour. Technol. 2016, 218, 1208–1214. [Google Scholar] [CrossRef]
- Wang, L.; Ou, M.S.; Nieves, I.; Erickson, J.E.; Vermerris, W.; Ingram, L.O.; Shanmugam, K.T. Fermentation of sweet sorghum derived sugars to butyric acid at high titer and productivity by a moderate thermophile Clostridium thermobutyricum at 50 °C. Bioresour. Technol. 2015, 198, 533–539. [Google Scholar] [CrossRef]
- Leja, K.; Myszka, K.; Czaczyk, K. The ability of Clostridium bifermentans strains to lactic acid biosynthesis in various environmental conditions. SpringerPlus 2013, 2, 44. [Google Scholar] [CrossRef] [PubMed]
| Light Intensity (lux) | Glucose (g/L) | Xylose (g/L) | Biomass (g/L) | Reducing Sugar (%) |
|---|---|---|---|---|
| 6000 | 1.26 ± 0.08 | 0.061 ± 0.001 | 12.60 ± 0.42 | 10.55 ± 0.42 |
| 8000 | (1.22 ± 0.11) ** | (0.058 ± 0.001) n.s. | (12.23 ± 0.30) n.s. | (11.36 ± 0.69) n.s. |
| 12,000 | (1.62 ± 0.01) ** | (0.055 ± 0.001) ** | (12.26 ± 0.71) n.s. | (13.73 ± 0.84) * |
| Light Intensity (lux) | Glucose (μmol) | Lactic Acid (μmol) | Butyric Acid (μmol) | Hydrogen Yield (μmol/μmol) | |
|---|---|---|---|---|---|
| Before culture | 6000 | 17.56 | 5.92 | 0 | / |
| 8000 | 18.51 | 5.46 | 0 | / | |
| 12,000 | 22.52 | 6.61 | 0 | / | |
| After culture | 6000 | 0 | 10.31 | 3.78 | 0.71 |
| 8000 | 0 | 11.84 | 5.39 | 0.70 | |
| 12,000 | 0 | 14.80 | 6.09 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, Y.; Xie, Z.; Li, L.; Wang, O.; Zheng, S.; Liu, F. Biohydrogen Production from Methane-Derived Biomass of Methanotroph and Microalgae by Clostridium. Fermentation 2024, 10, 383. https://doi.org/10.3390/fermentation10080383
Sang Y, Xie Z, Li L, Wang O, Zheng S, Liu F. Biohydrogen Production from Methane-Derived Biomass of Methanotroph and Microalgae by Clostridium. Fermentation. 2024; 10(8):383. https://doi.org/10.3390/fermentation10080383
Chicago/Turabian StyleSang, Yuxuan, Zhangzhang Xie, Liangyan Li, Oumei Wang, Shiling Zheng, and Fanghua Liu. 2024. "Biohydrogen Production from Methane-Derived Biomass of Methanotroph and Microalgae by Clostridium" Fermentation 10, no. 8: 383. https://doi.org/10.3390/fermentation10080383





