Optimizing Soaking and Boiling Time in the Development of Tempeh-like Products from Faba Bean (Vicia faba L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Microorganisms
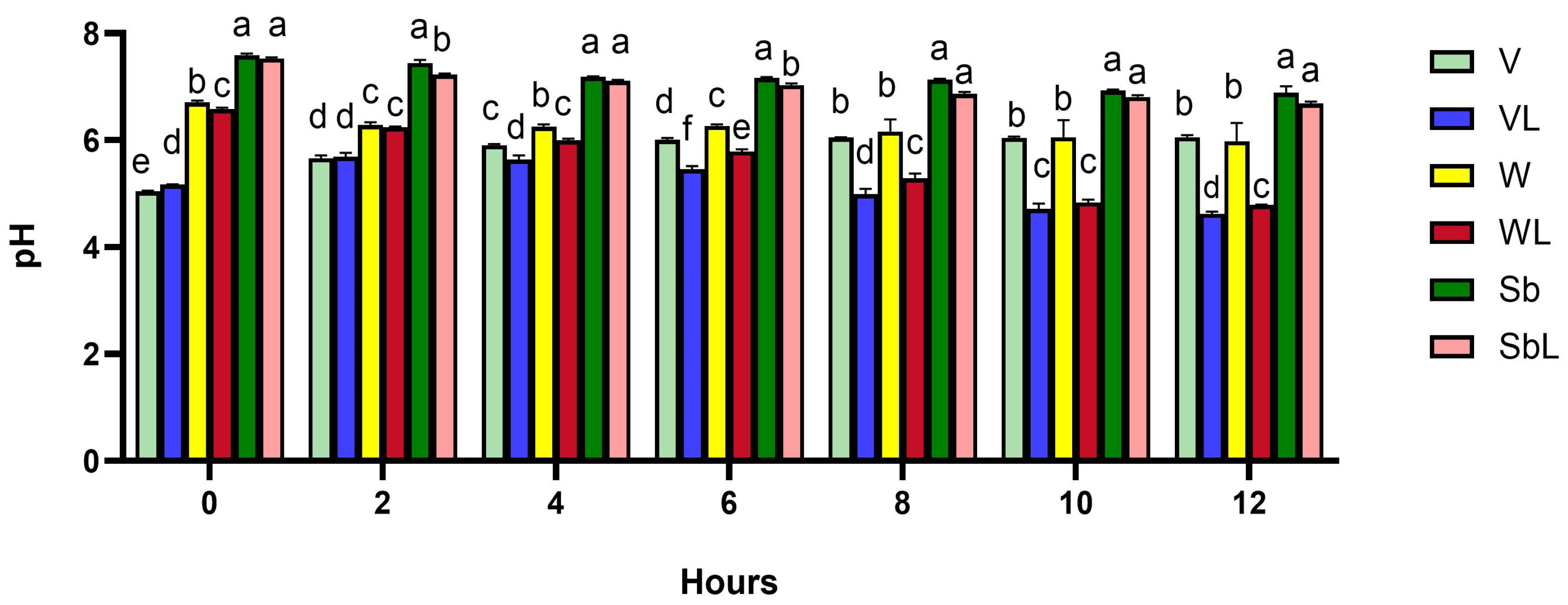
2.2. Soaking and pH Measurement
2.3. Boiling Time
2.4. Preparation of Tempeh
2.5. pH Measurement in Tempeh
2.6. Colour
2.7. Texture Analysis
2.8. Moisture Content
2.9. Statistical Analyses
3. Results and Discussion
3.1. Soaking and pH Measurement
3.2. pH Measurement in Tempeh
3.3. Colour
3.4. Texture Analysis
3.5. Moisture Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niva, M.; Vainio, A. Towards more environmentally sustainable diets? Changes in the consumption of beef and plant- and insect-based protein products in consumer groups in Finland. Meat Sci. 2021, 182, 108635. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Kolehmainen, M.; Landberg, R.; Poutanen, K. Traditional and new sources of grain protein in the healthy and sustainable Nordic diet. J. Cereal Sci. 2022, 105, 103462. [Google Scholar] [CrossRef]
- Cusworth, G.; Garnett, T.; Lorimer, J. Legume dreams: The contested futures of sustainable plant-based food systems in Europe. Glob. Environ. Chang. 2021, 69, 102321. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 16112. [Google Scholar] [CrossRef] [PubMed]
- Verni, M.; Pontonio, E.; Montemurro, M.; Giuseppe Rizzello, C. Fermentation as Strategy for Improving Nutritional, Functional, Technological, and Sensory Properties of Legumes. In Legumes Research—Volume 2; IntechOpen: London, UK, 2022. [Google Scholar]
- Adebo, J.A.; Njobeh, P.B.; Gbashi, S.; Oyedeji, A.B.; Ogundele, O.M.; Oyeyinka, S.A.; Adebo, O.A. Fermentation of Cereals and Legumes: Impact on Nutritional Constituents and Nutrient Bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Rahate, K.A.; Madhumita, M.; Prabhakar, P.K. Nutritional composition, anti-nutritional factors, pretreatments-cum-processing impact and food formulation potential of faba bean (Vicia faba L.): A comprehensive review. LWT 2021, 138, 110796. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legum. Sci. 2022, 4, e129. [Google Scholar] [CrossRef]
- Putri, S.P.; Ikram, M.M.M.; Sato, A.; Dahlan, H.A.; Rahmawati, D.; Ohto, Y.; Fukusaki, E. Application of gas chromatography-mass spectrometry-based metabolomics in food science and technology. J. Biosci. Bioeng. 2022, 133, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Yoon, H.; Shin, M.J.; Lee, S.; Yi, J.; Jeon, Y.A.; Wang, X.; Desta, K.T. Nutrient Levels, Bioactive Metabolite Contents, and Antioxidant Capacities of Faba Beans as Affected by Dehulling. Foods 2023, 12, 4063. [Google Scholar] [CrossRef]
- Kadar, A.D.; Aditiawati, P.; Astawan, M.; Putri, S.P.; Fukusaki, E. Gas chromatography coupled with mass spectrometry-based metabolomics for the classification of tempe from different regions and production processes in Indonesia. J. Biosci. Bioeng. 2018, 126, 411–416. [Google Scholar] [CrossRef]
- Yang, Z.; Piironen, V.; Lampi, A. Lipid-modifying enzymes in oat and faba bean. Food Res. Int. 2017, 100, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Mayer Labba, I.C.; Frøkiær, H.; Sandberg, A.S. Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res. Int. 2021, 140, 110038. [Google Scholar] [CrossRef] [PubMed]
- Wikandari, R.; Millati, R.; Lennartsson, P.R.; Harmayani, E.; Taherzadeh, M.J. Isolation and characterization of zygomycetes fungi from tempe for ethanol production and biomass applications. Appl. Biochem. Biotechnol. 2012, 167, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.Q.; Chin, N.L.; Chong, C.W.; Ripen, A.M.; How, S.; Lim, J.J.L. A review on health benefits and processing of tempeh with outlines on its functional microbes. Future Foods 2024, 9, 100330. [Google Scholar] [CrossRef]
- Pramudito, T.E.; Desai, K.; Voigt, C.; Smid, E.J.; Schols, H.A. Dextran and levan exopolysaccharides from tempeh-associated lactic acid bacteria with bioactivity against enterotoxigenic Escherichia coli (ETEC). Carbohydr. Polym. 2024, 328, 121700. [Google Scholar] [CrossRef] [PubMed]
- Ahnan-Winarno, A.D.; Cordeiro, L.; Winarno, F.G.; Gibbons, J.; Xiao, H. Tempeh: A semicentennial review on its health benefits, fermentation, safety, processing, sustainability, and affordability. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1717–1767. [Google Scholar] [CrossRef] [PubMed]
- Romulo, A.; Surya, R. Tempe: A traditional fermented food of Indonesia and its health benefits. Int. J. Gastron. Food Sci. 2021, 26, 100413. [Google Scholar] [CrossRef]
- Erkan, S.B.; Gürler, H.N.; Bilgin, D.G.; Germec, M.; Turhan, I. Production and characterization of tempehs from different sources of legume by Rhizopus oligosporus. LWT-Food Sci. Technol. 2020, 119, 108880. [Google Scholar] [CrossRef]
- Vadivel, V.; Pugalenthi, M. Effect of soaking in sodium bicarbonate solution followed by autoclaving on the nutritional and antinutritional properties of velvet bean seeds. J. Food Process. Preserv. 2009, 33, 60–73. [Google Scholar] [CrossRef]
- Torres, J.; Rutherfurd, S.M.; Muñoz, L.S.; Peters, M.; Montoya, C.A. The impact of heating and soaking on the in vitro enzymatic hydrolysis of protein varies in different species of tropical legumes. Food Chem. 2016, 194, 377–382. [Google Scholar] [CrossRef]
- Lestienne, I.; Icard-Vernière, C.; Mouquet, C.; Picq, C.; Trèche, S. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem. 2005, 89, 421–425. [Google Scholar] [CrossRef]
- Murphy, R.M.; Stanczyk, J.C.; Huang, F.; Loewen, M.E.; Yang, T.C.; Loewen, M.C. Reduction of phenolics in faba bean meal using recombinantly produced and purified Bacillus ligniniphilus catechol 2,3-dioxygenase. Bioresour. Bioprocess. 2023, 10, 13. [Google Scholar] [CrossRef]
- Jiménez, N.; Esteban-Torres, M.; Mancheño, J.M.; de Las Rivas, B.; Muñoz, R. Tannin Degradation by a Novel Tannase Enzyme Present in Some Lactobacillus plantarum Strains. Appl. Environ. Microbiol. 2014, 80, 2991–2997. [Google Scholar] [CrossRef]
- Adeyemo, S.M.; Onilude, A.A. Enzymatic Reduction of Anti-nutritional Factors in Fermenting Soybeans by Lactobacillus plantarum Isolates from Fermenting Cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Feng, X.M.; Eriksson, A.; Schnurer, J. Growth of lactic acid bacteria and Rhizopus oligosporus during barley tempeh fermentation. Int. J. Food Microbiol. 2005, 104, 249–256. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Coda, R.; Wang, Y.; Verni, M.; Kajala, I.; Katina, K.; Laitila, A. Characterization of indigenous Pediococcus pentosaceus, Leuconostoc kimchii, Weissella cibaria and Weissella confusa for faba bean bioprocessing. Int. J. Food Microbiol. 2019, 302, 24–34. [Google Scholar] [CrossRef]
- Ziarno, M.; Bryś, J.; Parzyszek, M.; Veber, A. Effect of lactic acid bacteria on the lipid profile of bean-based plant substitute of fermented milk. Microorganisms 2020, 8, 1348. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Losito, I.; Facchini, L.; Katina, K.; Palmisano, F.; Gobbetti, M.; Coda, R. Degradation of vicine, convicine and their aglycones during fermentation of faba bean flour. Sci. Rep. 2016, 6, 32452. [Google Scholar] [CrossRef]
- Verni, M.; Verardo, V.; Rizzello, C.G. How fermentation affects the antioxidant properties of cereals and legumes. Foods 2019, 8, 362. [Google Scholar] [CrossRef]
- Coda, R.; Melama, L.; Rizzello, C.G.; Curiel, J.A.; Sibakov, J.; Holopainen, U.; Pulkkinen, M.; Sozer, N. Effect of air classification and fermentation by Lactobacillus plantarum VTT E-133328 on faba bean (Vicia faba L.) flour nutritional properties. Int. J. Food Microbiol. 2015, 193, 34–42. [Google Scholar] [CrossRef]
- Rahmawati, D.; Astawan, M.; Putri, S.P.; Fukusaki, E. Gas chromatography-mass spectrometry-based metabolite profiling and sensory profile of Indonesian fermented food (tempe) from various legumes. J. Biosci. Bioeng. 2021, 132, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Kianjam, M.; Pontonio, E.; Verni, M.; Di Cagno, R.; Katina, K.; Rizzello, C.G.; Gobbetti, M. Sourdough-type propagation of faba bean flour: Dynamics of microbial consortia and biochemical implications. Int. J. Food Microbiol. 2017, 248, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kinyanjui, P.K.; Njoroge, D.M.; Makokha, A.O.; Christiaens, S.; Ndaka, D.S.; Hendrickx, M. Hydration properties and texture fingerprints of easy- and hard-to-cook bean varieties. Food Sci. Nutr. 2015, 3, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Świeca, M.; Gawlik-Dziki, U.; Złotek, U.; Baraniak, B. Nutritional quality, phenolics, and antioxidant capacity of mung bean paste obtained from seeds soaked in sodium bicarbonate. LWT 2018, 97, 456–461. [Google Scholar] [CrossRef]
- Huma, N.; Anjum, F.M.; Sehar, S.; Issa Khan, M.; Hussain, S. Effect of soaking and cooking on nutritional quality and safety of legumes. Nutr. Food Sci. 2008, 38, 570–577. [Google Scholar] [CrossRef]
- Schoeninger, V.; Coelho, S.R.M.; Christ, D.; Sampaio, S.C. Processing parameter optimization for obtaining dry beans with reduced cooking time. LWT 2014, 56, 49–57. [Google Scholar] [CrossRef]
- Kahala, M.; Ikonen, I.; Blasco, L.; Bragge, R.; Pihlava, J.-M.; Nurmi, M.; Pihlanto, A. Effect of Lactic Acid Bacteria on the Level of Antinutrients in Pulses: A Case Study of a Fermented Faba Bean–Oat Product. Foods 2023, 12, 3922. [Google Scholar] [CrossRef]
- Nyanga-Koumou, A.P.; Ouoba, L.I.I.; Kobawila, S.C.; Louembe, D. Response mechanisms of lactic acid bacteria to alkaline environments: A review. Crit. Rev. Microbiol. 2012, 38, 185–190. [Google Scholar] [CrossRef]
- Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation 2022, 8, 244. [Google Scholar] [CrossRef]
- Verni, M.; De Mastro, G.; De Cillis, F.; Gobbetti, M.; Rizzello, C.G. Lactic acid bacteria fermentation to exploit the nutritional potential of Mediterranean faba bean local biotypes. Food Res. Int. 2019, 125, 108571. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, I.; Pontonio, E.; Gobbetti, M.; Rizzello, C.G. Nutritional and functional effects of the lactic acid bacteria fermentation on gelatinized legume flours. Int. J. Food Microbiol. 2020, 316, 108426. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, J.; Pan, D.; Wu, Z.; Guo, Y.; Zeng, X.; Lian, L. Metabolomics analysis of Lactobacillus plantarum ATCC 14917 adhesion activity under initial acid and alkali stress. PLoS ONE 2018, 13, e0196231. [Google Scholar] [CrossRef] [PubMed]
- Seumahu, C.A.; Suwanto, A.; Rusmana, I.; Solihin, D.D. Bacterial and Fungal Communities in Tempeh as Reveal by Amplified Ribosomal Intergenic Sequence Analysis. HAYATI J. Biosci. 2013, 20, 65–71. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and Functional Properties of Fermented Soybean (Tempeh) by Using Rhizopus oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef]
- Verni, M.; Coda, R.; Rizzello, C.G. The Use of Faba Bean Flour to Improve the Nutritional and Functional Features of Cereal-Based Foods: Perspectives and Future Strategies. In Flour and Breads and their Fortification in Health and Disease Prevention, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128146392. [Google Scholar]
- Bakhsh, A.; Lee, S.J.; Lee, E.Y.; Sabikun, N.; Hwang, Y.H.; Joo, S.T. A novel approach for tuning the physicochemical, textural, and sensory characteristics of plant-based meat analogs with different levels of methylcellulose concentration. Foods 2021, 10, 560. [Google Scholar] [CrossRef]
- 313R-2013; Regional Standard for Tempe (CODEX STAN 313R-2013). Codex Alimentarius Commission: Rome, Italy, 2017; p. 3.




| Media Code | Media | Composition | Initial pH |
|---|---|---|---|
| V | Vinegar | 250 mL of tap water + 1 mL vinegar | 5.0 ± 0.02 |
| VL | Vinegar + L. plantarum | 250 mL of tap water + 1 mL vinegar + 0.1 g L. plantarum | 5.2 ± 0.01 |
| W | Water | 250 mL of tap water | 6.7 ± 0.03 |
| WL | Water + L. plantarum | 250 mL of tap water + 0.1 g L. plantarum | 6.6 ± 0.03 |
| Sb | Sodium bicarbonate | 250 mL of tap water + 1 g Sodium bicarbonate | 7.6 ± 0.03 |
| SbL | Sodium bicarbonate + L. plantarum | 250 mL of tap water + 1 g Sodium bicarbonate + 0.1 g L. plantarum | 7.5 ± 0.03 |
| Samples | L* | a* | b* |
|---|---|---|---|
| V8 | 85.2 ± 1.63 bc | −11.2 ± 0.45 e | 37.9 ± 0.09 cde |
| VL8 | 68.4 ± 1.32 h | −5.64 ± 1.31 ab | 40.2 ± 0.70 ab |
| V16 | 74.7 ± 1.34 ab | −7.67 ± 0.49 c | 41.3 ± 1.90 a |
| VL16 | 65.0 ± 2.51 cd | −4.86 ± 1.42 a | 39.7 ± 1.69 abcd |
| W8 | 84.0 ± 3.60 ef | −10.7 ± 0.96 e | 38.2 ± 0.75 bcde |
| WL8 | 72.6 ± 1.40 g | −7.80 ± 0.58 cd | 37.8 ± 0.60 cde |
| W16 | 81.1 ± 1.80 de | −9.76 ± 0.75 de | 37.7 ± 0.75 e |
| WL16 | 72.3 ± 3.38 fg | −7.58 ± 1.60 bc | 39.3 ± 1.65 abcde |
| Sb8 | 85.5 ± 1.60 a | −11.5 ± 0.64 e | 37.7 ± 0.66 abc |
| SbL8 | 60.7 ± 1.31 a | −4.91 ± 0.70 a | 33.7 ± 0.31 f |
| Sb16 | 78.6 ± 4.03 a | −8.26 ± 1.38 cd | 39.9 ± 1.05 abc |
| SbL16 | NA | NA | NA |
| Sample | Moisture (%) | Hardness (N) | Adhesiveness (N.s) | Cohesiveness | Springiness (%) | Chewiness (N) |
|---|---|---|---|---|---|---|
| V8 * | 64.6 ± 0.94 ab | 21.4 ± 1.91 ab | −0.08 ± 0.01 ab | 0.26 ± 0.03 e | 48.8 ± 1.24 a | 2.76 ± 0.41 abcd |
| VL8 * | 62.1 ± 1.81 b | 15.4 ± 1.85 bcd | −0.20 ± 0.05 bc | 0.33 ± 0.02 abcd | 39.9 ± 1.06 b | 2.03 ± 0.16 bcd |
| V16 | 69.5 ± 0.39 a | 8.5 ± 1.48 d | −0.05 ± 0.03 a | 0.31 ± 0.00 de | 50.3 ± 2.35 a | 1.29 ± 0.21 d |
| VL16 | 64.4 ± 2.98 ab | 27.6 ± 1.20 a | −0.16 ± 0.08 abc | 0.38 ± 0.00 ab | 43.0 ± 1.42 ab | 4.61 ± 0.10 cd |
| W8 * | 63.5 ± 3.00 ab | 26.3 ± 2.38 a | −0.14 ± 0.06 abc | 0.30 ± 0.03 de | 49.2 ± 3.09 a | 3.96 ± 0.39 ab |
| WL8 * | 63.3 ± 2.94 b | 24.7 ± 5.36 ab | −0.16 ± 0.03 abc | 0.39 ± 0.02 a | 42.4 ± 2.69 ab | 4.19 ± 1.21 ab |
| W16 | 66.9 ± 0.85 ab | 23.8 ± 4.94 ab | −0.09 ± 0.04 ab | 0.33 ± 0.02 bcd | 45.7 ± 2.00 ab | 3.60 ± 1.01 abc |
| WL16 | 67.4 ± 1.38 ab | 22.3 ± 5.92 ab | −0.19 ± 0.04 bc | 0.38 ± 0.02 abc | 43.8 ± 2.51 ab | 3.82 ± 1.32 abc |
| Sb8 | 67.1 ± 3.68 ab | 27.8 ± 3.77 a | −0.09 ± 0.06 ab | 0.32 ± 0.03 de | 46.1 ± 2.63 ab | 4.11 ± 1.13 ab |
| SbL8 | 63.9 ± 1.09 ab | 18.8 ± 2.32 abc | −0.23 ± 0.04 c | 0.34 ± 0.01 abcd | 43.7 ± 2.54 ab | 2.87 ± 0.25 abcd |
| Sb16 * | 67.9 ± 1.26 ab | 10.3 ± 1.83 cd | −0.1 ± 0.01 abc | 0.30 ± 0.01 cd | 50.3 ± 6.89 a | 1.72 ± 0.338 cd |
| SbL16 * | 65.9 ± 0.15 ab | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez Castaneda, L.A.; Auer, J.; Leong, S.-l.L.; Newson, W.R.; Passoth, V.; Langton, M.; Zamaratskaia, G. Optimizing Soaking and Boiling Time in the Development of Tempeh-like Products from Faba Bean (Vicia faba L.). Fermentation 2024, 10, 407. https://doi.org/10.3390/fermentation10080407
Fernandez Castaneda LA, Auer J, Leong S-lL, Newson WR, Passoth V, Langton M, Zamaratskaia G. Optimizing Soaking and Boiling Time in the Development of Tempeh-like Products from Faba Bean (Vicia faba L.). Fermentation. 2024; 10(8):407. https://doi.org/10.3390/fermentation10080407
Chicago/Turabian StyleFernandez Castaneda, Laura Alejandra, Jaqueline Auer, Su-lin L. Leong, William R. Newson, Volkmar Passoth, Maud Langton, and Galia Zamaratskaia. 2024. "Optimizing Soaking and Boiling Time in the Development of Tempeh-like Products from Faba Bean (Vicia faba L.)" Fermentation 10, no. 8: 407. https://doi.org/10.3390/fermentation10080407







