The Bioaugmentation of Electroactive Microorganisms Enhances Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
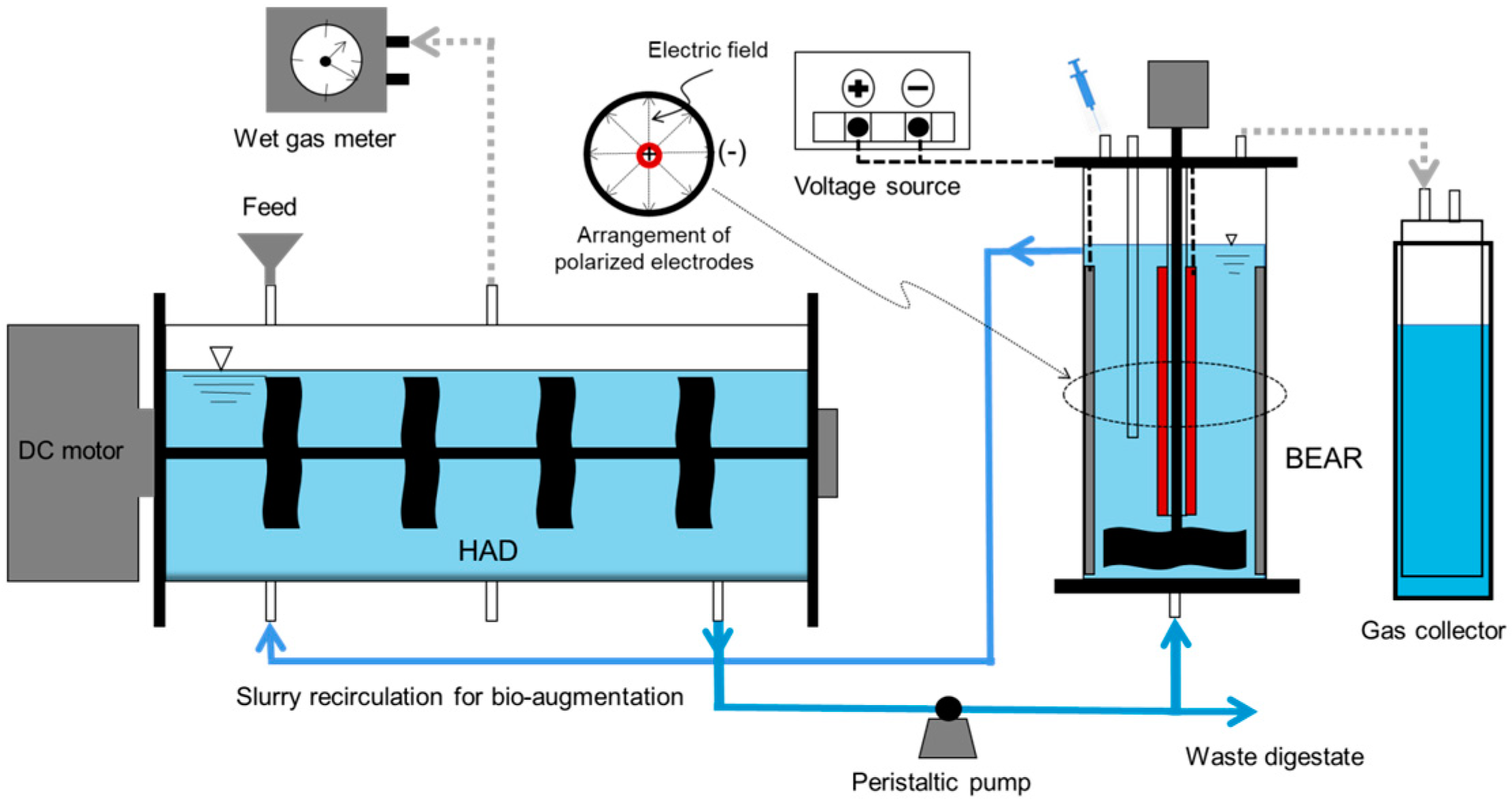
2.2. Set-Up for the Anaerobic Digestion System and Experimental Design
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. Enrichment of Electroactive Microorganisms
3.2. Bio-Augmented Anaerobic Digestion with Electroactive Microorganisms
3.3. Correlation Analysis for Enrichment of EAMs and Its Impact
3.4. Optimal Manipulated Variables
3.5. Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Liu, Y.; Yang, X.; Zhang, J.; Lu, B.; Chen, R. Kinetic and Thermodynamic Effects of Temperature on Methanogenic Degradation of Acetate, Propionate, Butyrate and Valerate. Chem. Eng. 2020, 396, 125366. [Google Scholar] [CrossRef]
- Saha, S.; Kurade, M.B.; Ha, G.-S.; Lee, S.S.; Roh, H.-S.; Park, Y.-K.; Jeon, B.-H. Syntrophic Metabolism Facilitates Methanosarcina-Led Methanation in the Anaerobic Digestion of Lipidic Slaughterhouse Waste. Bioresour. Technol. 2021, 335, 125250. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.-C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Polarized Electrode Enhances Biological Direct Interspecies Electron Transfer for Methane Production in Upflow Anaerobic Bioelectrochemical Reactor. Chemosphere 2018, 204, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Nzila, A.; Razzak, S.; Zhu, J. Bioaugmentation: An Emerging Strategy of Industrial Wastewater Treatment for Reuse and Discharge. Int. J. Environ. Res. Public Health 2016, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Song, Y.-C.; Yoo, K.; Kuppanan, N.; Subudhi, S.; Lal, B. Bioelectrochemical Enhancement of Direct Interspecies Electron Transfer in Upflow Anaerobic Reactor with Effluent Recirculation for Acidic Distillery Wastewater. Bioresour. Technol. 2017, 241, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Ai, W.; Dong, W. Lignocellulose Degradation, Biogas Production and Characteristics of the Microbial Community in Solid-State Anaerobic Digestion of Wheat Straw Waste. Life Sci. Space Res. 2022, 32, 1–7. [Google Scholar] [CrossRef]
- Yu, H.; Song, Y.-C.; Bae, B.-U.; Li, J.; Jang, S.-H. Electrostatic Fields Promote Methanogenesis More than Polarized Bioelectrodes in Anaerobic Reactors with Conductive Materials. ACS Omega 2021, 6, 29703–29712. [Google Scholar] [CrossRef]
- Wang, D.; Han, H.; Han, Y.; Li, K.; Zhu, H. Enhanced Treatment of Fischer-Tropsch (F-T) Wastewater Using the Up-Flow Anaerobic Sludge Blanket Coupled with Bioelectrochemical System: Effect of Electric Field. Bioresour. Technol. 2017, 232, 18–26. [Google Scholar] [CrossRef]
- Li, Y.; Ni, J.; Cheng, H.; Zhu, A.; Guo, G.; Qin, Y.; Li, Y.-Y. Methanogenic Performance and Microbial Community during Thermophilic Digestion of Food Waste and Sewage Sludge in a High-Solid Anaerobic Membrane Bioreactor. Bioresour. Technol. 2021, 342, 125938. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, B.; Kong, Z.; Yang, C.; Li, L.; Feng, B.; Luo, Z.; Xu, K.-Q.; Kobayashi, T.; Li, Y.-Y. Improved Stability of Up-Flow Anaerobic Sludge Blanket Reactor Treating Starch Wastewater by Pre-Acidification: Impact on Microbial Community and Metabolic Dynamics. Bioresour. Technol. 2021, 326, 124781. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, R.; Luo, H.; Liu, G.; Kim, Y.; Yu, S.; Zeng, J. Microbial Electrolysis Cell with Spiral Wound Electrode for Wastewater Treatment and Methane Production. Process Biochem. 2015, 50, 1103–1109. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, A.; Jia, J.; Liang, Q.; Liu, Q.; Xing, D.; Ren, N. Characterization of Methane Production and Microbial Community Shifts during Waste Activated Sludge Degradation in Microbial Electrolysis Cells. Bioresour. Technol. 2015, 175, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Wang, L.; Quan, X. Potential for Direct Interspecies Electron Transfer in an Electric-Anaerobic System to Increase Methane Production from Sludge Digestion. Sci. Rep. 2015, 5, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Leang, C.; Qian, X.; Mester, T.; Lovley, D.R. Alignment of the c-Type Cytochrome OmcS along Pili of Geobacter Sulfurreducens. Appl. Environ. Microbiol. 2010, 76, 4080–4084. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Live Wires: Direct Extracellular Electron Exchange for Bioenergy and the Bioremediation of Energy-Related Contamination. Energy Environ. Sci. 2011, 4, 4896–4906. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Edwards, S.R.; Dolfing, J.; Cotterill, S.E.; Curtis, T.P. Performance of a Pilot Scale Microbial Electrolysis Cell Fed on Domestic Wastewater at Ambient Temperatures for a 12 Month Period. Bioresour. Technol. 2014, 173, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, M.R.; Torabi, A.R. Tensile fracture in notched polycrystalline graphite specimens. Carbon 2010, 48, 2255–2265. [Google Scholar] [CrossRef]
- Cao, H.; Sun, J.; Wang, K.; Zhu, G.; Li, X.; Lv, Y.; Wang, Z.; Feng, Q.; Feng, J. Performance of Bioelectrode Based on Different Carbon Materials in Bioelectrochemical Anaerobic Digestion for Methanation of Maize Straw. Sci. Total Environ. 2022, 832, 154997. [Google Scholar] [CrossRef]
- Rousseau, R.; Etcheverry, L.; Roubaud, E.; Basséguy, R.; Délia, M.-L.; Bergel, A. Microbial Electrolysis Cell (MEC): Strengths, Weaknesses and Research Needs from Electrochemical Engineering Standpoint. Appl. Energy 2020, 257, 113938. [Google Scholar] [CrossRef]
- Cristiani, L.; Leobello, L.; Zeppilli, M.; Villano, M. Role of C/N ratio in a pilot scale Microbial Electrolysis Cell (MEC) for biomethane production and biogas upgrading. Renew. Energy 2023, 210, 355–363. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Patil, S.A. Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies. Biotechnol. Adv. 2020, 39, 107468. [Google Scholar] [CrossRef]
- Wang, B.; Liu, W.; Liang, B.; Jiang, J.; Wang, A. Microbial fingerprints of methanation in a hybrid electric-biological anaerobic digestion. Water Res. 2022, 226, 119270. [Google Scholar] [CrossRef]
- Slepetiene, A.; Kochiieru, M.; Skersiene, A.; Mankeviciene, A.; Belova, O. Changes in Stabile Organic Carbon in Differently Managed Fluvisol Treated by Two Types of Anaerobic Digestate. Energies 2022, 15, 5876. [Google Scholar] [CrossRef]
- Gahlot, P.; Ahmed, B.; Tiwari, S.B.; Aryal, N.; Khursheed, A.; Kazmi, A.A.; Tyagi, V.K. Conductive Material Engineered Direct Interspecies Electron Transfer (DIET) in Anaerobic Digestion: Mechanism and Application. Environ. Technol. 2020, 20, 101056. [Google Scholar] [CrossRef]
- An, Z.; Feng, Q.; Zhao, R.; Wang, X. Bioelectrochemical Methane Production from Food Waste in Anaerobic Digestion Using a Carbon-Modified Copper Foam Electrode. Processes 2020, 8, 416. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.-C.; Kim, D.-H.; Kim, M.-S.; Kim, D.-H. Influence of the Temperature and Hydraulic Retention Time in Bioelectrochemical Anaerobic Digestion of Sewage Sludge. Int. J. Hydrog. Energy 2019, 44, 2170–2179. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, B.; Yin, C.; Zhang, C.; Dai, X.; Yuan, H.; Zhu, N. Biostimulation by direct voltage to enhance anaerobic digestion of waste activated sludge. RSC Adv. 2016, 6, 1581–1588. [Google Scholar] [CrossRef]
- Flores-Rodriguez, C.; Reddy, C.N.; Min, B. Enhanced methane production from acetate intermediate by bioelectrochemical anaerobic digestion at optimal applied voltages. Biomass Bioenergy 2019, 127, 105261. [Google Scholar] [CrossRef]
- Shabib, A.; Abdallah, M.; Shanableh, A.; Sartaj, M. Effect of substrates and voltages on the performance of bio-electrochemical anaerobic digestion. Renew. Energy 2022, 198, 16–27. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Sheng, L.; Xing, Y.; Liu, G.; Yao, G.; Ngo, H.H.; Li, Q.; Wang, X.C.; Li, Y.Y.; et al. A review on facilitating bio-wastes degradation and energy recovery efficiencies in anaerobic digestion systems with biochar amendment. Bioresour. Technol. 2020, 314, 123777. [Google Scholar] [CrossRef]
- Alibardi, L.; Bernava, N.; Cossu, R.; Spagni, A. Anaerobic dynamic membrane bioreactor for wastewater treatment at ambient temperature. Chem. Eng. J. 2016, 284, 130–138. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.-J.; Lei, Z. Integrating Anaerobic Digestion with Microbial Electrolysis Cell for Performance Enhancement: A Review. Bioresour. Technol. 2022, 344, 126321. [Google Scholar] [CrossRef] [PubMed]
- Priyadharshini, M.; Fidal, V.T.; Kyle, B.; Chandra, T.S.; Taj, K.; Godfrey, K. Development of an electroactive aerobic biocathode for microbial fuel cell applications. Environ. Microbiol. Rep. 2020, 12, 607–612. [Google Scholar] [CrossRef]
- Hassan, M.; Zhu, G.; Lu, Y.; AL-Falahi, A.H.; Lu, Y.; Huang, S.; Wan, Z. Removal of antibiotics from wastewater and its problematic effects on microbial communities by bioelectrochemical Technology: Current knowledge and future perspectives. Environ. Eng. Res. 2021, 26, 190405. [Google Scholar] [CrossRef]
- Han, J.; Lin, X.; Liang, H.; Zhang, S.; Zhu, B.; Ji, C. Improving the safety and quality of Roucha using amine-degrading lactic acid bacteria starters. Food Res. Int. 2022, 161, 111918. [Google Scholar] [CrossRef]
- Xu, J.; Kumar, K.; Kang, Y.; Zhu, J.; Huang, X.; Zong, Y.; Pang, W.; Surendra, K.C.; Xie, L. Role of interspecies electron transfer stimulation in enhancing anaerobic digestion under ammonia stress: Mechanisms, advances, and perspectives. Bioresour. Technol. 2022, 360, 127558. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Grudzień, J.; Kamiński, K.; Gawlak, K.; Wolski, K.; Nowakowska, M.; Sulka, G.D. Novel bioelectrodes based on polysaccharide modified gold surfaces and electrochemically active Lactobacillus rhamnosus GG biofilms. Electrochim. Acta 2019, 296, 999–1008. [Google Scholar] [CrossRef]
- Zhang, X.; Prévoteau, A.; Louro, R.O.; Paquete, G.M.; Rabaey, K. Periodic polarization of electroactive biofilms increases current density and charge carriers concentration while modifying biofilm structure. Biosens. Bioelectron. 2018, 121, 183–191. [Google Scholar] [CrossRef]
- Mottet, A.; François, E.; Latrille, E.; Steyer, J.P.; Déléris, S.; Vedrenne, F.; Carrère, H. Estimating Anaerobic Biodegradability Indicators for Waste Activated Sludge. Chem. Eng. J. 2010, 160, 488–496. [Google Scholar] [CrossRef]
- Berninghaus, A.E.; Radniecki, T.S. Shock Loads Change the Resistance, Resiliency, and Productivity of Anaerobic Co-Digestion of Municipal Sludge and Fats, Oils, and Greases. J. Clean. Prod. 2022, 362, 132447. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Anaerobic Digestion and Electromethanogenic Microbial Electrolysis Cell Integrated System: Increased Stability and Recovery of Ammonia and Methane. Renew. Energy 2018, 120, 178–189. [Google Scholar] [CrossRef]
- Abadikhah, M.; Rodriguez, M.C.; Persson, F.; Wilén, B.M.; Farewell, A.; Modin, O. Evidence of competition between electrogens shaping electroactive microbial communities in microbial electrolysis cells. Front. Microbiol. 2022, 13, 959211. [Google Scholar] [CrossRef] [PubMed]
- Asuero, A.G.; Sayago, A.; González, A.G. The Correlation Coefficient: An Overview. Crit. Rev. Anal. Chem. 2006, 36, 41–59. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.-T.; Chen, K.-Y.; Wang, Z.-H.; Xu, X.-J.; Zhou, X.; Xing, D.-F.; Ren, N.-Q.; Lee, D.-J.; Chen, C. The Underlying Mechanism of Enhanced Methane Production Using Microbial Electrolysis Cell Assisted Anaerobic Digestion (MEC-AD) of Proteins. Water Res. 2021, 201, 117325. [Google Scholar] [CrossRef] [PubMed]
- Chang Bejarano, A.; Champagne, P. Optimization of Biogas Production during Start-up with Electrode-Assisted Anaerobic Digestion. Chemosphere 2022, 302, 134739. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chaudhary, S.; Yadav, S.; Patil, S.A. Protocol for Bioelectrochemical Enrichment, Cultivation, and Characterization of Extreme Electroactive Microorganisms. STAR Protoc. 2022, 3, 101114. [Google Scholar] [CrossRef]
- Yee, M.O.; Deutzmann, J.; Spormann, A.; Rotaru, A.-E. Cultivating Electroactive Microbes—From Field to Bench. Nanotechnology 2020, 31, 174003. [Google Scholar] [CrossRef]
- Oh, G.-G.; Song, Y.-C.; Bae, B.-U.; Lee, C.-Y. Electric Field-Driven Direct Interspecies Electron Transfer for Bioelectrochemical Methane Production from Fermentable and Non-Fermentable Substrates. Processes 2020, 8, 1293. [Google Scholar] [CrossRef]





| Parameters | HLS | PFW | Mixture | Inoculum |
|---|---|---|---|---|
| pH | 7.64 ± 0.02 | 5.46 ± 0.04 | 5.73 ± 0.04 | 7.58 ± 0.03 |
| Alkalinity (g/L CaCO3) | 12.80 ± 1.00 | 2.60 ± 1.00 | 4.66 ± 0.53 | 3.00 ± 0.10 |
| Total VFAs (g COD/L) | 8.00 ± 0.60 | 1.40 ± 0.40 | 3.30 ± 0.19 | 0.70 ± 0.00 |
| TCOD (g/L) | 64.90 ± 2.00 | 112.10 ± 8.40 | 88.07 ± 6.68 | 12.50 ± 0.00 |
| SCOD (g/L) | 58.50 ± 3.40 | 42.80 ± 7.30 | 47.79 ± 6.09 | 53.50 ± 1.60 |
| TS (g/L) | 58.40 ± 1.70 | 103.60 ± 4.40 | 72.76 ± 1.74 | 118.70 ± 3.00 |
| VS (g/L) | 43.70 ± 1.30 | 87.02 ± 2.50 | 56.27 ± 2.29 | 65.70 ± 2.20 |
| HAD | BEAR | |||||
|---|---|---|---|---|---|---|
| Day | Qr (L/d) | OLR (g COD/L.d) | Day | MET (d) | EFI (V/cm) | OLR (g COD/L.d) |
| 350~ | - | 3.11 ± 0.17 | - | - | - | - |
| 377 | - | 14.24 | - | - | - | - |
| 378~ | - | 3.17 ± 0.18 | - | - | - | - |
| 431~ | 0.17 | 2.85 ± 0.30 | 0 | 30 | 2 | 1.32 ± 0.14 |
| 482~ | 0.85 | 2.57 ± 0.34 | 52 | 6 | 2 | 5.17 ± 0.46 |
| 507~ | 1.7 | 2.59 ± 0.19 | 78 | 3 | 2 | 7.84 ± 0.60 |
| 528~ | 3.4 | 2.52 ± 0.14 | 97 | 1.5 | 2 | 13.91 ± 0.83 |
| 547~ | 1.7 | 2.63 ± 0.17 | 115 | 3 | 1 | 7.26 ± 0.42 |
| 554~ | 1.7 | 2.92 ± 0.03 | 127 | 3 | 0.5 | 7.74 ± 0.53 |
| 559~ | 1.7 | 2.94 ± 0.17 | 136 | 3 | 0 | 8.41 ± 0.11 |
| 564~ | 1.7 | 3.13 ± 0.09 | 148 | 3 | 3 | 7.51 ± 0.63 |
| Coefficient | Estimates | Std. Error | t-Value | Pr(>|t|) |
|---|---|---|---|---|
| β0 | 1031.669 | 16.240 | 63.5269 | 2.994 × 10−13 |
| β1 | 16.536 | 12.839 | 1.2880 | 0.229885 |
| β2 | 93.275 | 12.839 | 7.2651 | 4.739 × 10−5 |
| β3 | 32.332 | 12.827 | 2.5205 | 0.032742 |
| β1: β3 | 69.064 | 18.141 | 3.807 | 0.004171 |
| β12 | −171.632 | 17.697 | −9.6984 | 4.614 × 10−6 |
| β22 | −280.982 | 17.697 | −15.8774 | 6.879 × 10−8 |
| β32 | −164.243 | 17.666 | −9.2972 | 6.540 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Z.-K.; Song, Y.-C.; Kim, K.-T.; Lee, C.-Y.; Jang, S.-H.; Bae, B.-U. The Bioaugmentation of Electroactive Microorganisms Enhances Anaerobic Digestion. Fermentation 2023, 9, 988. https://doi.org/10.3390/fermentation9110988
An Z-K, Song Y-C, Kim K-T, Lee C-Y, Jang S-H, Bae B-U. The Bioaugmentation of Electroactive Microorganisms Enhances Anaerobic Digestion. Fermentation. 2023; 9(11):988. https://doi.org/10.3390/fermentation9110988
Chicago/Turabian StyleAn, Zheng-Kai, Young-Chae Song, Keug-Tae Kim, Chae-Young Lee, Seong-Ho Jang, and Byung-Uk Bae. 2023. "The Bioaugmentation of Electroactive Microorganisms Enhances Anaerobic Digestion" Fermentation 9, no. 11: 988. https://doi.org/10.3390/fermentation9110988
APA StyleAn, Z.-K., Song, Y.-C., Kim, K.-T., Lee, C.-Y., Jang, S.-H., & Bae, B.-U. (2023). The Bioaugmentation of Electroactive Microorganisms Enhances Anaerobic Digestion. Fermentation, 9(11), 988. https://doi.org/10.3390/fermentation9110988









