Abstract
The experiment aimed to evaluate the effects of varying levels of Laurus nobilis leaves [0% (control), 0.5%, 1%, 1.5%, and 2%] on the in vitro ruminal fermentation of a ruminant diet consisting of a 50% concentrate mixture, 40% berseem hay (Trifolium alexandrinum), and 10% rice straw (Oryza sativa). The in vitro incubation lasted 48 h, during which gas production (GP), methane (CH4), carbon dioxide (CO2), total and individual short-chain fatty acids (SCFA), and nutrient degradability were measured. The experiment utilized a randomized block design and consisted of two incubation runs. Gas chromatography analysis revealed that 1,8-cineole (81%) was the primary volatile compound in the L. nobilis leaves. The 0.5% inclusion level exhibited the highest (linear, p = 0.006) asymptotic GP and lowest lag of GP (linear, p = 0.002), while the 2% inclusion level had the highest lag of GP. The 2% inclusion level significantly lowered CH4 (linear, p = 0.003) compared to the control, and all levels of the leaves linearly decreased in the proportional CH4 production (p = 0.001), with the lowest value at the 0.5% inclusion level. The highest asymptotic CO2 production was observed with the 0.5% inclusion level (linear, p = 0.002), while the 0.5%, 1%, and 1.5% inclusion levels significantly increased (quadratic, p = 0.006) the proportion of CO2 compared to the control. The 0.5% inclusion level showed the highest (p < 0.001) degradable DM and fiber fractions compared to the control, whereas the 2% level decreased them. The 0.5% inclusion level resulted in the highest (p < 0.01) production of total SCFA, acetate, and propionate. Additionally, the 0.5% inclusion level demonstrated the highest (p < 0.05) metabolizable energy and microbial crude protein, while the 2% level reduced these measures compared to the control. It is concluded that L. nobilis leaves can be included at 0.5% of the ruminant diet (e.g., sheep) to improve ruminal fermentation and reduce CH4 production.
1. Introduction
Greenhouse gas emissions from livestock contribute to about 14.5% of global anthropogenic gas emissions, posing a significant environmental concern [1]. Methane (CH4) is particularly problematic, being 28 times more potent than carbon dioxide (CO2) in terms of its heat-trapping capacity [2]. Moreover, CH4 production also reduces animal energy efficiency, with 10% of energy being lost through CH4 production [3]. Therefore, mitigating CH4 emissions from ruminal fermentation is crucial, as approximately 71% of the CH4 produced by animals originates from this process [4]. Over the past decade, intensive research has focused on identifying anti-methanogenic feed additives (e.g., plant secondary metabolites) to reduce enteric CH4 emissions [5,6,7,8]. Nonetheless, only a few dietary approaches have been shown to be safe and effective in both in vitro and in vivo settings by the US Food and Drug Administration.
The use of feed additives derived from natural sources such as plant leaves and seeds, essential oils, plant extract, microalgae, etc., has garnered increased interest for organic and sustainable animal production to improve animal performance and reduce CH4 emissions [7,9,10,11]. These organic feed additives are favored because they avoid concerns related to antibiotic residues or chemical additives [12,13]. Laurus nobilis is one of these additives that may be sustainably used in feed to improve animal performance.
Laurus nobilis L. is a tree native to Mediterranean regions and belongs to the Lauraceae family [14]. L. nobilis, known as sweet bay, is an evergreen tree known for its glossy green, spicy leaves and berries. L. nobilis leaves have been used for many years in the diet of human beings as a food flavor due to their functional effects on human health [14]. The leaves have strong antibacterial, anti-inflammatory, antioxidant, and other health-promoting qualities because of their abundant bioactive compounds [15]. The additive contains up to 4% methyleugenol [16]. The main components in the leaves are 1,8-cineole (known as eucalyptol), estragole, and α-terpinyl acetate [14].
Recently, L. nobilis leaves gained interest from animal nutritionists to evaluate their efficacy as a sensory additive for all animal species [16]. The EU is currently authorized to use L. nobilis leaves as a feed additive under European Commission No. 1831/2003 [16]. Khayyal et al. [17] fed Rahmani × Finnish Landrace lambs a diet supplemented with L. nobilis leaves at 0.5%, 1.5%, and 2% for 20 weeks, and observed no effects on ruminal pH, ammonia-N (NH3-N), or short-chain fatty acids (SCFA) concentrations. Sızmaz [18] used the RUSITC technique to investigate the in vitro fermentation of a diet with a 48:52 ratio of hay to concentrate, supplemented with laurel essential oil at concentrations of 50 and 100 mg/L of the fermenter liquid. This study found that the essential oil did not impact rumen fermentation measured by pH, NH3-N, and SCFA, apparent nutrient degradation, CH4 emissions, bacterial number, and protozoa, including Holotrichs and Entodiniomorphs. By administering eucalyptus oil with eucalyptol (1,8-cineole) as the primary active component, Sallam et al. [19] evaluated its effect on in vitro fermentation. They observed a significant reduction in gas production (GP) and CH4 emissions (up to 85.3%) without impacting DM and organic matter (OM) degradation.
The suitability of utilizing L. nobilis leaves as a supplement in animal feed has only been the subject of a small number of research to date. Therefore, the aims of this experiment were to assess the effects of varying the amount of dried L. nobilis leaves in a total mixed diet on the in vitro fermentation of ruminants, CH4 and CO2 productions, and GP. Our hypothesis was that the dried L. nobilis leaves’ phytochemicals would influence the ruminal fermentation and enhance nutrient degradability.
2. Materials and Methods
2.1. Ingredients and Treatments
To be utilized as substrates, a basal total mixed ration (TMR) containing (per kg DM) 500 g concentrate feed mixture, 400 g berseem hay (Trifolium alexandrinum), and 100 g rice straw (Oryza sativa) was formulated. The incubated substrate or diet is the same as the control diet previously used by others [6,7,9,20]. Table 1 displays the nutrients’ concentrations in L. nobilis leaves as well as the ingredients and TMR.

Table 1.
Chemical composition of Laurus nobilis leaves and incubated diet (g/kg DM).
Dry and clean L. nobilis leaves were purchased from a local supplier in Egypt. Before use, the leaves were ground and mixed. At the Central Laboratory of National Research Centre (Dokki, Giza, Egypt), the volatile compounds in the leaves were quantified using a capillary column ZB-5 (60 m × 0.32 mm i.d.; J & W Agilent Technologies Inc., Palo Alto, CA, USA) and a Perkin Elmer Auto System XL GC/MS (J & W Agilent Technologies Inc., Palo Alto, CA, USA). The analysis was carried out in accordance with Qin et al. [21] with some modifications. The temperature was set for three minutes at 100 °C, and then it was raised to 240 °C at a pace of 2.5 °C per minute, where it remained for ten minutes. The injector and flame ionization detector (FID) were calibrated to operate at 250 °C and 285 °C, respectively. With a split vent flow ratio of 1:10, helium was utilized as the carrier gas at a rate of 1 mL/min. Direct introduction of the GC column effluent to the MS source was made. Spectra with an ionization energy of 70 eV were observed in the EI mode. A one-second scan from 40 to 300 amu was programmed into the sector mass analyzer. By comparing the compounds’ relative retention time and mass spectra with those of the NIST and WILLY libraries, a preliminary identification of the compounds was carried out.
2.2. In Vitro Fermentation and Biodegradation
Ruminal contents from three fattened Barki male sheep (42 ± 0.6 kg body weight, 25 ± 3 weeks old) were collected from a nearby abattoir in Cairo, Egypt. The sheep were allowed unrestricted access to water and fed a diet consisting of concentrates, berseem hay, and rice straw at a ratio of 500:400:100 (DM basis) for about 15 weeks prior to slaughter. Sheep were fasted for twenty-four hours before being slaughtered. The standardized process for sampling, storing, and using ruminal contents that Fortina et al. [22] advocated was followed while collecting the rumen contents. Less than ten minutes passed at the slaughterhouse between the animal’s slaughtering and the collection of rumen fluid. Using a colander, around 150–250 g of the rumen contents were manually sampled and squeezed into a plastic beaker. This technique was repeated until about 1000 mL of rumen fluid was collected. Large feed particles were removed from the collected ruminal contents by filtering it through two layers of cheesecloth. The inoculum’s initial pH ranged from 6.8 to 6.9.
Goering and Van Soest’s method [23] was followed in the preparation of the in vitro fermentation medium. Just before the rumen fluid was introduced, 2 mL of a sodium sulfide reduction solution was added to the buffer. A mixture of 20 mL ruminal inoculum and 80 mL buffer solution was contained in each 250 mL bottle.
An automatic wireless in vitro GP module (Ankom RF Gas Production System, Ankom Technology, Macedon, NY, USA) with pressure sensors (Ankom Technology, Macedon, NY, USA) was installed in 250 mL ANKOM bottles after a 1 g ± 10 mg sample of TMR was weighed into filter bags (ANKOM F57; Ankom Technology, Macedon, NY, USA). The amount of L. nobilis leaves added to the diet was 0 (control), 0.5% (0.005 g), 1% (0.01 g), 1.5% (0.015 g), and 2% (0.02 g). The experiment was repeated twice (2 incubations) in two different weeks. The doses of L. nobilis leaves were carefully weighed into the filter bags using a Luna Analytical Balance (LAB 124e, Adam Scales and Balances, Thetford, UK). To establish baseline fermentation GP, two bottles with inoculum but no feed (blanks) were also added to each incubation run (5 treatments × 3 replicates × 2 incubation runs + 2 blank bottles). The average of the 3 bottles was the experimental unit, which provided a total of three experimental units for testing each of the additive’s levels. For 48 h, pressure was measured every 10 min. The total pressure was computed using these readings. At standard pressure and temperature, the gas pressure was translated into volume (mL). To calculate net GP, the gas volume in the blank units was deducted. To quantify the concentration of CH4 and CO2, gas samples (5 mL) were obtained from the sampling vent and infused into a Gas-Pro detector (Gas Analyzer CROWCON Model Tetra3, Abingdon, UK) at 2, 4, 6, 8, 10, 12, 24, 36, and 48 h of incubation.
2.3. Sampling and Analysis of Fermentation Variables
At the end of the 48 h incubation period, the bottles were placed on ice for five minutes to stop the fermentation. A pH meter (Thermo Scientific, Orion StarTM A121, Beverly, MA, USA) was used to measure the pH. After that, the ANKOM F57 filter bags were dried for 48 h at 55 °C in a forced air oven. The weights of the dried residue and the initial weight of the dried substrate were subtracted to determine the DM, and neutral detergent fiber (NDF), acid detergent fiber (ADF), and degradation as:
Following a 48 h incubation period, the total gas, CH4, and CO2 produced were expressed in relation to the degraded DM (dDM), NDF (dNDF), and ADF (dADF).
Glass tubes were used to collect samples (5 mL) of the supernatant fermented fluid from each bottle to measure the amounts of NH3-N, total SCFAs, and individual SCFAs. In accordance with AOAC [24], a subsample of 3 mL was preserved with 3 mL of 0.2 M hydrochloric acid for the determination of NH3-N concentration. To prepare an aliquot (0.8 mL) for SCFA analysis using steam distillation and the titration method, 0.2 mL of a metaphosphoric acid solution (250 g/L) was combined with it.
2.4. Chemical Analysis
According to AOAC [24] methods, samples of L. nobilis leaves, ingredients, and TMR were dried at 55 °C for 48 h to determine DM concentration (method ID 930.15). The samples were burned at 550 °C for 12 h in a muffle furnace to measure ash concentration (method ID 942.05). Crude protein (CP) was measured using the Kjeldahl method (method ID 954.01), and ether extract (EE) was measured using diethyl ether in Soxhlet extractors (method ID 920.39). The samples were then analyzed for ash content. Using sodium sulfite and alpha amylase, the NDF content was ascertained by following Van Soest et al.’s protocol [25]. The ADF content was measured using the AOAC [24] (method ID 973.18), and the results were expressed without accounting for residual ash. The concentrations of OM (100 − ash) and non-structural carbohydrates (1000 − NDF − CP − EE − ash) were determined.
2.5. Calculations and Statistical Analyses
The NLIN procedure of SAS (Version 9.4, SAS Institute Inc., Cary, NC, USA) was used to fit data of total GP, CH4, and CO2 (mL/g DM) in accordance with the model of France et al. [26]: y = A × [1 − e−c(t−Lag)] for the estimation of GP, CH4, and CO2 kinetics, where A is the asymptotic GP, CH4, or CO2 (mL/g DM); c is the fractional rate of GP, CH4, or CO2 (per h); Lag (h) is the discrete lag time before any gas, CH4, or CO2 release; and y is the volume of total GP CH4 or CO2 production at time t (h).
According to Blümmel et al. [27], the partitioning factor during 24 h of incubation (PF24) was estimated as mg dDM/mL gas. The volume of gas produced (mL/200 mg DM) at 24 h incubation (GY24) was calculated. Menke et al.’s formula was used for metabolizable energy (ME) calculation [28]. The production of microbial crude protein (MCP) was estimated in accordance with Blümmel et al. [27].
Data were analyzed using the mixed procedure of SAS in a randomized block design. Each run formed a block, and the experimental unit was specified as the additive level within each block. The model, Yijk = μ + Li + Rj + (L × R)ij + εijk, was employed where Yijk is the observation, μ is the population mean, Li is the L. nobilis leaves’ level effect, Rj is the run (block) effect, (L × R)ij is the interaction between run and additive level, and εij is the residual error. Linear and quadratic contrasts were used to determine the level responses (increasing L. nobilis leaves’ levels). The effect of run and interaction between run and additive level were nonsignificant (i.e., p > 0.05) for most of the measurements; thus, only the main effects of additives are reported.
3. Results
3.1. Laurus nobilis
The essential oil in the laurel leaves was about 3.29% of its weight. The GC analysis showed that the leaves of L. nobilis leaves contained 12 volatile compounds (Table 2). Eucalyptol (1,8-cineole) (81.01%), estragole (5.9%), and α-terpinyl acetate (3.91%) were the major compounds.

Table 2.
Volatile compounds in Laurus nobilis leaves identified by GC-MS analysis.
3.2. Biogases Production
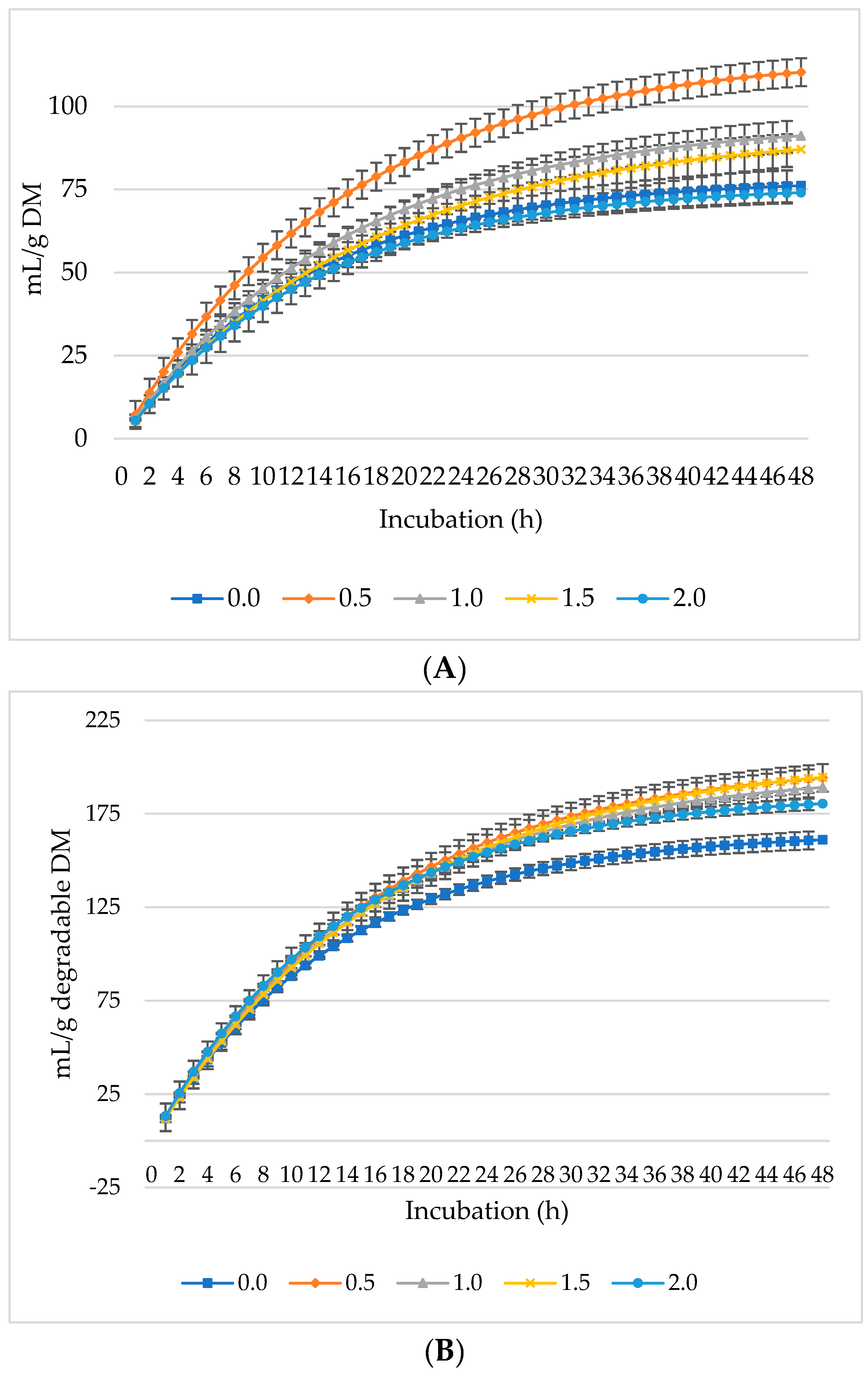
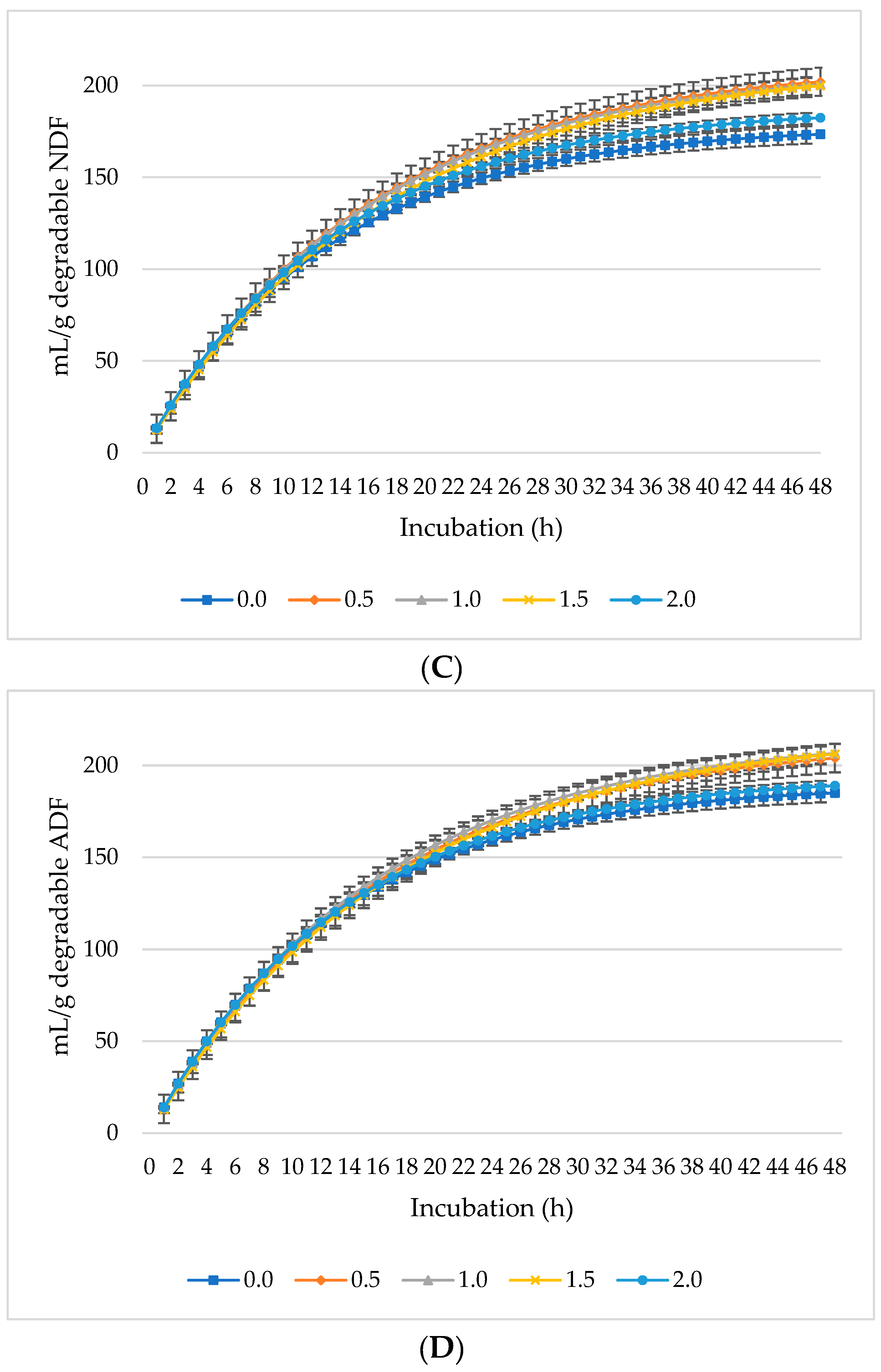
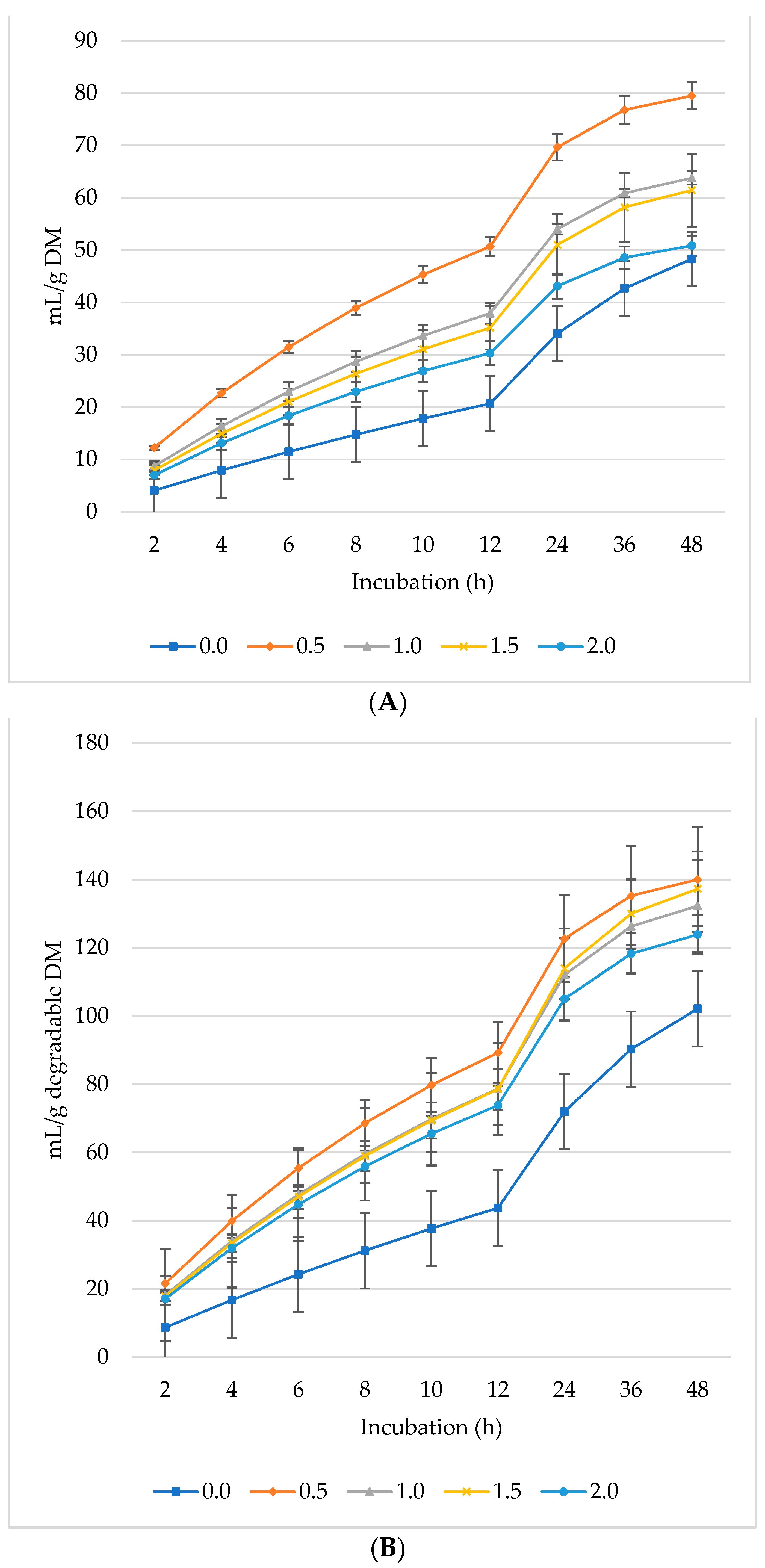
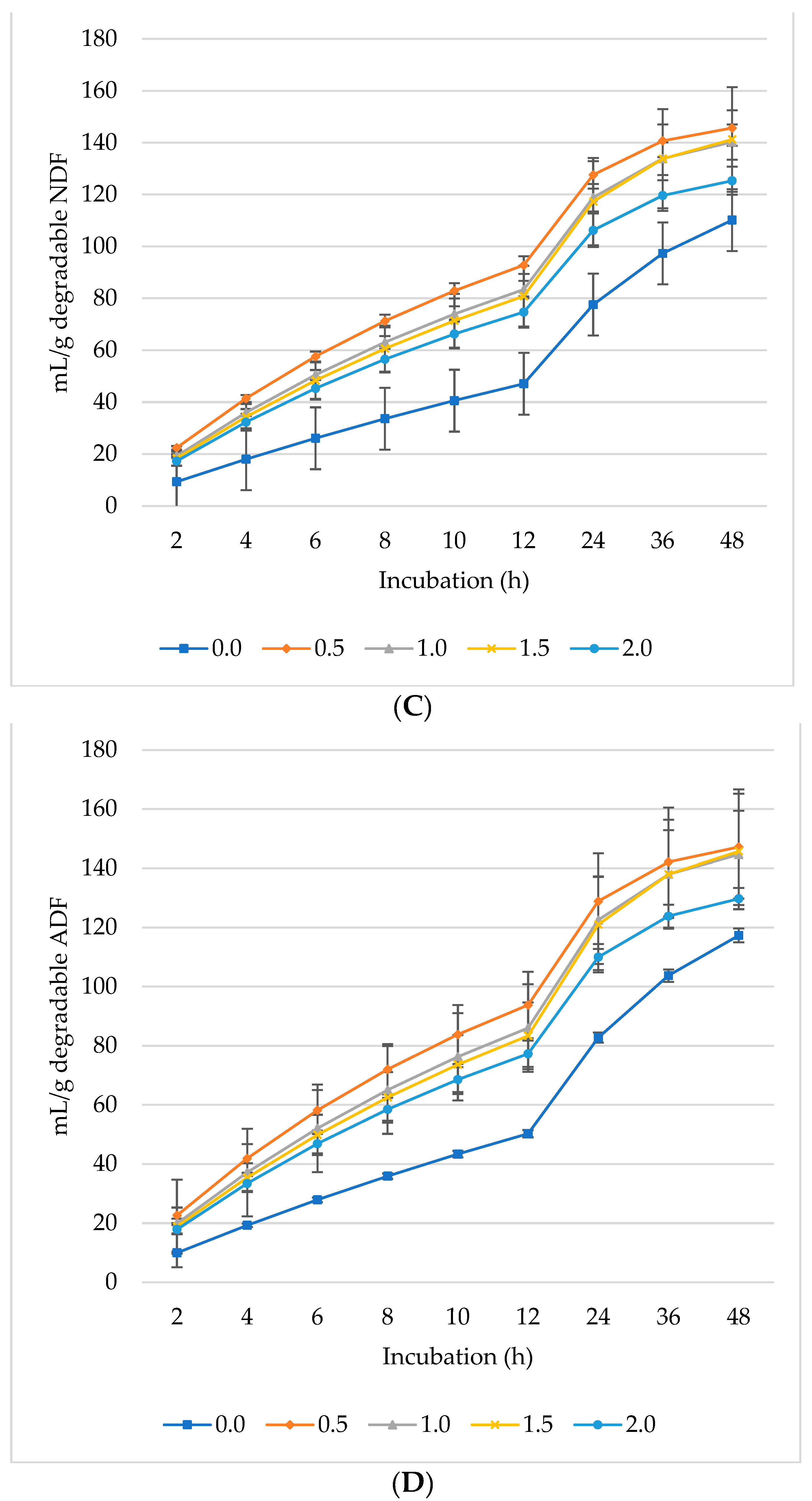
Figure 1, Figure 2 and Figure 3 represent GP, CH4, and CO2 as mL/g DM, dDM, dNDF, and dADF. The maximum GP was noted at 0.5% inclusion of L. nobilis leaves, followed by a 1% and 1.5% dietary inclusion. Gas production (mL/g DM) rose linearly with incubation hours (Figure 1A). The lowest GP observed at the 2% L. nobilis leaves inclusion. Conversely, 0.5%, 1%, and 1.5% inclusion of L. nobilis leaves resulted in optimal GP per gram of dDM (Figure 1B), dNDF (Figure 1C), and dADF (Figure 1D). The 0.5% inclusion level achieved the highest (linear, p = 0.006; quadratic, p < 0.001) asymptotic GP, followed by 1% and 1.5%, while the 2% inclusion did not affect it compared to the control level (Table 3). Without affecting the rate of GP, the level 0.5% showed the lowest lag time, while the level 2% showed the highest one (linear, p = 0.002, quadratic, p = 0.039).
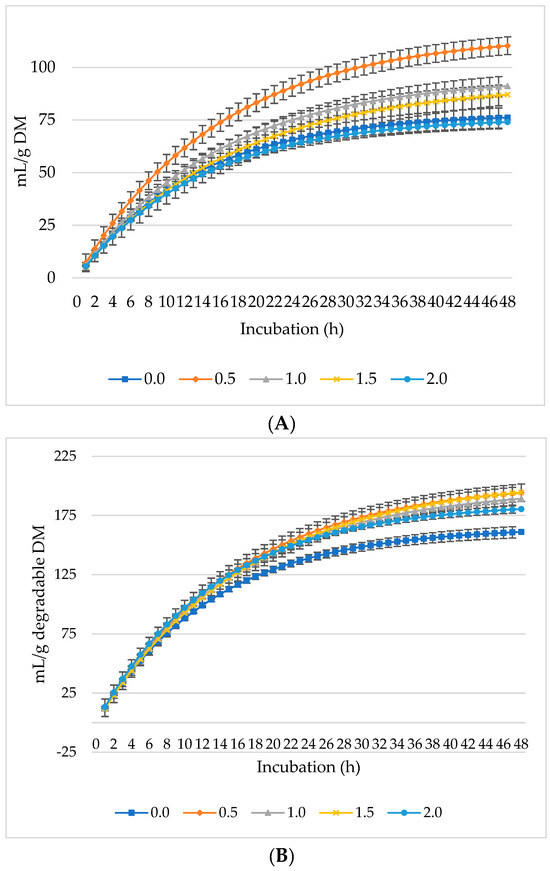
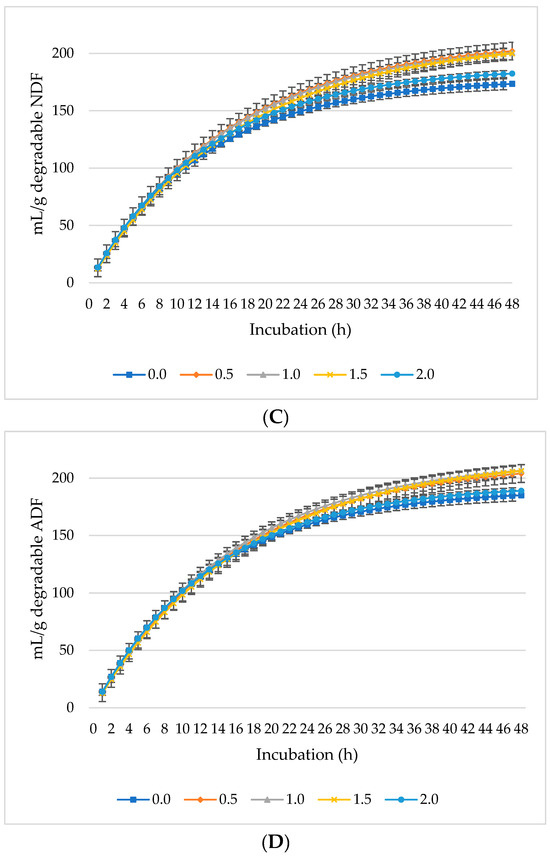
Figure 1.
In vitro ruminal gas production: mL/g incubated DM (A), mL/g degradable DM (B), mL/g degradable NDF (C), mL/g degradable ADF (D) of a total mixed ration supplemented with different levels of Laurus nobilis leaves. ADF refers to Acid detergent fiber, and NDF refers to neutral detergent fiber.
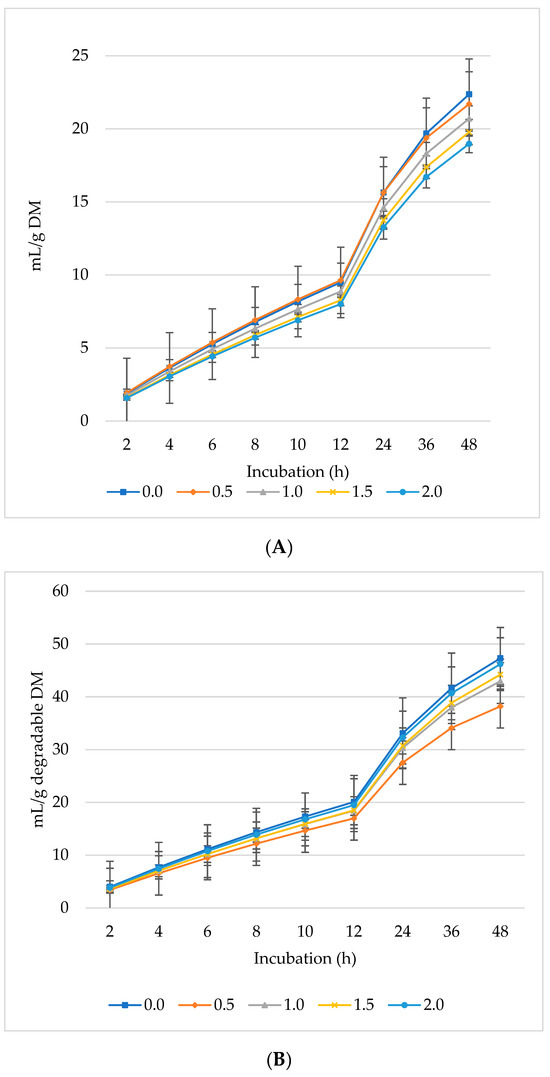
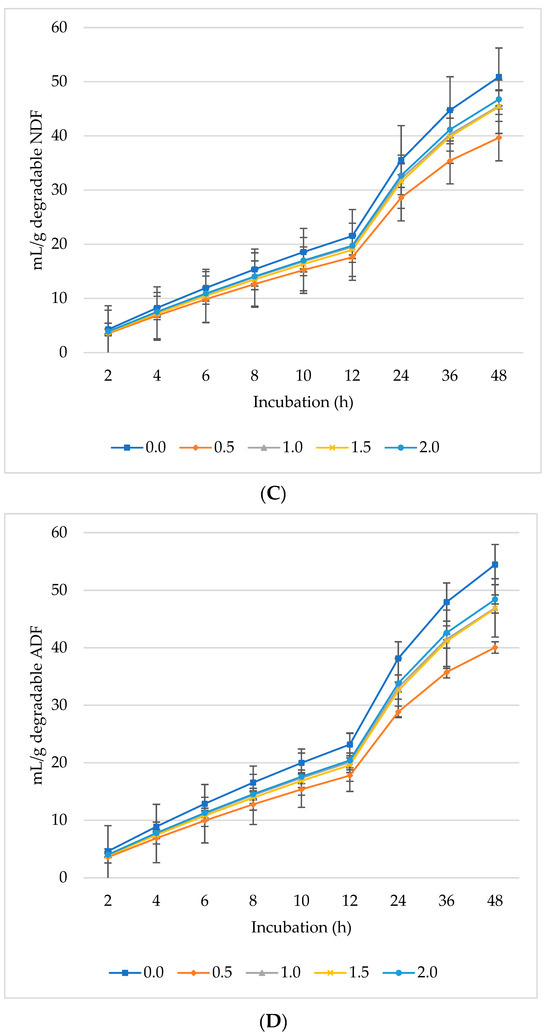
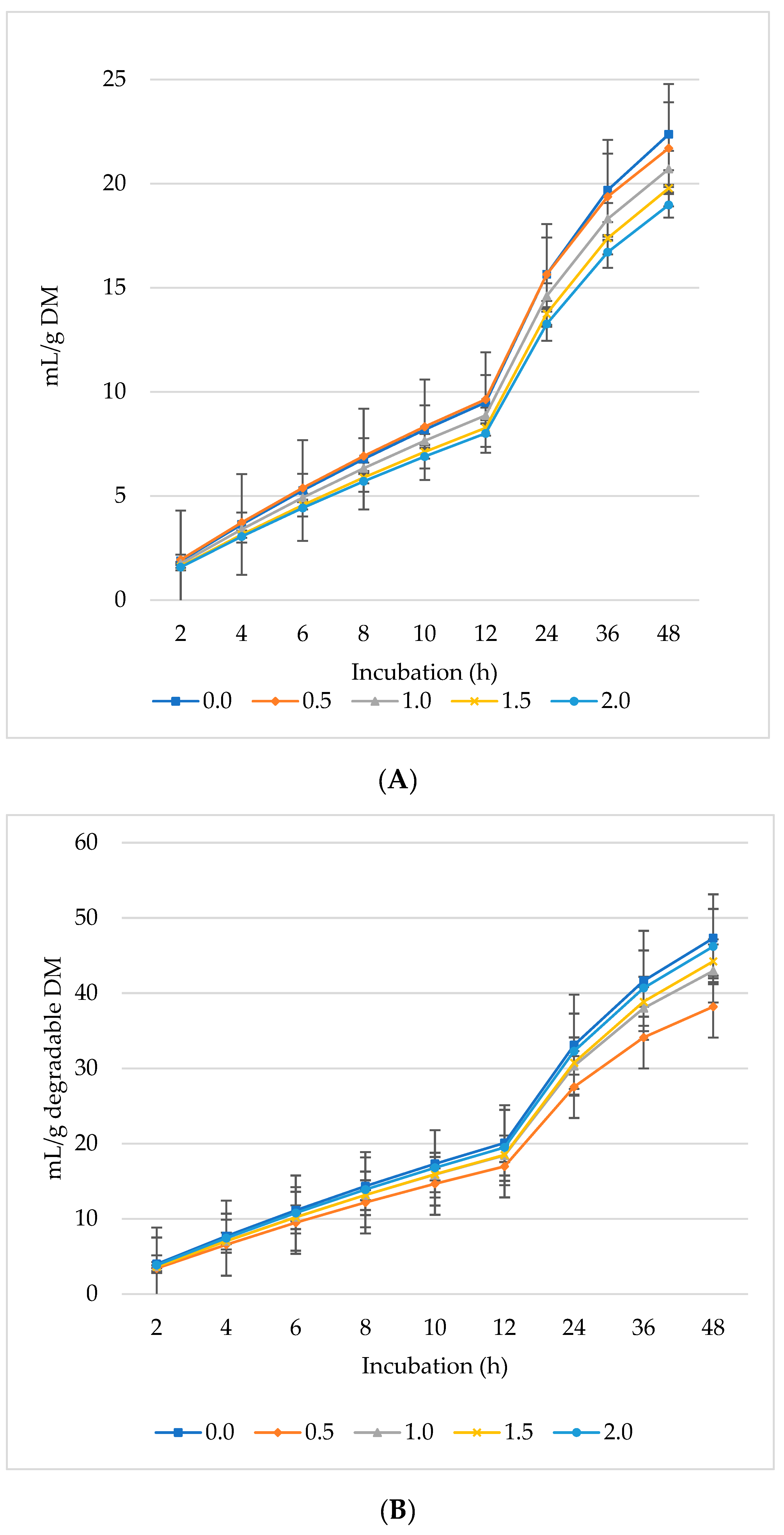
Figure 2.
In vitro ruminal methane (CH4): mL/g incubated DM (A), mL/g degradable DM (B), mL/g degradable NDF (C), mL/g degradable ADF (D) of a total mixed ration supplemented with different levels of Laurus nobilis leaves. ADF refers to Acid detergent fiber, and NDF refers to neutral detergent fiber.
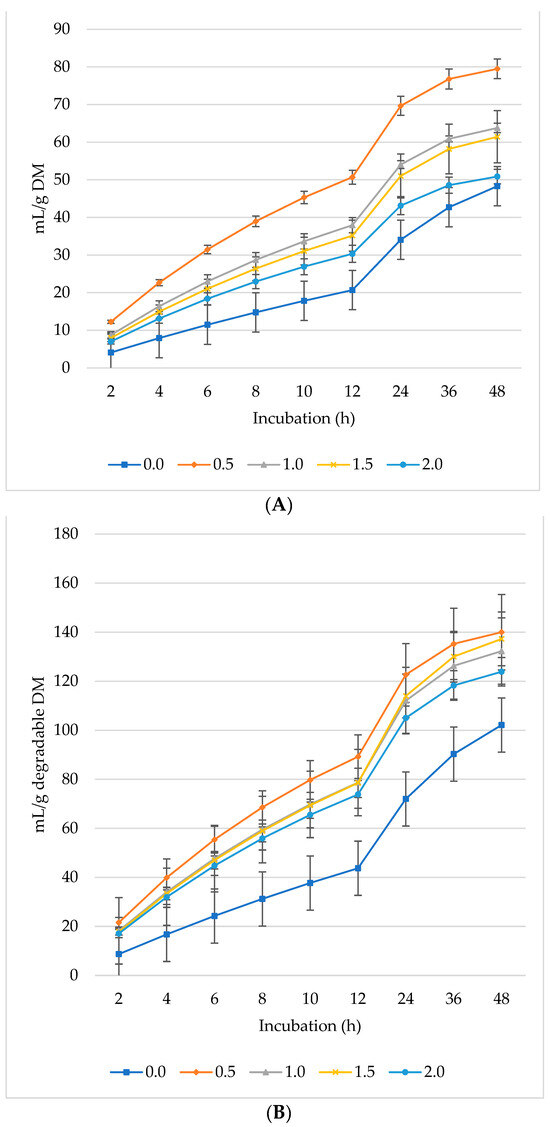
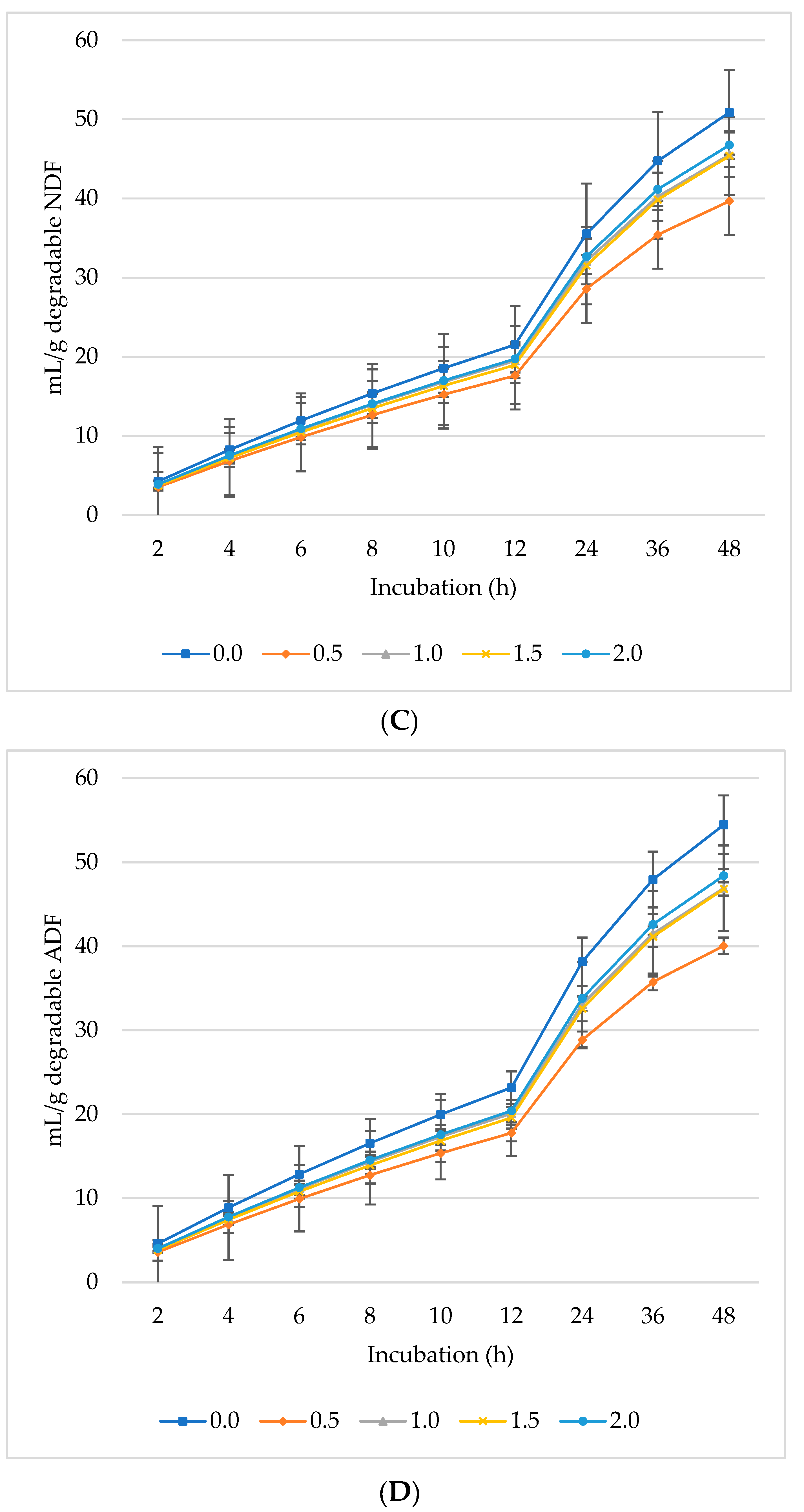
Figure 3.
In vitro ruminal carbon dioxide (CO2): mL/g incubated DM (A), mL/g degradable DM (B), mL/g degradable NDF (C), mL/g degradable ADF (D) of a total mixed ration supplemented with different levels of Laurus nobilis leaves. ADF refers to Acid detergent fiber, and NDF refers to neutral detergent fiber.

Table 3.
In vitro rumen gas production (GP), methane (CH4), and carbon dioxide (CO2) kinetics as affected by increasing levels of Laurus nobilis leaves (%, DM).
Ruminal CH4 production decreased per gram DM (Figure 2A), dDM (Figure 2B), dNDF (Figure 2C), and dADF (Figure 2D) with increasing levels of L. nobilis leaves in the diet. Without significant differences compared to the control, the 0.5%, 1%, and 1.5% inclusion levels gradually decreased asymptotic CH4 production, while the 2% inclusion level significantly showed the lowest production (p = 0.0032) compared to the control (Table 3). All levels of L. nobilis leaves linearly decreased proportional CH4 production (p = 0.001), with the lowest value observed at the 0.5% inclusion level. None of the L. nobilis leaves’ inclusion levels affected the rate; however, the levels 1.5% and 2% linearly increased (p = 0.001) the lag phase of CH4 production.
The production of CO2 increased per gram DM (Figure 3A), dDM (Figure 3B), dNDF (Figure 3C), and dADF (Figure 3D) with all levels of L. nobilis leaves in the diet. The 0.5% inclusion level significantly (linear, p = 0.002; quadratic, p < 0.001) increased asymptotic CO2 production compared to the control, with no significant differences observed between the other inclusion levels compared to the control (Table 3). All inclusion levels increased the rate of CO2 production (linear, p = 0.002; quadratic, p < 0.001) compared to the control. The 0.5%, 1%, and 1.5% inclusion levels significantly increased (p = 0.006) the proportion of CO2 compared to the control. None of the inclusion levels affected the rate or the lag phase of CO2 production.
3.3. Degradability and Fermentation
The 0.5% level of L. nobilis leaves exhibited the highest dDM (linear and quadratic p < 0.001), dNDF (linear p = 0.045, quadratic p = 0.043), and dADF (linear p = 0.041, quadratic p = 0.42) compared to the control, whereas the 2% level decreased all these parameters (Table 4).

Table 4.
In vitro rumen fermentation profile of diet with increasing levels of Laurus nobilis leaves (%, DM).
Compared to the control, the additive did not affect the proportions of individual SCFA; however, both the 0.5% and 1% levels increased the production of total SCFA (linear p = 0.001, quadratic p < 0.001) (Table 4). The highest acetate concentration (linear p = 0.006, quadratic p = 0.009) was observed at the 0.5% level, with no significant differences between other levels and the control. Additionally, the 0.5%, 1%, and 1.5% levels increased the propionate concentration (linear p = 0.039, quadratic p = 0.004). However, the treatments did not affect the butyrate concentration.
Without affecting pH, NH3-N, PF24, and GY24, the 0.5% level demonstrated the highest ME (linear p < 0.001, quadratic p = 0.012) and MCP (linear p = 0.002, quadratic p = 0.034), while the 2% level reduced them compared to the control (Table 4).
4. Discussion
4.1. Laurus nobilis
Many pharmacological characteristics, such as antibacterial, anti-inflammatory, and antioxidant properties, are possessed by plant volatile molecules [29]. The extraction of essential oil from the leaves revealed a yield of 3.29% by weight, which is aligned with the findings of Fidan et al. [30], who reported that the essential oil content in laurel leaves could reach up to 3.25% by weight, and with Khayyal et al. [17], who observed a 3% essential oil content in the leaves. In the present experiment, 1,8-cineole at about 81% and estragole at about 6% were the major compounds in the L. nobilis leaves. However, Fidan et al. [30] stated that 1,8-cineole (41.0%), α-terpinyl acetate (14.4%), sabinene (8.8%), methyl eugenole (6.0%), β-linalool (4.9%), and α-terpineol (3.1%) were the essential oils found in L. nobilis leaves. Others [31] showed that 1,8-cineole (18.2%), α-phellandrene (15%), β-pinene (9.4%), α-pinene (9.1%), α-terpinyl acetate (7.9%), sabinene (6.3%), camphene (4.2%), germacrene D (3.7%), and β-caryophyllene (3%) were the major constituents of L. nobilis essential oils. Sızmaz [18] reported that limonene was the major constituent of the laurel oil (64.6%). Choudhary et al. [32] reported that L. nobilis leaves from India and Nepal were found to have linalool as a significant component, followed by 1,8-cineole and α-pinene. Under the Egyptian conditions, Khayyal et al. [17] reported that 1,8-cineole (38.9%), α- pinene (17.0%), and terpinene-4-ol (15.01%) are the major volatile compounds in L. nobilis leaves. The variations across research could be attributed to environmental factors such as the kind of soil, temperature, season, location, and timing of plant harvesting, as well as strategies for drying, extracting, and analyzing data [15,33].
Due to their effect on ruminal bacteria, plant volatile compounds that modify the rumen microbiome offer a variety of dietary interventions that may affect and modulate rumen fermentation [34]. For example, rumen microbes’ growth and activity have been shown to be strongly inhibited by terpenoids, such as 1,8-cineole [35]. According to de Sousa et al. [29], these compounds also have antibacterial properties against bacteria, protozoa, and fungus. They influence enzyme activity, signal transduction pathways, bacterial colonization, and cell membrane integrity. The ruminal fermentation profile, however, varies depending on the source and concentration of volatile chemicals, which affect ruminal bacteria through different ways.
4.2. Gas Production
In order to evaluate the effect of any feed addition on ruminal microbes, GP kinetics offer important insights into the digestibility of feedstuffs, ruminal fermentation processes, and the activity of ruminal microorganisms [36,37]. The additives did not affect the lag time of GP, indicating that the additives did not affect the microbial activity on the incubated substrate [38]. L. nobilis leaves at 0.5% increased asymptotic GP by about 48%, indicating that L. nobilis leaves improved the ruminal fermentation. The presence of volatile compounds in the leaves may be the main reason for the increased GP [39,40]. Essential oils have multiple mechanisms through which they affect GP; however, their primary mechanism is their antimicrobial properties. Due to the nature of the plants, the amount and concentration of bioactive components in essential oils might vary, which affects how efficient their antibacterial qualities are [41,42]. In light of the specific chemical composition of certain feed additives, higher doses may inhibit a broad spectrum of microorganisms in the rumen, consequently altering GP characteristics. In the present study, it was anticipated that the highest level of L. nobilis leaves would reduce GP due to the antimicrobial impact of essential oils present in L. nobilis leaves on various ruminal microorganisms. However, contrary to expectations, minimal differences were observed between the highest level of L. nobilis leaves and the control treatment. The increased dDM, dNDF, and dADF at 0.5% inclusion also explains the highest GP compared to other levels. Furthermore, there is evidence that certain components in essential oils from plant extracts, especially those with lower antimicrobial potential like monoterpenoids (e.g., 1,8-cineole, estragole, and α-terpinyl acetate) with hydrocarbon and alcohol structures, could act as a carbon source for specific rumen microorganisms. This means that the high concentration of volatile compounds in L. nobilis leaves, which are sources of monoterpene hydrocarbons, may potentially alter rumen fermentation and GP [43].
Kumar et al. [44] reported that administering eucalyptus fresh leaf extracts rich in 1,8-cineole at 0.5 mL per 30 mL of buffered rumen fluid containing oats hay increased GP per gram DM or dDM. However, increasing the dose to 2 mL resulted in decreased GP. However, Sallam et al. [19] conducted an incubation study of a total mixed ration (1:1 roughage to concentrate) with eucalyptus oil containing mainly 1,8-cineole added at concentrations of 25, 50, 100, and 150 µL per 75 mL of buffered rumen fluid. They observed a reduction in GP by 5.3%, 24.2%, 44.6%, and 56.7%, respectively, with increasing levels of eucalyptus oil.
All levels of L. nobilis leaves’ administration did not affect the rate of GP; however, numerical differences were observed between different treatments. Moreover, the level 2% NLM increased the lag time of GP by 26.5% compared to the control level, confirming the inverse relationship between the lag phase and asymptotic GP [6]. A period of adjustment is required for the bacteria to acclimate to the addition of L. nobilis leaves, which may be the cause of the extended lag time seen at 2% level inclusion. This leads to a delayed beginning of fermentation and GP.
4.3. Methane and Carbon Dioxide Production
It was expected that varying concentrations of L. nobilis leaves would demonstrate anti-methanogenic activity by improving the ruminal fermentation profile and substrate degradability while suppressing methanogenic archaea and lowering the generation of CH4. Without affecting the rate of CH4, all levels of L. nobilis leaves lowered the asymptotic CH4 production and proportion with the lowest CH4 production at 2% (reduced CH4 by about 18%) and lowest proportion at 0.5% (reduced CH4 proportion by about 33%). Moreover, the 1.5 and 2% levels increased the lag of CH4 production by 38.7% and 31.7%, respectively, indicating that methanogens needed more time to start producing CH4. The impact of essential oils (e.g., 1,8-cineole) on reducing the methanogenic archaeal population and methanogenesis was previously approved [45,46]. The molar proportion of each individual SCFA and the total SCFA concentration may also contribute to the decreasing CH4 generation with L. nobilis leaves. For instance, the levels 0.5% and 1% increased the propionate concentration which can act as a hydrogen sink, limiting the availability of CH4 production, thereby decreasing methanogenesis [47,48]. Previous research has demonstrated that secondary metabolites from plants can alter the metabolic processes of hydrogen-consuming bacteria and methanogens, as well as affect protozoa populations [49,50]. Similar results were observed by Sallam et al. [19] when they administered eucalyptus oil rich in 1,8-cineole at concentrations of 25, 50, 100, and 150 µL per 75 mL of buffered rumen fluid containing a diet with equal portions of concentrates and roughages. The production of CH4 was shown to have dropped by 26.0%, 46.8%, 77.3%, and 85.3%, respectively. Moreover, Kumar et al. [44] reported that administering eucalyptus fresh leaf extracts rich in 1,8-cineole at 0.5 or 2 mL per 30 mL of buffered rumen fluid containing oats hay decreased CH4 production at both doses. However, as previously mentioned, the higher dose reduced GP.
The inclusion of 0.5% of L. nobilis leaves showed the highest asymptotic CO2 production by about 38%. Kholif et al. [6] found comparable outcomes when applying Salvia officinalis, which is abundant in volatile compounds, to the substrate employed in our study. Moreover, the administration of L. nobilis leaves increased the rate of CO2 production by about 83% to 128% compared to the control. Increasing the propionate concentration at the expense of acetate could increase CO2 levels [51]. Although the highest propionate level was found at 1% L. nobilis leaves’ inclusion without a corresponding decrease in acetate or butyrate (both acetate and butyrate increased alongside propionate), the mechanism underlying the increased CO2 with rising levels of L. nobilis leaves in this study is difficult to reconcile. Therefore, the inhibition of CH4 generation by the plant secondary compounds in L. nobilis leaves may be responsible for the decreased utilization of CO2.
4.4. Degradability and Fermentation
The ruminal pH, which normally ranges from 5.0 to 7.5, should be measured in order to evaluate the stability and balance of the rumen environment in ruminant animals [52,53]. The administration of L. nobilis leaves at different levels did not affect pH and NH3-N. The values of pH [54] and concentration of NH3-N [55] were within the reference ranges required for optimal microflora growth and activity for nutrient digestion. Similar results were observed by Khayyal et al. [17] fed growing lambs were fed diets supplemented with L. nobilis leaves. It was expected that the high levels of L. nobilis leaves and its active component 1,8-cineole will decrease the concentration of ruminal NH3-N [19,44] because essential oils at a high concentration inhibit the hyper-NH3 producing bacteria [45]. But this was not observed, which may be related to the concentration of 1,8-cineole in their treatments or the incubated substrates.
All of dDM, dNDF, and dADF exhibited a dose-dependent response, with higher values observed at 0.5% inclusion of L. nobilis leaves. This level improved the degradability of DM by 20%, NDF by 25%, and ADF by 32%, whereas the 2% level decreased them by about 13%, 8%, and 5%, respectively, indicating the importance of defining the optimal doses of this feed additive. Essential oils at appropriate doses, typically low, are beneficial for ruminal microbial activity and growth, especially fibrolytic bacterial activity [45], which enhance the degradation and fermentation of substrates. Higher concentrations of essential oils derived from plant extracts have been demonstrated in earlier studies to potentially inhibit the growth of cellulolytic bacteria in the rumen and decrease the feedstuffs’ capacity to degrade feeds [44,56]. Lee et al. [57] reported that administering San wormwood essential oil, containing 56.7% 1,8-cineole, to Bermuda grass hay increased the populations of Ruminococcus albus and Streptococcus bovis, which are directly linked to cellulose/hemicellulose digestion [58]. The negative effects of increasing the level of L. nobilis leaves, and subsequently the level of 1,8-cineole, were previously reported by Kumar et al. [44]. They observed that a low level of Eucalyptus fresh leaf extracts rich in 1,8-cineole, at 0.5 mL per 30 mL of buffered rumen fluid containing oats hay, enhanced dDM, while increasing the dose to 2 mL resulted in decreased dDM, dNDF, and dADF. Khayyal et al. [17] reported that the inclusion of L. nobilis leaves in diets of growing lambs did not affect nutrient digestibility. The presence of active compounds like 1,8-cineole and other components in L. nobilis leaves may act as stimulants for rumen microflora, enhancing their efficiency in producing essential vitamins and enzymes needed to optimize digestibility [45]. Moreover, administering San wormwood essential oil, containing 56.7% 1,8-cineole, at 5 mg/kg improved dDM of Bermuda grass during fermentation [57]. Sızmaz [18] reported that laurel essential oil at concentrations of 50 and 100 mg/L of fermenter liquid did not affect apparent nutrient degradation. The low doses used in Sızmaz’s experiment (equal to 2.92 and 5.84 mg/g DM) and the continuous dilution of the fermenter liquid with buffer may be the main reason for the weak effects on nutrient degradability [18].
The total SCFA (by about 18% and 11%, respectively), acetate (by about 18% and 9%, respectively), and propionate (by about 18% and 19%, respectively) increased with the administration of L. nobilis leaves at 0.5% and 1% levels. The enhancements in the production of the total SCFA may be due to the positive effects of the essential oils in L. nobilis leaves on nutrient digestion [7,45]. Administering Eucalyptus fresh leaf extracts rich in 1,8-cineole at 0.5 mL per 30 mL of buffered rumen fluid containing oats hay increased the production of total SCFA, acetate, propionate, and butyrate; however, increasing the dose to 2 mL reduced their production [44].
Without affecting PF24 or GY24, increased estimated ME, by about 7%, and MCP, by about 12%, were observed when L. nobilis leaves were administered at 0.5%, while 2% administration lowered them by about 13% and 17%, respectively. These findings point to the ideal ratio of protein to energy that encourages higher microbial protein production [6,7,20]. Since L. nobilis leaves include phytochemicals that regulate both pathways, phenolic compounds in L. nobilis leaves at a concentration of 0.5% may interact with the biosynthesis of aromatic amino acids [59]. The increase in MCP indicates that a significant portion of NH3-N and SCFA were utilized for microbial protein synthesis [60].
5. Conclusions
By adding 0.5% L. nobilis leaves (DM basis) to a diet that included concentrate and roughages at a 1:1 ratio, it was possible to minimize ruminal CH4 production and boost GP in vitro, which could help reduce the environmental impact of ruminants (e.g., sheep) and promote sustainability. Additionally, this study showed that supplementing the diet with 0.5% L. nobilis leaves improved the total and individual SCFA, primarily acetate and propionate, as well as the nutrient degradability (dDM, dNDF, and dADF). Higher quantities of L. nobilis leaf supplementation in vivo should be investigated further to evaluate their effects on ruminant animal production performance and rumen microbiota alterations. These studies will yield important information about how to best utilize L. nobilis leaves in livestock farming practices to enhance animal health and environmental sustainability. In addition, methods for gathering leaves of L. nobilis should be assessed to create a more uniform product that can handle possible issues with broad distribution.
Funding
This research received no external funding.
Institutional Review Board Statement
This was an in vitro experiment using ruminal fluid obtained from nearby butcher facilities; therefore, ethical reviews and permission were not needed. The Assistant Editor received a formal letter of exemption signed by the Dean of the Faculty of Agriculture at New Valley University in Egypt as well as the Head of Department. There was no further use of live animals during any of the in vitro experimental processes. Following the guidelines set forth by the animal welfare laws, the animals were killed without experiencing any kind of pain, suffering, or mistreatment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
The author wishes to thank Tarek A. Morsy, Gouda A. Gouda, and Mahmoud Fahmy from the Dairy Science Department at the National Research Centre (Egypt) for their assistance during the experimental work.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 978-92-5-107920-1. [Google Scholar]
- Johannisson, J. Prospective Environmental Assessment of Technologies for Mitigating Methane Emissions. Ph.D. Thesis, Universität Ulm, Ulm, Germany, 2023. [Google Scholar]
- Niu, M.; Kebreab, E.; Hristov, A.N.; Oh, J.; Arndt, C.; Bannink, A.; Bayat, A.R.; Brito, A.F.; Boland, T.; Casper, D.; et al. Prediction of Enteric Methane Production, Yield, and Intensity in Dairy Cattle Using an Intercontinental Database. Glob. Chang. Biol. 2018, 24, 3368–3389. [Google Scholar] [CrossRef] [PubMed]
- Opio, C.; Gerber, P.; Mottet, A.; Falcucci, A.; Tempio, G.; MacLeod, M.; Vellinga, T.; Henderson, B.; Steinfeld, H. Greenhouse Gas Emissions from Ruminant Supply Chains—A Global Life Cycle Assessment; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Morsy, T.A.; Gouda, G.A.G.A.; Kholif, A.E. In Vitro Fermentation and Production of Methane and Carbon Dioxide from Rations Containing Moringa oleifera Leave Silage as a Replacement of Soybean Meal: In Vitro Assessment. Environ. Sci. Pollut. Res. 2022, 29, 69743–69752. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Rahman, M.A.; Abo El-Nor, S.A.H.; Morsy, T.A.; Gouda, G.A.; Fahmy, M.; Chahine, M. Efficacy of Salvia officinalis Shrub as a Sustainable Feed Additive for Reducing Ruminal Methane Production and Enhancing Fermentation in Ruminants. Animals 2024, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Morsy, T.A.; Kholif, A.E.; Adegbeye, M.J.; Olafadehan, O.A.; Gouda, G.A.; Fahmy, M.; Chahine, M. Lupin Seed Supplementation as a Functional Feed Additive: In Vitro Ruminal Gas, Methane and Carbon Dioxide Production, Fermentation Kinetics, and Nutrient Degradability. Animals 2024, 14, 2119. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Salem, A.Z.M.; Montes de Oca, R.; Barbabosa, A.; Mariezcurrena, M.; Olafadehan, O.A. Addressing Sustainable Ruminal Methane and Carbon Dioxide Emissions of Soybean Hulls by Organic Acid Salts. J. Clean. Prod. 2016, 135, 194–200. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Fahmy, M.; Morsy, T.A.; Abdelsattar, M.M.; Vargas-Bello-Pérez, E. Fennel Seeds Dietary Inclusion as a Sustainable Approach to Reduce Methane Production and Improve Nutrient Utilization and Ruminal Fermentation. Anim. Sci. J. 2024, 95, e13910. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Barbabosa, A.; Márquez, O.; Odongo, N.E. The Effects of Three Total Mixed Rations with Different Concentrate to Maize Silage Ratios and Different Levels of Microalgae Chlorella vulgaris on in Vitro Total Gas, Methane and Carbon Dioxide Production. J. Agric. Sci. 2017, 155, 494–507. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Kholif, A.E.; Salem, A.Z.M.; Olafadehan, O.A.; Kholif, A.M. Sustainable Anaerobic Rumen Methane and Carbon Dioxide Productions from Prickly Pear Cactus Flour by Organic Acid Salts Addition. J. Clean. Prod. 2016, 139, 1362–1369. [Google Scholar] [CrossRef]
- Ghimpețeanu, O.M.; Pogurschi, E.N.; Popa, D.C.; Dragomir, N.; Drăgotoiu, T.; Mihai, O.D.; Petcu, C.D. Antibiotic Use in Livestock and Residues in Food—A Public Health Threat: A Review. Foods 2022, 11, 1430. [Google Scholar] [CrossRef]
- Mouliom Mouiche, M.M.; Okah-Nnane, N.H.; Moffo, F.; Djibo, I.; Mapiefou, N.P.; Mpouam, S.E.; Mfopit, Y.M.; Mingoas, J.-P.K.; Tebug, S.F.; Ndukum, J.A. Antibiotic Residues in Foods of Animal Origin in Cameroon: Prevalence, Consumers’ Risk Perceptions, and Attitudes. J. Food Prot. 2024, 87, 100237. [Google Scholar] [CrossRef]
- Awada, F.; Hamade, K.; Kassir, M.; Hammoud, Z.; Mesnard, F.; Rammal, H.; Fliniaux, O. Laurus nobilis Leaves and Fruits: A Review of Metabolite Composition and Interest in Human Health. Appl. Sci. 2023, 13, 4606. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Repajić, M.; Dragović-Uzelac, V.; Elez Garofulić, I. Isolation of Laurus nobilis Leaf Polyphenols: A Review on Current Techniques and Future Perspectives. Foods 2022, 11, 235. [Google Scholar] [CrossRef]
- EFSA. Safety and Efficacy of a Feed Additive Consisting of an Essential Oil from the Leaves of Laurus nobilis L. (Laurel Leaf Oil) for All Animal Species (FEFANA Asbl). EFSA J. 2023, 21, e07875. [Google Scholar] [CrossRef]
- Khayyal, A.; El-Badawy, M.; Ashmawy, T. Effect of Rosemary or Laurel Leaves as Feed Additives on Performance of Growing Lambs. Egypt. J. Nutr. Feed. 2021, 24, 343–356. [Google Scholar] [CrossRef]
- Sızmaz, Ö. Assessment of in Vitro Rumen Fermentation Patterns, Gas Formation and Nutrient Degradation of Laurel Oil. J. Turk. Vet. Med. Soc. 2016, 87, 1–10. [Google Scholar]
- Sallam, S.M.A.; Bueno, I.C.S.; Brigide, P.; Godoy, P.B.; Vitti, D.M.S.; Abdalla, A.L. Production in Efficacy of Eucalyptus Oil on in Vitro Ruminal Fermentation and Methane Production. In Options Méditerranéennes: Série A. Séminaires Méditerranéens; Papachristou, T.G., Parissi, Z.M., Ben Salem, H., Morand-Fehr, P., Eds.; CIHEAM/FAO/NAGREF: Zaragoza, Spain, 2009; Volume 272, pp. 267–272. [Google Scholar]
- Kholif, A.E.; Olafadehan, O.A.; Gouda, G.A.; Fahmy, M.; Morsy, T.A.; Ammar, H.; Hamdon, H.A.; Chahine, M. Turmeric Rhizomes Reduced in Vitro Methane Production and Improved Gas Production and Nutrient Degradability. Anim. Biotechnol. 2024, 35, 2371519. [Google Scholar] [CrossRef]
- Qin, D.-M.; Wang, X.-B.; Zou, N.; Han, C.; Xu, J. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the Volatile Oil of Cichorium Glandulosum boiss et Huet and Its Effects on Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Med. Sci. Monit. 2019, 25, 3591–3604. [Google Scholar] [CrossRef]
- Fortina, R.; Glorio Patrucco, S.; Barbera, S.; Tassone, S. Rumen Fluid from Slaughtered Animals: A Standardized Procedure for Sampling, Storage and Use in Digestibility Trials. Methods Protoc. 2022, 5, 59. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses; ARS-USDA: Washington, DC, USA, 1975. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC International: Washington, DC, USA, 1997; ISBN 9780935584547. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the Extent of Degradation of Ruminant Feeds from a Description of Their Gas Production Profiles Observed in Vitro: Derivation of Models and Other Mathematical Considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef]
- Blümmel, M.; Steingaβ, H.; Becker, K. The Relationship between in Vitro Gas Production, in Vitro Microbial Biomass Yield and 15 N Incorporation and Its Implications for the Prediction of Voluntary Feed Intake of Roughages. Br. J. Nutr. 1997, 77, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor in Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Fidan, H.; Stefanova, G.; Kostova, I.; Stankov, S.; Damyanova, S.; Stoyanova, A.; Zheljazkov, V.D. Chemical Composition and Antimicrobial Activity of Laurus nobilis L. Essential Oils from Bulgaria. Molecules 2019, 24, 804. [Google Scholar] [CrossRef] [PubMed]
- Petkova, Z.; Stefanova, G.; Girova, T.; Antova, G.; Stoyanova, M.; Damianova, S.; Gochev, V.; Stoyanova, A.; Zheljazkov, V.D. Phytochemical Investigations of Laurel Fruits (Laurus nobilis). Nat. Prod. Commun. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Choudhary, D.; Kala, S.P.; Todaria, N.P.; Dasgupta, S.; Kinhal, G.; Kollmair, M. Essential Oil from Bay Leaves in India and Nepal: An Analysis for Quality Oriented Value Chain Development. Int. J. Med. Aromat. Plants 2013, 3, 11–17. [Google Scholar]
- Paparella, A.; Nawade, B.; Shaltiel-Harpaz, L.; Ibdah, M. A Review of the Botany, Volatile Composition, Biochemical and Molecular Aspects, and Traditional Uses of Laurus nobilis. Plants 2022, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.L.; Waghorn, G.C.; Ulyatt, M.J.; Lassey, K.R. Early Indications That Feeding Lotus Will Reduce Methane Emissions from Ruminants. Proc. N. Z. Soc. Anim. Prod. 2001, 61, 23–26. [Google Scholar]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A Review of Plant-Derived Essential Oils in Ruminant Nutrition and Production. Anim. Feed. Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Benchaar, C.; Hassanat, F.; Yang, W.Z. Effects of Active Dried Yeast (Saccharomyces cerevisiae), a Non-Ionic Surfactant, or Their Combination on Gas Production, Rumen Microbial Fermentation and Methane Production in Vitro. Anim. Feed. Sci. Technol. 2024, 307, 115844. [Google Scholar] [CrossRef]
- Salem, A.Z.M.; Kholif, A.E.; Elghandour, M.M.Y.; Hernandez, S.R.; Domínguez-Vara, I.A.; Mellado, M. Effect of Increasing Levels of Seven Tree Species Extracts Added to a High Concentrate Diet on in Vitro Rumen Gas Output. Anim. Sci. J. 2014, 85, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, G.; Simeonidis, K.; Faustini, M.; Le Mura, A.; Cavalleri, M.; Serra, V.; Attard, E. Chemical Characterization and In Vitro Gas Production Kinetics of Alternative Feed Resources for Small Ruminants in the Maltese Islands. Metabolites 2023, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Elghandour, M.M.Y.; Kholif, A.E.; Bastida, A.Z.; Martínez, D.L.P.; Salem, A.Z.M. In Vitro Gas Production of Five Rations of Different Maize Silage and Concentrate Ratios Influenced by Increasing Levels of Chemically Characterized Extract of Salix babylonica. Turk. J. Vet. Anim. Sci. 2015, 39, 186–194. [Google Scholar] [CrossRef]
- Cedillo, J.; Vázquez-Armijo, J.F.; González-Reyna, A.; Salem, A.Z.M.; Kholif, A.E.; Hernández-Meléndez, J.; Martínez-González, J.C.; de Oca Jiménez, R.M.; Rivero, N.; López, D. Effects of Different Doses of Salix babylonica Extract on Growth Performance and Diet in Vitro Gas Production in Pelibuey Growing Lambs. Ital. J. Anim. Sci. 2014, 13, 609–613. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited Review: Essential Oils as Modifiers of Rumen Microbial Fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [PubMed]
- Macheboeuf, D.; Morgavi, D.P.; Papon, Y.; Mousset, J.-L.; Arturo-Schaan, M. Dose–Response Effects of Essential Oils on in Vitro Fermentation Activity of the Rumen Microbial Population. Anim. Feed. Sci. Technol. 2008, 145, 335–350. [Google Scholar] [CrossRef]
- Broudiscou, L.P.; Papon, Y.; Broudiscou, A.F. Effects of Dry Plant Extracts on Fermentation and Methanogenesis in Continuous Culture of Rumen Microbes. Anim. Feed. Sci. Technol. 2000, 87, 263–277. [Google Scholar] [CrossRef]
- Kumar, K.; Dey, A.; Rose, M.K.; Dahiya, S.S. Modulating Feed Digestion and Methane Production by Eucalyptus (Eucalyptus citriodora) Leaves Essential Oils in Water Buffalo (Bubalus bubalis). Buffalo Bull. 2022, 41, 41–47. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential Oils and Phytogenic Feed Additives in Ruminant Diet: Chemistry, Ruminal Microbiota and Fermentation, Feed Utilization and Productive Performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Capasso, V.; Lotito, D.; Pugliese, G.; Ruocco, R.A.; Musco, N. Essential Oils and Methanogenesis. A Review. J. Nutr. Ecol. Food Res. 2017, 4, 107–127. [Google Scholar] [CrossRef]
- Wang, K.; Xiong, B.; Zhao, X. Could Propionate Formation Be Used to Reduce Enteric Methane Emission in Ruminants? Sci. Total Environ. 2023, 855, 158867. [Google Scholar] [CrossRef]
- Kamra, D.N.; Patra, A.K.; Chatterjee, P.N.; Kumar, R.; Agarwal, N.; Chaudhary, L.C. Effect of Plant Extracts on Methanogenesis and Microbial Profile of the Rumen of Buffalo: A Brief Overview. Aust. J. Exp. Agric. 2008, 48, 175–178. [Google Scholar] [CrossRef]
- Zmora, P.; Cieślak, A.; Pers-Kamczyc, E.; Szyszka, P.; Szumacher-Strabel, M. An in Vitro Study on the Effect of Sage, Salvia officinalis L., on Rumen Fermentation. J. Anim. Feed. Sci. 2012, 21, 613–623. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Rezaei, J.; Rouzbehan, Y. Nutritive Value of Adiantum capillus-veneris and Salvia officinalis L. Forages and the Effect of Their Dietary Levels on in Vitro Digestibility, Methane Production, Antioxidant Capacity, and Fermentation Parameters. Anim. Prod. Res. 2022, 11, 1–15. [Google Scholar] [CrossRef]
- Haisan, J.; Sun, Y.; Guan, L.L.; Beauchemin, K.A.; Iwaasa, A.; Duval, S.; Barreda, D.R.; Oba, M. The Effects of Feeding 3-Nitrooxypropanol on Methane Emissions and Productivity of Holstein Cows in Mid Lactation. J. Dairy Sci. 2014, 97, 3110–3119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dagar, S.S.; Sirohi, S.K.; Upadhyay, R.C.; Puniya, A.K. Microbial Profiles, in Vitro Gas Production and Dry Matter Digestibility Based on Various Ratios of Roughage to Concentrate. Ann. Microbiol. 2013, 63, 541–545. [Google Scholar] [CrossRef]
- Khattab, M.S.A.; Kholif, A.E.; Abd El Tawab, A.M.; Shaaban, M.M.; Hadhoud, F.I.; El-Fouly, H.A.; Olafadehan, O.A. Effect of Replacement of Antibiotics with Thyme and Celery Seed Mixture on the Feed Intake and Digestion, Ruminal Fermentation, Blood Chemistry, and Milk Lactation of Lactating Barki Ewes. Food Funct. 2020, 11, 6889–6898. [Google Scholar] [CrossRef] [PubMed]
- Ryle, M.; Ørskov, E.R. Energy Nutrition in Ruminants; Springer Netherlands: Dordrecht, The Netherlands, 1990; ISBN 978-94-010-6823-9. [Google Scholar]
- Satter, L.D.; Slyter, L.L. Effect of Ammonia Concentration on Rumen Microbial Protein Production in Vitro. Br. J. Nutr. 1974, 32, 199–208. [Google Scholar] [CrossRef]
- Agarwal, N.; Shekhar, C.; Kumar, R.; Chaudhary, L.C.; Kamra, D.N. Effect of Peppermint (Mentha piperita) Oil on in Vitro Methanogenesis and Fermentation of Feed with Buffalo Rumen Liquor. Anim. Feed. Sci. Technol. 2009, 148, 321–327. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, D.H.; Paradhipta, D.H.V.; Lee, H.J.; Yoon, H.; Joo, Y.H.; Adesogan, A.T.; Kim, S.C. Effects of Wormwood (Artemisia montana) Essential Oils on Digestibility, Fermentation Indices, and Microbial Diversity in the Rumen. Microorganisms 2020, 8, 1605. [Google Scholar] [CrossRef]
- Arjun, S.; Neha, P.; Mohith Sai, S.R.; Ravi, L. Microbial Symbionts in Ruminants. In Microbial Symbionts: Functions and Molecular Interactions on Host; Dharumadurai, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 493–509. [Google Scholar]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic Acids Act as Signaling Molecules in Plant-Microbe Symbioses. Plant Signal Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Boucher, S.E.; Ordway, R.S.; Whitehouse, N.L.; Lundy, F.P.; Kononoff, P.J.; Schwab, C.G. Effect of Incremental Urea Supplementation of a Conventional Corn Silage-Based Diet on Ruminal Ammonia Concentration and Synthesis of Microbial Protein. J. Dairy Sci. 2007, 90, 5619–5633. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).