Investigation of Biofilm Formation on Air Cathodes with Quaternary Ammonium Compounds in Microbial Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Gas Diffusion Electrodes
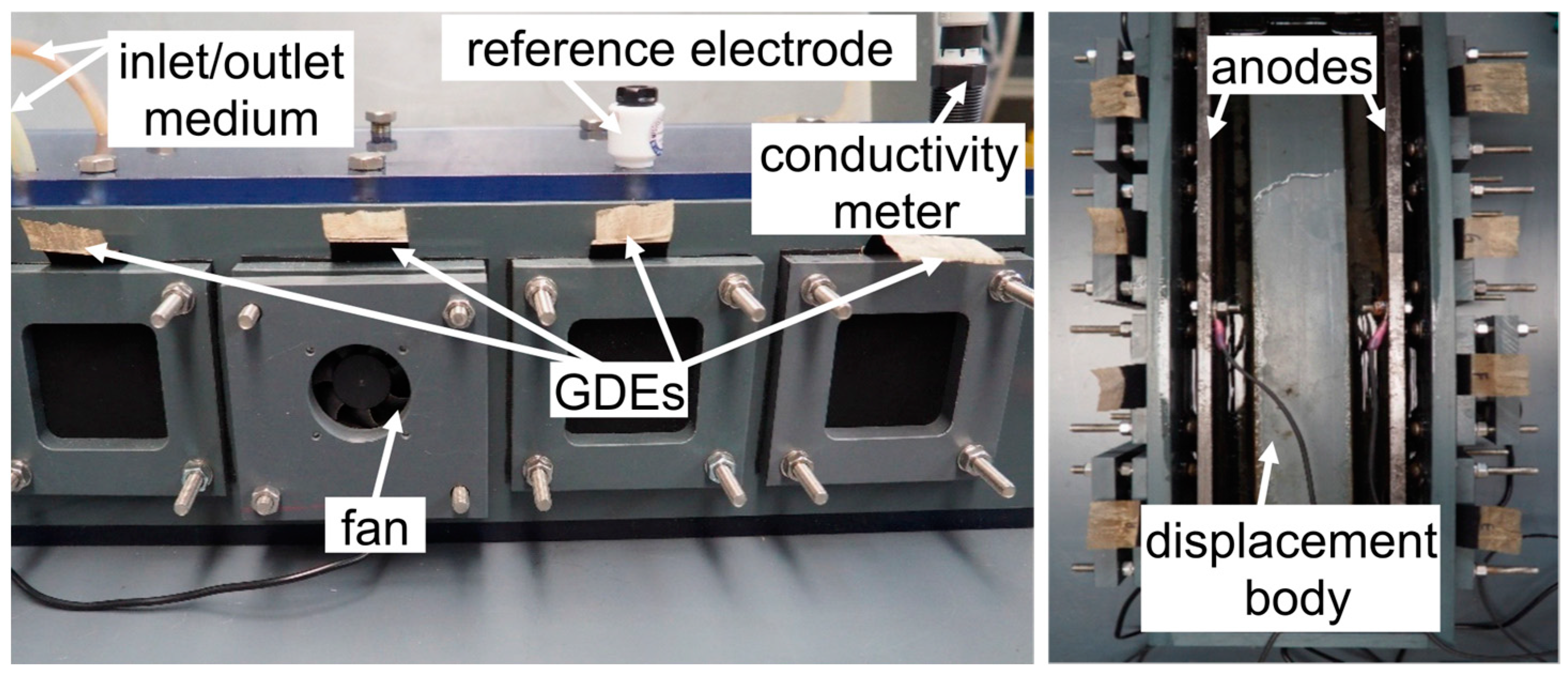
2.2. Reactor Design and Components
2.3. Measurement and Calculation
3. Results
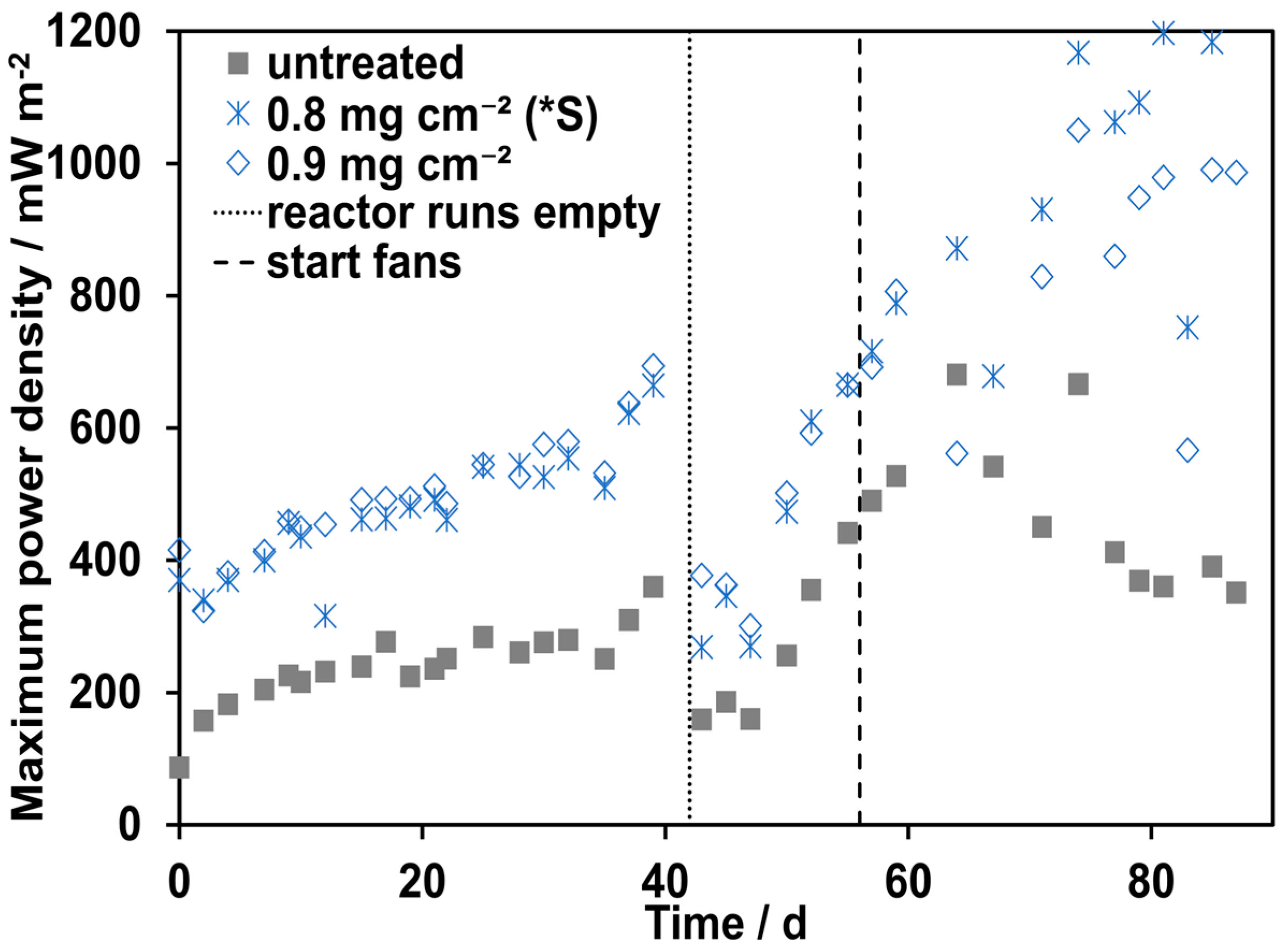
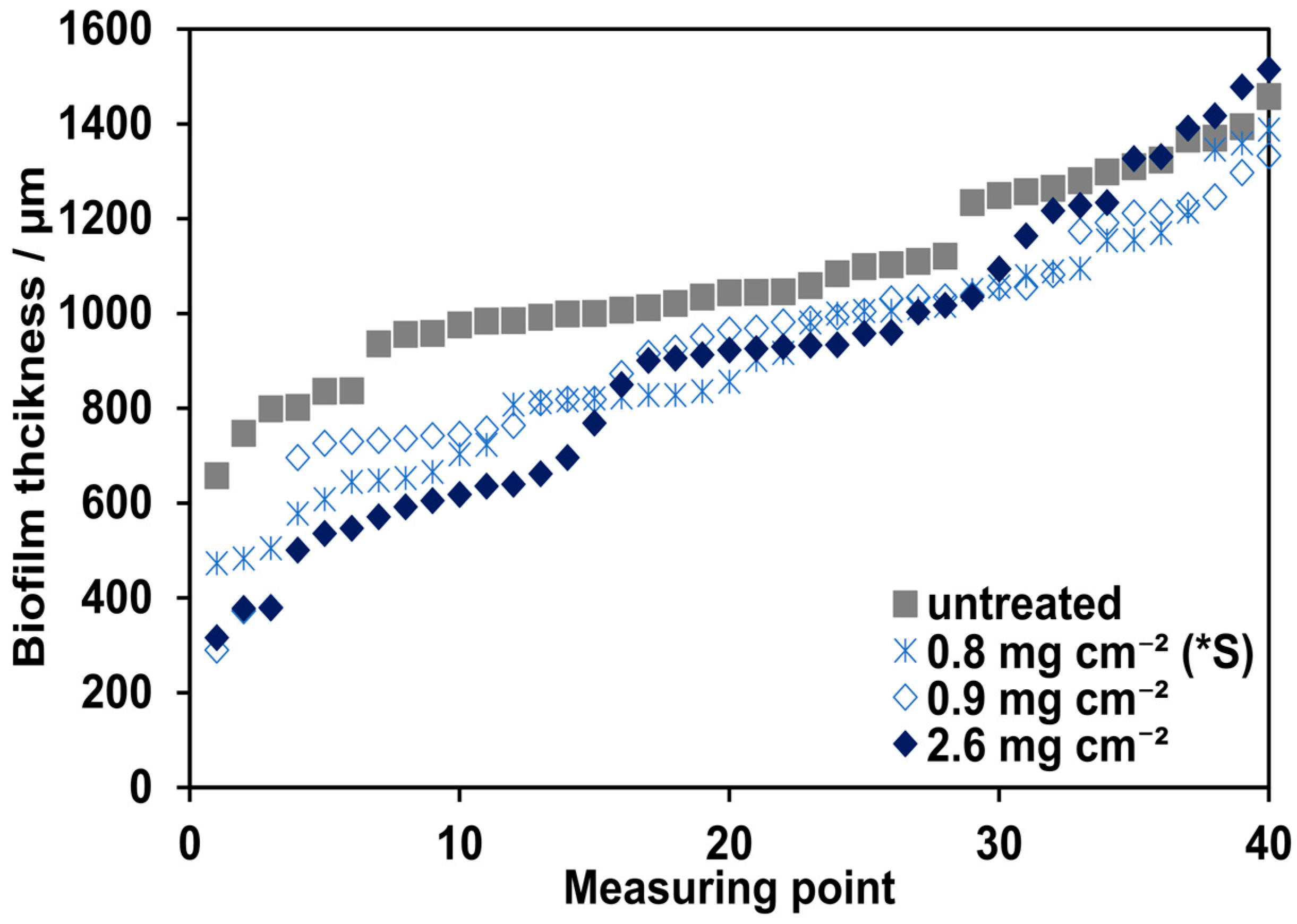
3.1. Influence of Different Ionomer Loadings
3.2. Influence of the Application Method and Additional Aeration
3.3. Influence of the Biofilm on the Electrical Resistance of the System
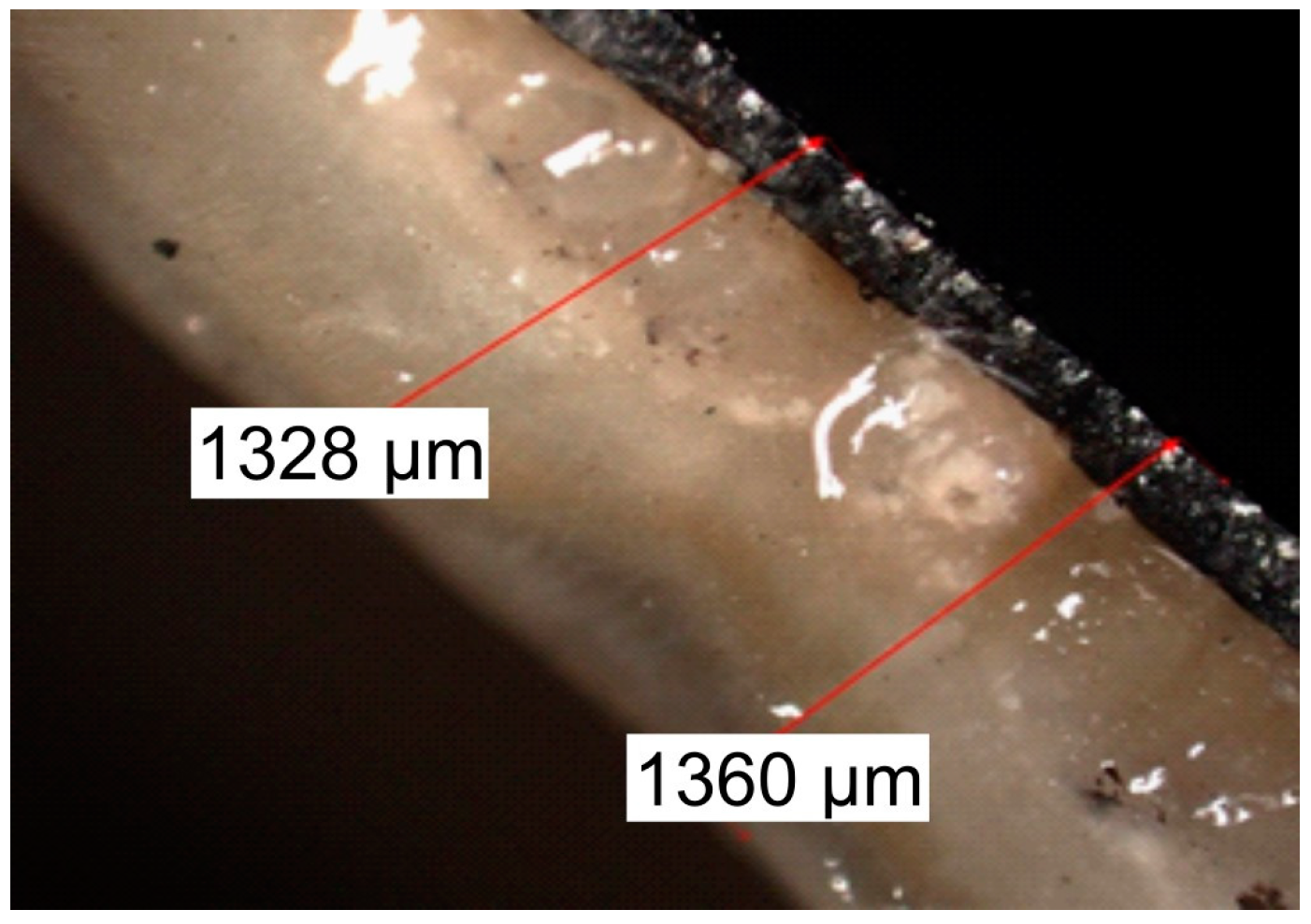
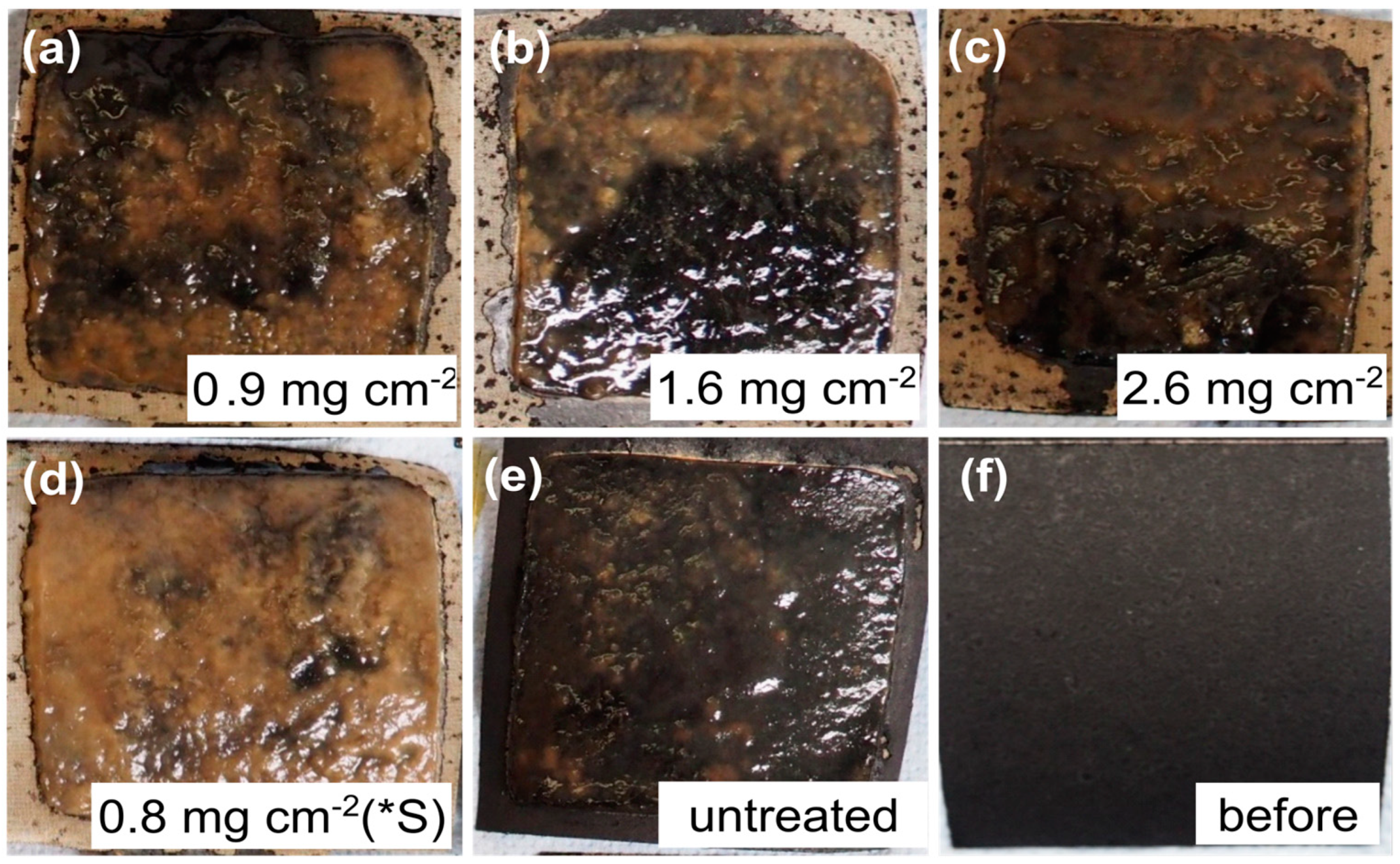
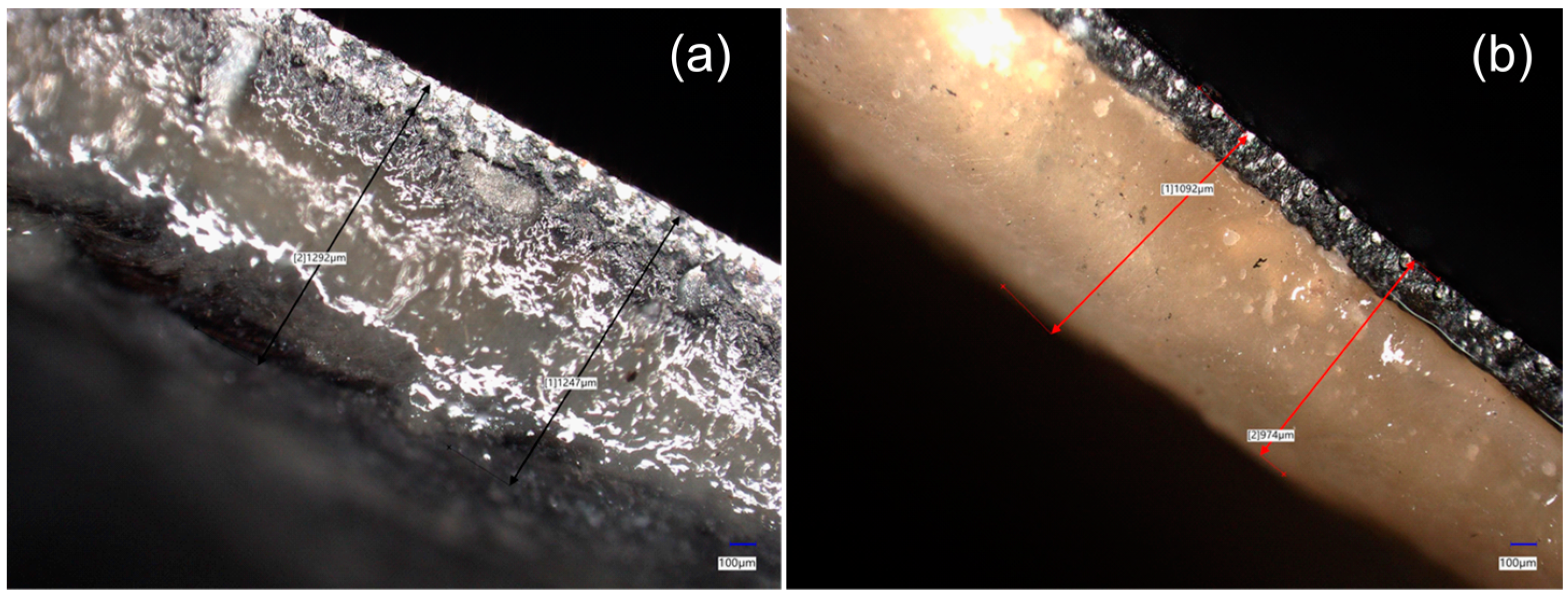
3.4. Evaluation of the Biofilm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Köroğlu, E.Q.; Yörüklü, H.C.; Demir, A.; Ozkaya, B. Scale-Up and Commercialization Issues of the MFCs: Challenges and Implications. In Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 565–580. [Google Scholar]
- Gil, G.; Chang, I.; Kim, B.H.; Kim, M.; Jang, J.; Park, H.S.; Kim, H.J. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Carver, S.M.; Christya, A.D.; Tuovinen, O.H. Cathodic limitations in microbial fuel cells: An overview. J. Power Sources 2008, 180, 683–694. [Google Scholar] [CrossRef]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the internal resistance distribution of microbial fuel cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, S.; Yin, L.; Sun, Y.; Yu, L. Influence of soluble microbial products on the long-term stability of air cathodes in microbial fuel cells. Electrochim. Acta 2018, 261, 557–564. [Google Scholar] [CrossRef]
- Zhang, F.; Pant, D.; Logan, B.E. Long-term performance of activated carbon air cathodes with different diffusion layer porosities in microbial fuel cells. Biosens. Bioelectron. 2011, 30, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Fan, Y.; Wang, L.; Long, F.; Deng, D.; Liu, H. Accelerated tests for evaluating the air-cathode aging in microbial fuel cells. Bioresour. Technol. 2020, 297, 122479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wang, F.; Yu, Q.; Scott, K.; Wang, X.; Diao, G. Durability and regeneration of activated carbon air-cathodes in long-term operated microbial fuel cells. J. Power Sources 2017, 360, 21–27. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, S.; Sun, D.; Huang, H.; Chen, J.; Cen, K. Inhibition of microbial growth on air cathodes of single chamber microbial fuel cells by incorporating enrofloxacin into the catalyst layer. Biosens. Bioelectron. 2015, 72, 44–50. [Google Scholar] [CrossRef]
- An, J.; Li, N.; Wan, L.; Zhou, L.; Du, Q.; Li, T.; Wang, X. Electric field induced salt precipitation into activated carbon air-cathode causes power decay in microbial fuel cells. Water Res. 2017, 123, 369–377. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Qu, Y.; Wang, H.; Feng, Y. Analysis of the effect of biofouling distribution on electricity output in microbial fuel cells. RSC Adv. 2016, 33, 27494–27500. [Google Scholar] [CrossRef]
- Kiely, P.D.; Rader, G.; Regan, J.M.; Logan, B.E. Long-term cathode performance and the microbial communities that develop in microbial fuel cells fed different fermentation endproduct. Bioresour. Technol. 2011, 102, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Ghangrekar, M.M. Preparation of a fouling-resistant sustainable cathode for a single-chambered microbial fuel cell. Water Sci. Technol. 2014, 69, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Oliot, M.; Etcheverry, L.; Bergel, A. Removable air-cathode to overcome cathode biofouling in microbial fuel cells. Bioresour. Technol. 2016, 221, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pant, D.; Zhang, J.; Liu, W.; He, B.; Logan, B.E. Long-Term Performance of Chemically and Physically Modified Activated Carbons in Air Cathodes of Microbial Fuel Cells. ChemElectroChem 2014, 1, 1859–1866. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, H.; Qu, Y.; Zhang, J.; Feng, Y. Enhanced catalytic activity and inhibited biofouling of cathode in microbial fuel cells through controlling hydrophilic property. J. Power Source 2016, 332, 454–460. [Google Scholar] [CrossRef]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/nanosilver composite coatings for antibacterial applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Noori, M.T.; Tiwari, B.R.; Mukherjee, C.K.; Ghangrekar, M.M. Enhancing the performance of microbial fuel cell using Ag-Pt bimetallic alloy as cathode catalyst and anti-biofouling agent. Int. J. Hydrogen Energy 2018, 43, 19650–19660. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, Y.; Duan, Y.; Xu, X.; LV, Y.; Yang, L.; Zou, J. Biofouling inhibition on nano-silver/ferrous sulfide/partly-graphitized carbon cathode with enhanced catalytic activity and durability for microbial fuel cells. Carbon 2017, 119, 394–402. [Google Scholar] [CrossRef]
- Noori, M.T.; Jain, S.C.; Ghangrekar, M.M.; Mukherjee, C.K. Biofouling inhibition and enhancing performance of microbial fuel cell using silver nano-particles as fungicide and cathode catalyst. Bioresour. Technol. 2016, 220, 183–189. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, S.; Zhang, S.; Zheng, S.; Xu, Y.; Nie, W.; Zhou, Y.; Xu, T.; Chen, P. Quaternary Ammonium Salts: Insights into Synthesis and New Directions in Antibacterial Applications. Bioconjugate Chem. 2023, 34, 302–325. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Tang, C.Y.; Ma, J.; Liu, M.; Ping, M.; Chen, M.; Wu, Z. Modification of microfiltration membranes by alkoxysilane polycondensation induced quaternary ammonium compounds grafting for biofouling mitigation. J. Membr. Sci. 2018, 549, 165–172. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, B.; Yu, B.; Ma, B.; Hu, C.; Ulbricht, M.; Qu, J. Maintaining Antibacterial Activity against Biofouling Using a Quaternary Ammonium Membrane Coupling with Electrorepulsion. Environ. Sci. Technol. 2023, 57, 1520–1528. [Google Scholar] [CrossRef]
- Haupt, D.R.; Landwehr, L.; Schumann, R.; Hahn, L.; Issa, M.; Coskun, C.; Kunz, U.; Sievers, M. A New Reactor Concept for Single-Chamber Microbial Fuel Cells and Possible Anti-Fouling Strategies for Long-Term Operation. Microorganisms 2022, 10, 2421. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Y.; An, J.; Feng, C.; Wang, X. Bifunctional quaternary ammonium compounds to inhibit biofilm growth and enhance performance for activated carbon air-cathode in microbial fuel cells. J. Power Source 2014, 272, 895–899. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Zhuang, L.; Li, W.; Zhou, S.; Zhang, J. Manganese dioxide as an alternative cathodic catalyst to platinum in microbial fuel cells. Biosens. Bioelectron. 2009, 24, 2825–2829. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Tartakovsky, B.; Savadogo, O. Cathode materials evaluation in microbial fuel cells: A comparison of carbon, Mn2O3, Fe2O3 and platinum materials. Electrochim. Acta 2011, 58, 58–66. [Google Scholar] [CrossRef]
- Roche, I.; Katuri, K.; Scott, K. A microbial fuel cell using manganese oxide oxygen reduction catalysts. J. Appl. Electrochem. 2009, 40, 13–21. [Google Scholar] [CrossRef]
- Jiang, B.; Muddemann, T.; Kunz, U.; Bormann, H.; Niedermeiser, M.; Haupt, D.; Schläfer, O.; Sievers, M. Evaluation of Microbial Fuel Cells with Graphite Plus MnO2 and MoS2 Paints as Oxygen Reduction Cathode Catalyst. J. Electrochem. Soc. 2017, 164, 3083–3090. [Google Scholar] [CrossRef]
- Kenawy, E.; Worley, S.D.M.; Broughton, R. The Chemistry and Applications of Antimicrobial Polymers: A State-of-the-Art Review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landwehr, L.; Haupt, D.R.; Sievers, M.; Kunz, U. Investigation of Biofilm Formation on Air Cathodes with Quaternary Ammonium Compounds in Microbial Fuel Cells. Fermentation 2024, 10, 408. https://doi.org/10.3390/fermentation10080408
Landwehr L, Haupt DR, Sievers M, Kunz U. Investigation of Biofilm Formation on Air Cathodes with Quaternary Ammonium Compounds in Microbial Fuel Cells. Fermentation. 2024; 10(8):408. https://doi.org/10.3390/fermentation10080408
Chicago/Turabian StyleLandwehr, Laura, Dennis R. Haupt, Michael Sievers, and Ulrich Kunz. 2024. "Investigation of Biofilm Formation on Air Cathodes with Quaternary Ammonium Compounds in Microbial Fuel Cells" Fermentation 10, no. 8: 408. https://doi.org/10.3390/fermentation10080408
APA StyleLandwehr, L., Haupt, D. R., Sievers, M., & Kunz, U. (2024). Investigation of Biofilm Formation on Air Cathodes with Quaternary Ammonium Compounds in Microbial Fuel Cells. Fermentation, 10(8), 408. https://doi.org/10.3390/fermentation10080408





