Optimization of Microbial Glycogen Production by Saccharomyces cerevisiae CEY1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Fermentation Conditions
2.3. Analysis of Substrates and Metabolites Produced during Fermentation
2.4. Cell Growth and Quantitation of Glycogen Production
2.5. Fermentation Kinetics
2.6. Statistical Analyses
3. Results
3.1. Effect of Initial Glucose Concentration on Glycogen Production
3.2. Effect of Mixed Sugar Fermentation on Glycogen Production
3.3. Effect of the Type of Media Used for Fermentation on Glycogen Production
3.4. Effect of Temperature on Glycogen Production
3.5. Effect of Initial Ethanol Concentration on Glycogen Production
3.6. Effect of Aeration on Glycogen Production
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Preiss, J. Glycogen: Biosynthesis and regulation. EcoSal Plus 2014, 6. [Google Scholar] [CrossRef]
- Prats, C.; Graham, T.E.; Shearer, J. The dynamic life of the glycogen granule. J. Biol. Chem. 2018, 293, 7089–7098. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Colleoni, C.; Cenci, U.; Raj, J.N.; Tirtiaux, C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J. Exp. Bot. 2011, 62, 1775–1801. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Q.; Tan, X.; Yang, T.; Tang, D.; Wang, W.; Wise, M.J. Bacterial glycogen as a durable energy reserve contributing to persistence: An updated bibliography and mathematical model. bioRxiv 2019, 536110. [Google Scholar] [CrossRef]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Jirátová, M.; Pospíšilová, A.; Rabyk, M.; Pařízek, M.; Kovář, J.; Gálisová, A.; Hrubý, M.; Jirák, D. Biological characterization of a novel hybrid copolymer carrier system based on glycogen. Drug Deliv. Transl. Res. 2018, 8, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R. A comparative study of molecular structures, solution properties and food applications for three branched polysaccharides: Amylopectin, glycogen, and dextran. Curr. Trends Polym. Sci. 2012, 16, 49–63. [Google Scholar]
- Gálisová, A.; Jirátová, M.; Rabyk, M.; Sticová, E.; Hájek, M.; Hrubý, M.; Jirák, D. Glycogen as an advantageous polymer carrier in cancer theranostics: Straightforward in vivo evidence. Sci. Rep. 2020, 10, 10411. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Chen, Y.; Zhang, T.; Miao, M. Fabrication, structure and functional characterizations of pH-responsive hydrogels derived from phytoglycogen. Foods 2021, 10, 2653. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Hassimotto, N.M.A.; Lajolo, F.M.; Fabi, J.P. Nanotechnology as a tool to mitigate the effects of intestinal microbiota on metabolization of anthocyanins. Antioxidants 2022, 11, 506. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Kormpa, A.; van der Maarel, M.J. The glycogen of Galdieria sulphuraria as alternative to starch for the production of slowly digestible and resistant glucose polymers. Carbohyd Polym. 2017, 169, 75–82. [Google Scholar] [CrossRef]
- Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Burgad, D.; Landay, A.; Weber, K.M.; Cohen, M.; Ravel, J.; Spear, G.T. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PLoS ONE 2014, 9, e102467. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, J.; Wang, T.; Hu, Q.; Luo, Y. Carboxymethylation of phytoglycogen and its interactions with caseinate for the preparation of nanocomplex. Food Hydrocoll. 2020, 100, 105390. [Google Scholar] [CrossRef]
- Furuyashiki, T.; Ogawa, R.; Nakayama, Y.; Honda, K.; Kamisoyama, H.; Takata, H.; Yasuda, M.; Kuriki, T.; Ashida, H. Enzymatically synthesized glycogen reduces lipid accumulation in diet-induced obese rats. Nutr. Res. 2013, 33, 743–752. [Google Scholar] [CrossRef]
- Kakutani, R.; Adachi, Y.; Kajiura, H.; Takata, H.; Ohno, N.; Kuriki, T. Stimulation of macrophage by enzymatically synthesized glycogen: The relationship between structure and biological activity. Biocatal. Biotransform. 2008, 26, 152–160. [Google Scholar] [CrossRef]
- Powell, P.O.; Sullivan, M.A.; Sweedman, M.C.; Stapleton, D.I.; Hasjim, J.; Gilbert, R.G. Extraction, isolation and characterisation of phytoglycogen from su-1 maize leaves and grain. Carbohydr. Polym. 2014, 101, 423–431. [Google Scholar] [CrossRef]
- Kajiura, H.; Kakutani, R.; Akiyama, T.; Takata, H.; Kuriki, T. A novel enzymatic process for glycogen production. Biocatal. Biotransform. 2008, 26, 133–140. [Google Scholar] [CrossRef]
- Kajiura, H.; Takata, H.; Akiyama, T.; Kakutani, R.; Furuyashiki, T.; Kojima, I.; Harui, T.; Kuriki, T. In vitro synthesis of glycogen: The structure, properties, and physiological function of enzymatically-synthesized glycogen. Biologia 2011, 66, 387–394. [Google Scholar] [CrossRef]
- Ugalde, J.E.; Parodi, A.J.; Ugalde, R.A. De novo synthesis of bacterial glycogen: Agrobacterium tumefaciens glycogen synthase is involved in glucan initiation and elongation. Proc. Natl. Acad. Sci. USA 2003, 100, 10659–10663. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Q.; Tan, X.; Wang, Z.; Wang, M.; Wise, M.J.; Li, C.; Ma, C.; Li, E.; Deng, B. Molecular structure of glycogen in Escherichia coli. Biomacromolecules 2019, 20, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S.; Johnston, M. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 1999, 63, 554–569. [Google Scholar] [CrossRef] [PubMed]
- François, J.; Parrou, J.L. Reserve carbohydrates metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Lomako, J.; Lomako, W.M.; Whelan, W.J. Glycogenin: The primer for mammalian and yeast glycogen synthesis. Biochim. Biophys. Acta-Gen. Subj. 2004, 1673, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Torija, M.-J.; Novo, M.; Lemassu, A.; Wilson, W.; Roach, P.J.; François, J.; Parrou, J.-L. Glycogen synthesis in the absence of glycogenin in the yeast Saccharomyces cerevisiae. FEBS Lett. 2005, 579, 3999–4004. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Q.; Wu, X.; Huang, Y.; Wise, M.J.; Liu, Z.; Wang, W.; Hu, J.; Wang, C. Bioinformatics analysis of metabolism pathways of archaeal energy reserves. Sci. Rep. 2019, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Lillie, S.H.; Pringle, J.R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: Responses to nutrient limitation. J. Bacteriol. 1980, 143, 1384–1394. [Google Scholar] [CrossRef]
- Paulillo, S.C.d.L.; Yokoya, F.; Basso, L.C. Mobilization of endogenous glycogen and trehalose of industrial yeasts. Braz. J. Microbiol. 2003, 34, 249–254. [Google Scholar] [CrossRef]
- Kavšček, M.; Stražar, M.; Curk, T.; Natter, K.; Petrovič, U. Yeast as a cell factory: Current state and perspectives. Microb. Cell Fact. 2015, 14, 94. [Google Scholar] [CrossRef]
- Eom, S.J.; Park, J.T.; Kang, M.C.; Lee, N.H.; Song, K.M. Use of ultrasound treatment to extract mannan polysaccharide from Saccharomyces cerevisiae. J. Food Process Eng. 2022, 45, e14105. [Google Scholar] [CrossRef]
- Krisman, C.R. A method for the colorimetric estimation of glycogen with lodine. Anal. Biochem. 1962, 4, 17–23. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Van Dijck, P.; Colavizza, D.; Smet, P.; Thevelein, J.M. Differential importance of trehalose in stress resistance in fermenting and nonfermenting Saccharomyces cerevisiae cells. Appl. Environ. Microbiol. 1995, 61, 109–115. [Google Scholar] [CrossRef]
- Dake, M.S.; Jadhv, J.P.; Patil, N.B. Variations of two pools of glycogen and carbohydrate in Saccharomyces cerevisiae grown with various ethanol concentrations. J. Ind. Microbiol. Biot. 2010, 37, 701–706. [Google Scholar] [CrossRef]
- Dake, M.S.; Patil, N.B. Effect of ethanol on three pools of glycogen in top flocculating strain of Saccharomyces cerevisiae-3441. Asian J. Microbiol. Biotechnol. Environ. Sci. 2016, 18, 227–232. [Google Scholar]
- Comer, A.D.; Abraham, J.P.; Steiner, A.J.; Korosh, T.C.; Markley, A.L.; Pfleger, B.F. Enhancing photosynthetic production of glycogen-rich biomass for use as a fermentation feedstock. Front. Energy Res. 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Liszkowska, W.; Berlowska, J. Yeast fermentation at low temperatures: Adaptation to changing environmental conditions and formation of volatile compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef] [PubMed]
- Paalman, J.W.; Verwaal, R.; Slofstra, S.H.; Verkleij, A.J.; Boonstra, J.; Verrips, C.T. Trehalose and glycogen accumulation is related to the duration of the G1 phase of Saccharomyces cerevisiae. FEMS Yeast Res. 2003, 3, 261–268. [Google Scholar] [PubMed]
- Joannis-Cassan, C.; Riess, J.; Jolibert, F.; Taillandier, P. Optimization of very high gravity fermentation process for ethanol production from industrial sugar beet syrup. Biomass Bioenergy 2014, 70, 165–173. [Google Scholar] [CrossRef]
- Rivera, E.C.; Yamakawa, C.K.; Saad, M.B.; Atala, D.I.; Ambrosio, W.B.; Bonomi, A.; Junior, J.N.; Rossell, C.E. Effect of temperature on sugarcane ethanol fermentation: Kinetic modeling and validation under very-high-gravity fermentation conditions. Biochem. Eng. J. 2017, 119, 42–51. [Google Scholar] [CrossRef]
- Dakal, T.C.; Solieri, L.; Giudici, P. Adaptive response and tolerance to sugar and salt stress in the food yeast Zygosaccharomyces rouxii. Int. J. Food Microbiol. 2014, 185, 140–157. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Zhu, B.; Zhang, G.; Wei, N. Co-fermentation of cellobiose and xylose by mixed culture of recombinant Saccharomyces cerevisiae and kinetic modeling. PLoS ONE 2018, 13, e0199104. [Google Scholar] [CrossRef] [PubMed]
- Hoang Nguyen Tran, P.; Ko, J.K.; Gong, G.; Um, Y.; Lee, S.M. Improved simultaneous co-fermentation of glucose and xylose by Saccharomyces cerevisiae for efficient lignocellulosic biorefinery. Biotechnol. Biofuels 2020, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ploessl, D.; Shao, Z. Enhancing the co-utilization of biomass-derived mixed sugars by yeasts. Front. Microbiol. 2019, 9, 3264. [Google Scholar] [CrossRef]
- Phowchinda, O.; Strehaiano, P. Utilization of mixed sugars for alcoholic fermentation by Saccharomyces cerevisiae. Sci. Technol. Asia 1999, 4, 23–31. [Google Scholar]
- Gancedo, J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 1998, 62, 334–361. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.; Tatchell, K. Hyperactive glycogen synthase mutants of Saccharomyces cerevisiae suppress the glc7-1 protein phosphatase mutant. J. Bacteriol. 2001, 183, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Duveau, F.; Cordier, C.; Chiron, L.; Le Bec, M.; Pouzet, S.; Seguin, J.; Llamosi, A.; Sorre, B.; Di Meglio, J.M.; Hersen, P. Yeast cell responses and survival during periodic osmotic stress are controlled by glucose availability. eLife 2024, 12, RP88750. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.S.; Hossack, J.; Rose, A. Plasma-membrane lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Arch. Microbiol. 1978, 117, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Nagodawithana, T.; Whitt, J.; Cutaia, A. Study of the feedback effect of ethanol on selected enzymes of the glycolytic pathway. J. Am. Soc. Brew. Chem. 1977, 35, 179–183. [Google Scholar] [CrossRef]
- Abbott, D.A.; Van Den Brink, J.; Minneboo, I.M.; Pronk, J.T.; Van Maris, A.J. Anaerobic homolactate fermentation with Saccharomyces cerevisiae results in depletion of ATP and impaired metabolic activity. FEMS Yeast Res. 2009, 9, 349–357. [Google Scholar] [CrossRef]
- Postmus, J.; Canelas, A.B.; Bouwman, J.; Bakker, B.M.; van Gulik, W.; de Mattos, M.J.T.; Brul, S.; Smits, G.J. Quantitative analysis of the high temperature-induced glycolytic flux increase in Saccharomyces cerevisiae reveals dominant metabolic regulation. J. Biol. Chem. 2008, 283, 23524–23532. [Google Scholar] [CrossRef] [PubMed]
- François, J.; Villanueva, M.E.; Hers, H.G. The control of glycogen metabolism in yeast: 1. Interconversion in vivo of glycogen synthase and glycogen phosphorylase induced by glucose, a nitrogen source or uncouplers. Eur. J. Biochem. 1988, 174, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Noventa-Jordão, M.A.; de Lourdes, M.; Polizeli, T.M.; Beatriz; Bonini, M.; Jorge, J.A.; Terenzi, H.F. Effects of temperature shifts on the activities of Neurospora crassa glycogen synthase, glycogen phosphorylase and trehalose-6-phosphate synthase. FEBS Lett. 1996, 378, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Lip, K.Y.F.; García-Ríos, E.; Costa, C.E.; Guillamón, J.M.; Domingues, L.; Teixeira, J.; van Gulik, W.M.J.B.R. Selection and subsequent physiological characterization of industrial Saccharomyces cerevisiae strains during continuous growth at sub- and- supra optimal temperatures. Biotechnol. Rep. 2020, 26, e00462. [Google Scholar] [CrossRef]
- Furukawa, K.; Heinzle, E.; Dunn, I. Influence of oxygen on the growth of Saccharomyces cerevisiae in continuous culture. Biotechnol. Bioeng. 1983, 25, 2293–2317. [Google Scholar] [CrossRef] [PubMed]
- Dephilippis, R.; Sili, C.; Vincenzini, M. Glycogen and Poly-Beta-Hydroxybutyrate Synthesis in Spirulina-Maxima. J. Gen. Microbiol. 1992, 138, 1623–1628. [Google Scholar] [CrossRef]
- Yao, C.H.; Ai, J.N.; Cao, X.P.; Xue, S.; Zhang, W. Enhancing starch production of a marine green microalga through nutrient limitation. Bioresour. Technol. 2012, 118, 438–444. [Google Scholar] [CrossRef]
- Aikawa, S.; Nishida, A.; Ho, S.H.; Chang, J.S.; Hasunuma, T.; Kondo, A. Glycogen production for biofuels by the euryhaline cyanobacteria Synechococcus sp. strain PCC 7002 from an oceanic environment. Biotechnol. Biofuels 2014, 7, 88. [Google Scholar] [CrossRef]
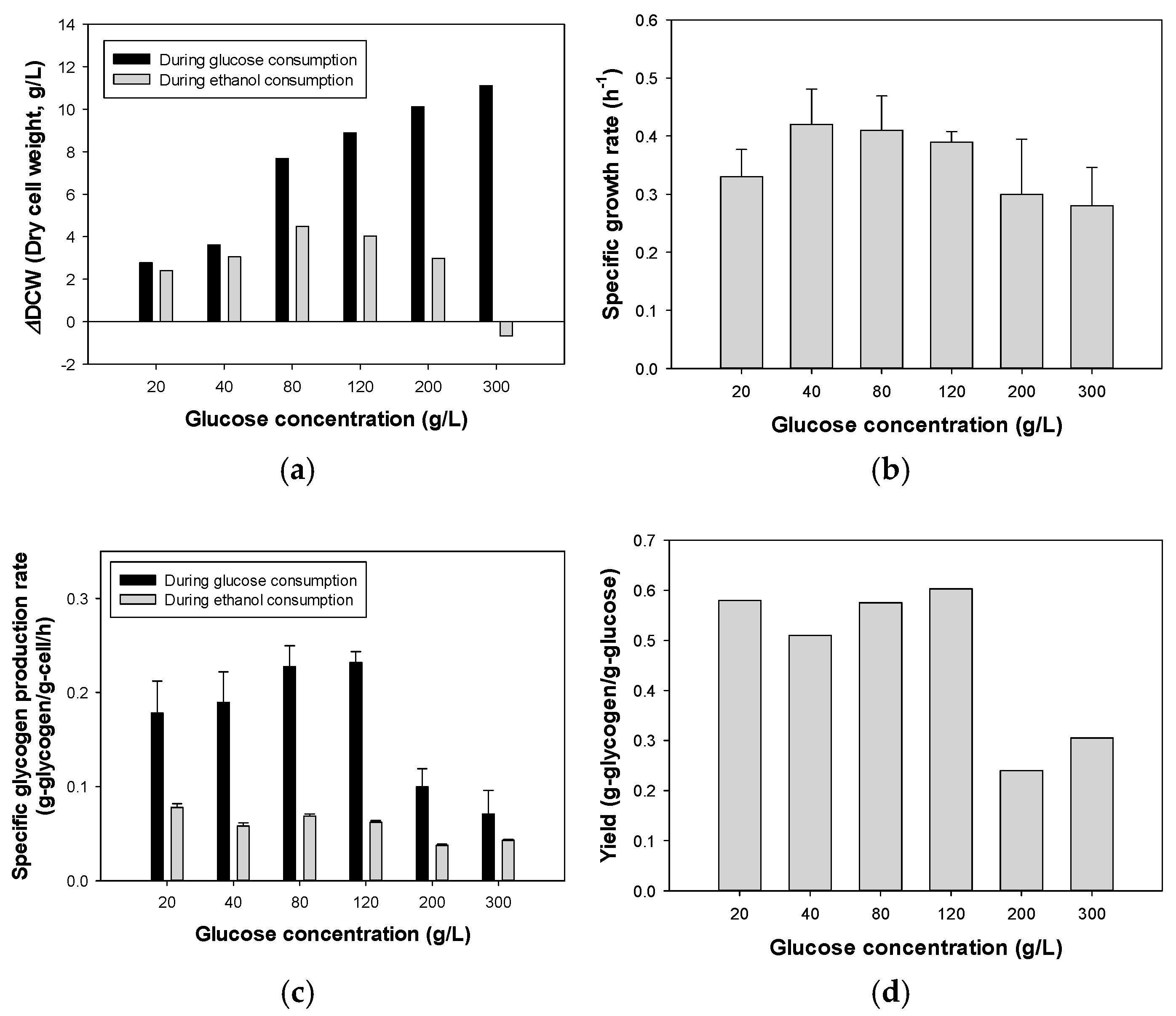
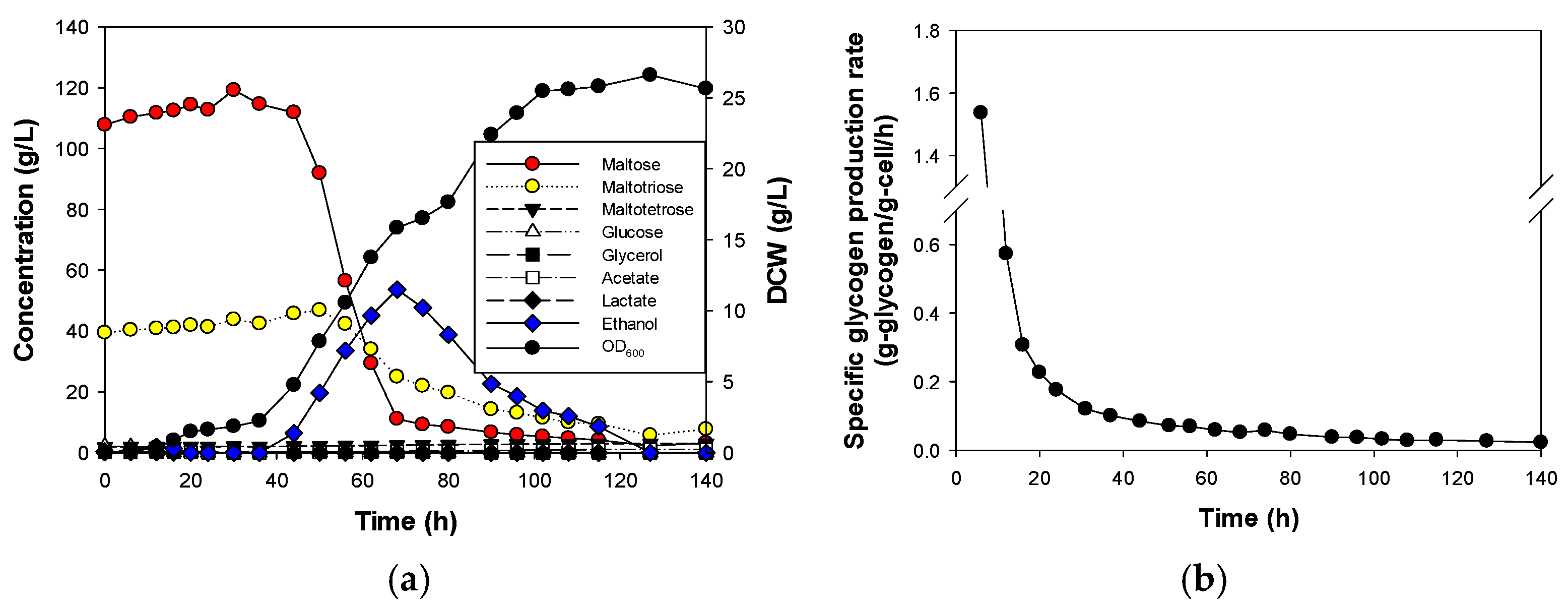

| Initial Ethanol Concentration (g/L) | Dry Cell Weight (g/L) | Glucose Consumption Time (h) | Ethanol Production (g/L) |
|---|---|---|---|
| 0 | 3.668 | 10 | 18.9 |
| 10 | 3.976 | 10 | 12.3 |
| 20 | 5.012 | 14 | 5.8 |
| 30 | 5.376 | 18 | −4.5 |
| Aeration (vvm) | Specific Growth Rate (/h) | Specific Glycogen Production Rate (g-glycogen/g-cell/h) | Glycogen Yield (g-glycogen/g-glucose) |
|---|---|---|---|
| 3 | 0.427 ± 0.005 | 0.199 ± 0.015 | 0.456 ± 0.061 |
| 1 | 0.354 ± 0.003 | 0.164 ± 0.002 | 0.403 ± 0.002 |
| 0 | 0.337 ± 0.019 | 0.174 ± 0.004 | 0.258 ± 0.007 |
| anaerobic | 0.137 ± 0.002 | 0.193 ± 0.001 | 0.278 ± 0.004 |
| Species | Glycogen Content (% of Dry Cell Weight) | Carbon Source | Titer (g/L) | Ref. |
|---|---|---|---|---|
| Arthrospira maxima SOSA 18 | 70.0 | 200 mM HCO3− | 0.91 | [56] |
| Tetraselmis subcordiformis | 47.8 | 3% CO2 | 2.7 | [57] |
| Synechococcus sp. PCC 7002 | 49.8 | 2% CO2 | 3.5 | [58] |
| 75 | 1% CO2 | 3.7 | [35] | |
| S. cerevisiae CEY1 | 69.1 | 120 g/L Glucose | 11.8 * | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Yeo, I.-S.; Mwiti, G.; Dinh, T.N.S.; Kang, H.; Kim, C.S.; Kim, J. Optimization of Microbial Glycogen Production by Saccharomyces cerevisiae CEY1. Fermentation 2024, 10, 388. https://doi.org/10.3390/fermentation10080388
Choi H, Yeo I-S, Mwiti G, Dinh TNS, Kang H, Kim CS, Kim J. Optimization of Microbial Glycogen Production by Saccharomyces cerevisiae CEY1. Fermentation. 2024; 10(8):388. https://doi.org/10.3390/fermentation10080388
Chicago/Turabian StyleChoi, Hyungseok, In-Seok Yeo, Godfrey Mwiti, Toan Nguyen Song Dinh, Hyein Kang, Chang Sup Kim, and Jaehan Kim. 2024. "Optimization of Microbial Glycogen Production by Saccharomyces cerevisiae CEY1" Fermentation 10, no. 8: 388. https://doi.org/10.3390/fermentation10080388






