Thermal Treatment and Fermentation of Legume Flours with Leuconostoc citreum TR116 for the Development of Spreadable Meat Alternatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compositional Analysis of Legume Flours
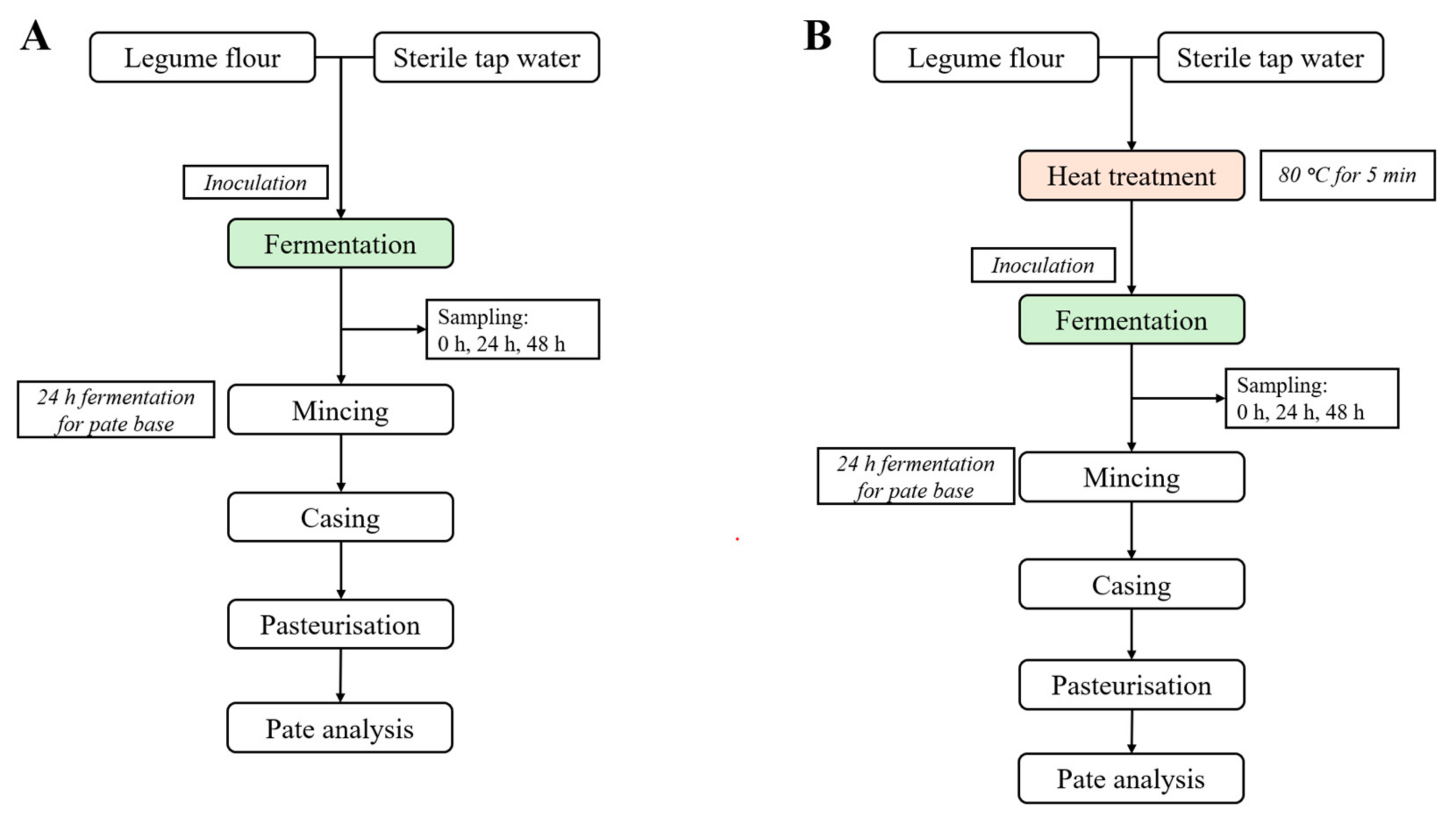
2.3. Experimental Design
2.4. Fermentation
2.5. Characterisation of Fermented Legume Flours
2.5.1. Microbial Growth
2.5.2. Acidification of Fermented Legume Flours
2.5.3. Quantification of Mono-, Di-, Trisaccharides and Organic Acids
2.6. Preparation of Pâté-like Base and Analyses of Physicochemical and Sensory Characteristics
2.6.1. Texture
2.6.2. Colour
2.6.3. Sensory
2.7. Statistics
3. Results and Discussion
3.1. Impact of Thermal Treatment on Fermentation Substrate
3.2. Fermentation Characteristics of Thermal-Treated Versus Non-Treated Legume Flours
3.3. Physicochemical and Sensory Characteristics of Spreadable Meat Alternative
3.3.1. Texture
3.3.2. Colour
3.3.3. Sensory
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiala, N. Meeting the Demand: An Estimation of Potential Future Greenhouse Gas Emissions from Meat Production. Ecol. Econ. 2008, 67, 412–419. [Google Scholar] [CrossRef]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat Alternatives: Life Cycle Assessment of Most Known Meat Substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Pavan Kumar, M.K.; Chatli, N.M.P.S.O.P.M.; Verma, A.K. Meat Analogues: Health Promising Sustainable Meat Substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Stagnari, F.; Maggio, A.; Galieni, A.; Pisante, M. Multiple Benefits of Legumes for Agriculture Sustainability: An Overview. Chem. Biol. Technol. Agric. 2017, 4, 2. [Google Scholar] [CrossRef]
- Hoehnel, A.; Zannini, E.; Arendt, E.K. Targeted Formulation of Plant-Based Protein-Foods: Supporting the Food System’s Transformation in the Context of Human Health, Environmental Sustainability and Consumer Trends. Trends Food Sci. Technol. 2022, 128, 238–252. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant Protein-Based Alternatives of Reconstructed Meat: Science, Technology, and Challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Ma, K.K.; Greis, M.; Lu, J.; Nolden, A.A.; McClements, D.J.; Kinchla, A.J. Functional Performance of Plant Proteins. Foods 2022, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Peng, S.; Green, J.; Koh, L.; Chen, X. Soybean Supply Chain Management and Sustainability: A Systematic Literature Review. J. Clean. Prod. 2020, 255, 120254. [Google Scholar] [CrossRef]
- Xia, J.; Zu, Q.; Yang, A.; Wu, Z.; Li, X.; Tong, P.; Yuan, J.; Wu, Y.; Fan, Q.; Chen, H. Allergenicity Reduction and Rheology Property of Lactobacillus-Fermented Soymilk. J. Sci. Food Agric. 2019, 99, 6841–6849. [Google Scholar] [CrossRef]
- Kumari, T.; Deka, S.C. Potential Health Benefits of Garden Pea Seeds and Pods: A Review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Abu Risha, M.; Rick, E.M.; Plum, M.; Jappe, U. Legume Allergens Pea, Chickpea, Lentil, Lupine and Beyond. Curr. Allergy Asthma Rep. 2024. [Google Scholar] [CrossRef]
- Del Borghi, A.; Tacchino, V.; Moreschi, L.; Matarazzo, A.; Gallo, M.; Arellano Vazquez, D. Environmental Assessment of Vegetable Crops towards the Water-Energy-Food Nexus: A Combination of Precision Agriculture and Life Cycle Assessment. Ecol. Indic. 2022, 140, 109015. [Google Scholar] [CrossRef]
- Boeck, T.; Sahin, A.W.; Zannini, E.; Arendt, E.K. Nutritional Properties and Health Aspects of Pulses and Their Use in Plant-Based Yogurt Alternatives. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3858–3880. [Google Scholar] [CrossRef] [PubMed]
- Marchini, M.; Carini, E.; Cataldi, N.; Boukid, F.; Blandino, M.; Ganino, T.; Vittadini, E.; Pellegrini, N. The Use of Red Lentil Flour in Bakery Products: How Do Particle Size and Substitution Level Affect Rheological Properties of Wheat Bread Dough? LWT 2021, 136, 110299. [Google Scholar] [CrossRef]
- Hajas, L.; Sipos, L.; Csobod, C.; Bálint, M.V.; Juhász, R.; Benedek, C. Lentil (Lens Culinaris Medik.) Flour Varieties as Promising New Ingredients for Gluten-Free Cookies. Foods 2022, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chan, E.; Masatcioglu, M.T.; Erkinbaev, C.; Paliwal, J.; Koksel, F. Effects of Extrusion Conditions and Nitrogen Injection on Physical, Mechanical, and Microstructural Properties of Red Lentil Puffed Snacks. Food Bioprod. Process. 2020, 121, 143–153. [Google Scholar] [CrossRef]
- Sinaki, N.Y.; Koksel, F. Effects of Dietary Fibre Source and Content and Extrusion Conditions on the Physicochemical Composition and Physical Quality of Fibre-Enriched Lentil Snacks. Int. J. Food Sci. Technol. 2024, 59, 2236–2248. [Google Scholar] [CrossRef]
- Boeck, T.; Zannini, E.; Sahin, A.W.; Bez, J.; Arendt, E.K. Nutritional and Rheological Features of Lentil Protein Isolate for Yoghurt-like Application. Foods 2021, 10, 1692. [Google Scholar] [CrossRef]
- Boeck, T.; Ispiryan, L.; Hoehnel, A.; Sahin, A.W.; Coffey, A.; Zannini, E.; Arendt, E.K. Lentil-Based Yogurt Alternatives Fermented with Multifunctional Strains of Lactic Acid Bacteria—Techno-Functional, Microbiological, and Sensory Characteristics. Foods 2022, 11, 2013. [Google Scholar] [CrossRef]
- Romano, A.; Gallo, V.; Ferranti, P.; Masi, P. Lentil Flour: Nutritional and Technological Properties, in Vitro Digestibility and Perspectives for Use in the Food Industry. Curr. Opin. Food Sci. 2021, 40, 157–167. [Google Scholar] [CrossRef]
- Lee, H.W.; Lu, Y.; Zhang, Y.; Fu, C.; Huang, D. Physicochemical and Functional Properties of Red Lentil Protein Isolates from Three Origins at Different PH. Food Chem. 2021, 358, 129749. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K. Chemistry and Implications of Antinutritional Factors in Dry Beans and Pulses. Dry Beans Pulses Prod. Process. Nutr. 2012, 359–377. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic Acid Bacteria as Functional Starter Cultures for the Food Fermentation Industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The Potential of Fermentation on Nutritional and Technological Improvement of Cereal and Legume Flours: A Review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Coffey, A. Genomic Analysis of Leuconostoc citreum TR116 with Metabolic Reconstruction and the Effects of Fructose on Gene Expression for Mannitol Production. Int. J. Food Microbiol. 2021, 354, 109327. [Google Scholar] [CrossRef]
- Hemme, D.; Foucaud-Scheunemann, C. Leuconostoc, Characteristics, Use in Dairy Technology and Prospects in Functional Foods. Int. Dairy J. 2004, 14, 467–494. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Zannini, E.; Axel, C.; Coffey, A.; Lynch, K.M.; Arendt, E.K. Leuconostoc citreum TR116: In-Situ Production of Mannitol in Sourdough and Its Application to Reduce Sugar in Burger Buns. Int. J. Food Microbiol. 2019, 302, 80–89. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Zannini, E.; Lynch, K.M.; Coffey, A.; Arendt, E.K. Sourdough Technology as a Novel Approach to Overcome Quality Losses in Sugar-Reduced Cakes. Food Funct. 2019, 10, 4985–4997. [Google Scholar] [CrossRef]
- Sahin, A.W.; Rice, T.; Zannini, E.; Lynch, K.M.; Coffey, A.; Arendt, E.K. The Incorporation of Sourdough in Sugar-Reduced Biscuits: A Promising Strategy to Improve Techno-Functional and Sensory Properties. Eur. Food Res. Technol. 2019, 245, 1841–1854. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Lynch, K.M.; Coffey, A.; Arendt, E.K. Polyol-Producing Lactic Acid Bacteria Isolated from Sourdough and Their Application to Reduce Sugar in a Quinoa-Based Milk Substitute. Int. J. Food Microbiol. 2018, 286, 31–36. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Heitmann, M.; Lynch, K.M.; Jacob, F.; Arendt, E.K.; Coffey, A. Application of Mannitol Producing Leuconostoc citreum TR116 to Reduce Sugar Content of Barley, Oat and Wheat Malt-Based Worts. Food Microbiol. 2020, 90, 103464. [Google Scholar] [CrossRef]
- AACC International AACC 44-17.01. In Approved Methods of the AACC; American Association of Cereal Chemists: St. Paul, MN, USA.
- AACC International AACC 46-12.01. In Approved Methods of the AACC; American Association of Cereal Chemists: St. Paul, MN, USA.
- AACC International AACC 30-25.01. In Approved Methods of the AACC; American Association of Cereal Chemists: St. Paul, MN, USA.
- AACC International AACC 08-01.01. In Approved Methods of the AACC; American Association of Cereal Chemists: St. Paul, MN, USA.
- Hoehnel, A.; Bez, J.; Sahin, A.W.; Coffey, A.; Arendt, E.K.; Zannini, E. Leuconostoc citreum TR116 as a Microbial Cell Factory to Functionalise High-Protein Faba Bean Ingredients for Bakery Applications. Foods 2020, 9, 1706. [Google Scholar] [CrossRef] [PubMed]
- Neylon, E.; Nyhan, L.; Zannini, E.; Monin, T.; Münch, S.; Sahin, A.W.; Arendt, E.K. Food Ingredients for the Future: In-Depth Analysis of the Effects of Lactic Acid Bacteria Fermentation on Spent Barley Rootlets. Fermentation 2023, 9, 78. [Google Scholar] [CrossRef]
- Waters, D.M.; Kingston, W.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Wheat Bread Biofortification with Rootlets, a Malting by-Product. J. Sci. Food Agric. 2013, 93, 2372–2383. [Google Scholar] [CrossRef]
- Blazek, J.; Copeland, L. Pasting and Swelling Properties of Wheat Flour and Starch in Relation to Amylose Content. Carbohydr. Polym. 2008, 71, 380–387. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional Properties of Soy Proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Shevkani, K.; Kaur, M.; Singh, N. Composition, Pasting, Functional, and Microstructural Properties of Flours from Different Split Dehulled Pulses (Dhals). J. Food Process. Preserv. 2021, 45, e15485. [Google Scholar] [CrossRef]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of Plant Proteins for Improved Functionality: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef]
- Mession, J.L.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of Globular Pea Proteins Fractionation on Their Heat-Induced Aggregation and Acid Cold-Set Gelation. Food Hydrocoll. 2015, 46, 233–243. [Google Scholar] [CrossRef]
- McBryde, W.A.E. The PH Meter as a Hydrogen-Ion Concentration Probe. Analyst 1969, 94, 337–346. [Google Scholar] [CrossRef]
- Ai, Y.; Jane, J.L. Gelatinization and Rheological Properties of Starch. Starch/Staerke 2015, 67, 213–224. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Chen, Y.; Li, M.; Liu, C.; Wu, X. Effect of Glycosylation Modification on Structure and Properties of Soy Protein Isolate: A Review. J. Food Sci. 2024, 89, 4620–4637. [Google Scholar] [CrossRef] [PubMed]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. Characterization of the FODMAP-Profile in Cereal-Product Ingredients. J. Cereal Sci. 2020, 92, 102916. [Google Scholar] [CrossRef]
- Ispiryan, L.; Heitmann, M.; Hoehnel, A.; Zannini, E.; Arendt, E.K. Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products. J. Agric. Food Chem. 2019, 67, 4384–4392. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, M.; Miljković, M. Chemistry of the Glycosidic Bond. In Carbohydrates: Synthesis, Mechanisms, and Stereoelectronic Effects; Springer: New York, NY, USA, 2009; pp. 323–421. [Google Scholar]
- Sushil Dhital Frederick, J.; Warren, P.J.B.P.R.E.; Gidley, M.J. Mechanisms of Starch Digestion by α-Amylase—Structural Basis for Kinetic Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 875–892. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering Capacity of Dairy Products. Int. Dairy. J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Wang, S.; Chao, C.; Guo, Q.; Gu, C. Protein Complexation with Carbohydrates and Lipids. In Functionality of Plant Proteins; Elsevier: Amsterdam, The Netherlands, 2024; pp. 221–251. [Google Scholar] [CrossRef]
- Tian, S.; Sun, Y. Influencing Factor of Resistant Starch Formation and Application in Cereal Products: A Review. Int. J. Biol. Macromol. 2020, 149, 424–431. [Google Scholar] [CrossRef]
- 58Hall, A.E.; Moraru, C.I. Comparative Effects of High Pressure Processing and Heat Treatment on in Vitro Digestibility of Pea Protein and Starch. NPJ Sci. Food 2022, 6, 2. [Google Scholar] [CrossRef]
- Tester, R.F.; Morrison, W.R. Swelling and Gelatinization of Cereal Starches. I. Effects of Amylopectin, Amylose, and Lipids. Cereal Chem. 1990, 67, 551–557. [Google Scholar]
- Devkota, L.; Kyriakopoulou, K.; Bergia, R.; Dhital, S. Structural and Thermal Characterization of Protein Isolates from Australian Lupin Varieties as Affected by Processing Conditions. Foods 2023, 12, 908. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, E.; Resta, D.; Brambilla, F.; Zacherl, C.; Arnoldi, A. The Effects of Various Processing Conditions on a Protein Isolate from Lupinus Angustifolius. Food Chem. 2010, 120, 496–504. [Google Scholar] [CrossRef]
- Choe, U.; Osorno, J.M.; Ohm, J.B.; Chen, B.; Rao, J. Modification of Physicochemical, Functional Properties, and Digestibility of Macronutrients in Common Bean (Phaseolus vulgaris, L.) Flours by Different Thermally Treated Whole Seeds. Food Chem. 2022, 382, 132570. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Hua, Y.; Chen, Y.; Kong, X.; Zhang, C. Effect of Preheating-Induced Denaturation during Protein Production on the Structure and Gelling Properties of Soybean Proteins. Food Hydrocoll. 2020, 105, 105846. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Gelation Properties of Salt-Extracted Pea Protein Isolate Induced by Heat Treatment: Effect of Heating and Cooling Rate. Food Chem. 2011, 124, 1011–1016. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Van Vliet, T. Heat-Induced Gel Formation by Soy Proteins at Neutral PH. J. Agric. Food Chem. 2002, 50, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Sahin, A.W.; Coffey, A.; Zannini, E. Functionalisation of Wheat and Oat Bran Using Single-Strain Fermentation and Its Impact on Techno-Functional and Nutritional Properties of Biscuits. Eur. Food Res. Technol. 2021, 247, 1825–1837. [Google Scholar] [CrossRef]
- Wang, R.; Hartel, R.W. Understanding Stickiness in Sugar-Rich Food Systems: A Review of Mechanisms, Analyses, and Solutions of Adhesion. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5901–5937. [Google Scholar] [CrossRef]
- Boucheham, N.; Galet, L.; Patry, S.; Zidoune, M.N. Physicochemical and Hydration Properties of Different Cereal and Legume Gluten-Free Powders. Food Sci. Nutr. 2019, 7, 3081–3092. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal Processing Effects on the Functional Properties and Microstructure of Lentil, Chickpea, and Pea Flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Rosenthal, A.J.; Thompson, P. What Is Cohesiveness?—A Linguistic Exploration of the Food Texture Testing Literature. J. Texture Stud. 2021, 52, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Cheng, Y.T.; Chan, Y.J.; Lu, W.C.; Li, P.H. Effect of Heat Treatment on Nutritional and Chromatic Properties of Mung Bean (Vigna radiata, L.). Agronomy 2022, 12, 1365. [Google Scholar] [CrossRef]
- Spaggiari, G.; Di Pizio, A.; Cozzini, P. Sweet, Umami and Bitter Taste Receptors: State of the Art of in Silico Molecular Modeling Approaches. Trends Food Sci. Technol. 2020, 96, 21–29. [Google Scholar] [CrossRef]
- Breslin, P.A.S. Interactions among Salty, Sour and Bitter Compounds. Trends Food Sci. Technol. 1996, 7, 390–399. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2016; ISBN 0429150830. [Google Scholar]
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; Van De Velde, F.; De Kok, P.M.T. Flavor Aspects of Pulse Ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Bühler, J.M.; Dekkers, B.L.; Bruins, M.E.; Van Der Goot, A.J. Modifying Faba Bean Protein Concentrate Using Dry Heat to Increase Water Holding Capacity. Foods 2020, 9, 1077. [Google Scholar] [CrossRef]




| Soy Flour (SF) | Red Lentil Flour (LF) | Yellow Pea Flour (PF) | |
|---|---|---|---|
| Protein [g/100 g] | 35.85 ± 0.27 | 22.39 ± 0.17 | 21.93 ± 0.06 |
| Moisture [g/100 g] | 9.37 ± 0.09 | 10.98 ± 0.26 | 10.20 ± 0.08 |
| Fat [g/100 g] | 21.51 ± 0.12 | 1.60 ± 0.11 | 1.58 ± 0.22 |
| Carbohydrates [g/100 g] | 28.83 | 62.73 | 63.60 |
| of which total starch [g/100 g] | 1.47 ± 0.02 (c) | 48.19 ± 0.42 (a) | 43.40 ± 0.75 (b) |
| of which digestible starch [g/100 g] | 1.47 ± 0.02 (c) | 38.47 ± 0.22 (a) | 31.39 ± 0.58 (b) |
| Ash [g/100 g] | 4.44 ± 0.02 | 2.30 ± 0.02 | 2.69 ± 0.01 |
| pH [43,44] | TTA [mL 0.1 M NaOH/10 g] | Maximum Growth Rate (µmax) [43,44] | |
|---|---|---|---|
| SF (t0) | 6.81 ± 0.04 (j) | 5.87 ± 0.19 (d) | 0.13 ± 0.01 (a) |
| SF (t24) | 5.44 ± 0.01 (e) | 14.77 ± 0.16 (f) | |
| SF (t48) | 5.35 ± 0.01 (d,e) | 16.08 ± 0.12 (g) | |
| LF (t0) | 6.54 ± 0.04 (h) | 6.06 ± 0.09 (d) | 0.12 ± 0.01 (a) |
| LF (t24) | 4.80 ± 0.01 (b) | 20.96 ± 0.29 (h) | |
| LF (t48) | 4.69 ± 0.05 (a) | 24.98 ± 0.33 (i) | |
| LF-H (t0) | 6.74 ± 0.01 (i,j) | 2.19 ± 0.02 (a) | 0.09 ± 0.01 (b) |
| LF-H (t24) | 5.62 ± 0.07 (e) | 4.20 ± 0.05 (c) | |
| LF-H (t48) | 5.61 ± 0.08 (e) | 4.45 ± 0.13 (c) | |
| PF (t0) | 6.48 ± 0.03 (i) | 5.72 ± 0.12 (d) | 0.11 ± 0.01 (a) |
| PF (t24) | 4.99 ± 0.05 (c) | 19.84 ± 0.41 (h) | |
| PF (t48) | 4.83 ± 0.04 (b) | 24.35 ± 0.29 (i) | |
| PF-H (t0) | 6.78 ± 0.05 (j) | 3.14 ± 0.13 (b) | 0.06 ± 0.01 (b) |
| PF-H (t24) | 5.34 ± 0.06 (d,e) | 7.18 ± 0.04 (e) | |
| PF-H (t48) | 5.32 ± 0.08 (d) | 5.97 ± 0.23 (d) |
| Citric Acid | Succinic Acid | Lactic Acid | Acetic Acid | Σ | |
|---|---|---|---|---|---|
| SF (t0) | 0.62 ± 0.01 (a) | 0.00 ± 0.00 (d) | 0.00 ± 0.00 (e) | 0.16 ± 0.02 (d) | 0.79 ± 0.04 (e) |
| SF (t24) | 0.36 ± 0.02 (c) | 0.80 ± 0.04 (a) | 5.15 ± 0.14 (a) | 3.31 ± 0.01 (a) | 7.60 ± 0.16 (a) |
| LF (t0) | 0.19 ± 0.01 (d) | 0.00 ± 0.00 (d) | 1.47 ± 0.02 (d) | 0.00 ± 0.00 (e) | 1.66 ± 0.03 (d) |
| LF-H (t0) | 0.22 ± 0.01 (d) | 0.00 ± 0.00 (d) | 1.74 ± 0.04 (c,d) | 0.00 ± 0.00 (e) | 1.87 ± 0.05 (d) |
| LF (t24) | 0.00 ± 0.00 (f) | 0.35 ± 0.01 (b) | 5.48 ± 0.15 (a) | 1.07 ± 0.03 (c) | 6.91 ± 0.19 (a) |
| LF-H (t24) | 0.17 ± 0.01 (d,e) | 0.14 ± 0.01 (c) | 3.14 ± 0.08 (b) | 1.30 ± 0.17 (b) | 4.76 ± 0.16 (b) |
| PF (t0) | 0.56 ± 0.02 (b) | 0.00 ± 0.00 (d) | 0.00 ± 0.00 (e) | 0.00 ± 0.00 (e) | 0.56 ± 0.02 (e) |
| PF-H(t0) | 0.27 ± 0.02 (d) | 0.00 ± 0.00 (d) | 0.00 ± 0.00 (e) | 0.00 ± 0.00 (e) | 0.27 ± 0.02 (e) |
| PF (t24) | 0.22 ± 0.01 (d) | 0.35 ± 0.01 (b) | 3.05 ± 0.09 (b) | 1.29 ± 0.10 (b) | 4.91 ± 0.22 (b) |
| PF-H (t24) | 0.13 ± 0.00 (e) | 0.15 ± 0.01 (c) | 2.23 ± 0.12 (c) | 1.46 ± 0.07 (b) | 3.97 ± 0.20 (c) |
| Glucose | Fructose | Sucrose | Maltose | Galactose | Σ Mono- and Disaccharides | Raffinose/ Stachyose | Verbascose | Σ Tri- and Tetrasaccharides | Mannitol | |
|---|---|---|---|---|---|---|---|---|---|---|
| SF (t0) | 0.00 ± 0.00 (c) | 0.13 ± 0.05 (a) | 6.67 ± 0.35 (a) | <LoQ | 0.00 ± 0.00 (g) | 6.82 ± 0.41 (a) | 3.45 ± 0.18 (a) | <LoQ | 3.49 ± 0.19 (a) | 0.00 ± 0.00 (e) |
| SF (t24) | 0.00 ± 0.00 (c) | 0.22 ± 0.02 (a) | 0.00 ± 0.00 (d) | 0.00 ± 0.00 (b) | 0.00 ± 0.00 (g) | 0.22 ± 0.02 (h) | 3.80 ± 0.24 (a) | 0.06 ± 0.01 (f) | 3.86 ± 0.25 (a) | 1.62 ± 0.06 (a) |
| LF (t0) | 0.18 ± 0.01 (a) | 0.00 ± 0.00 (b) | 1.25 ± 0.13 (c) | 0.16 ± 0.04 (a) | 0.15 ± 0.02 (f) | 1.74 ± 0.20 (d) | 0.62 ± 0.21 (e) | 0.15 ± 0.05 (e) | 0.77 ± 0.25 (e) | 0.00 ± 0.00 (e) |
| LF-H (t0) | 0.07 ± 0.00 (b) | 0.00 ± 0.00 (b) | 1.82 ± 0.01 (c) | <LoQ | 0.50 ± 0.01 (c,d) | 2.40 ± 0.02 (c) | 1.03 ± 0.18 (d) | 0.33 ± 0.04 (c) | 1.36 ± 0.22 (d) | 0.00 ± 0.00 (e) |
| LF (t24) | 0.00 ± 0.00 (c) | <LoQ | <LoQ | <LoQ | 1.15 ± 0.06 (a) | 1.29 ± 0.07 (e) | 1.11 ± 0.06 (c,d) | 0.26 ± 0.02 (d) | 1.37 ± 0.08 (d) | 0.59 ± 0.01 (c) |
| LF-H (t24) | 0.00 ± 0.00 (c) | 0.00 ± 0.00 (b) | 0.00 ± 0.00 (d) | 0.00 ± 0.00 (b) | 0.56 ± 0.03 (c) | 0.56 ± 0.03 (g) | 0.94 ± 0.04 (e) | 0.24 ± 0.01 (d) | 1.18 ± 0.04 (e) | 0.14 ± 0.02 (d) |
| PF (t0) | 0.18 ± 0.00 (a) | <LoQ | 2.21 ± 0.20 (b) | 0.15 ± 0.01 (a) | 0.35 ± 0.01 (e) | 2.89 ± 0.23 (c) | 1.55 ± 0.05 (b) | 0.36 ± 0.03 (c) | 1.91 ± 0.08 (b,c) | 0.00 ± 0.00 (e) |
| PF-H(t0) | 0.12 ± 0.01 (a) | <LoQ | 3.06 ± 0.08 (b) | 0.00 ± 0.00 (b) | 0.73 ± 0.02 (b,c) | 3.94 ± 0.01 (b) | 1.54 ± 0.16 (b,c) | 0.61 ± 0.03 (a) | 2.16 ± 0.20 (b) | 0.00 ± 0.00 (e) |
| PF (t24) | <LoQ | <LoQ | <LoQ | <LoQ | 1.33 ± 0.04 (a) | 1.44 ± 0.07 (d,e) | 1.10 ± 0.08 (d) | 0.25 ± 0.01 (d) | 1.34 ± 0.08 (d) | 0.76 ± 0.08 (b) |
| PF-H (t24) | 0.00 ± 0.00 (c) | <LoQ | 0.00 ± 0.00 (d) | 0.00 ± 0.00 (b) | 0.48 ± 0.02 (d,e) | 0.89 ± 0.10 (f,g) | 1.28 ± 0.09 (c) | 0.42 ± 0.03 (b) | 1.70 ± 0.12 (c) | 0.48 ± 0.02 (c) |
| Firmness (N) | Adhesiveness (N) | Cohesiveness (N) | Colour Change ΔE | |
|---|---|---|---|---|
| SF (t0) | 20.73 ± 1.13 (c,a) | 10.86 ± 2.22 (a) | 0.68 ± 0.02 (a) | - |
| SF (t24) | 19.52 ± 1.20 (b,c) | 10.85 ± 1.30 (a) | 0.69 ± 0.04 (a) | 3.09 ± 1.04 (c) |
| LF (t0) | 2.10 ± 0.15 (g) | 1.27 ± 0.23 (b) | 0.66 ± 0.00 (a) | - |
| LF-H (t0) | 15.86 ± 1.34 (e,d) | 0.39 ± 0.09 (e) | 0.29 ± 0.03 (c) | 24.43 ± 0.93 (a) |
| LF (t24) | 5.28 ± 0.59 (f) | 5.64 ± 1.98 (c) | 0.65 ± 0.01 (a) | 2.36 ± 0.71 (c) |
| LF-H (t24) | 13.89 ± 1.27 (e) | 0.23 ± 0.05 (f) | 0.26 ± 0.01 (c) | 21.45 ± 0.67 (a) |
| PF (t0) | 4.11 ± 0.69 (g,f) | 0.81 ± 0.07 (d) | 0.42 ± 0.02 (b) | - |
| PF-H(t0) | 19.24 ± 0.76 (b,c) | 0.38 ± 0.06 (e) | 0.25 ± 0.02 (c) | 14.62 ± 0.99 (b) |
| PF (t24) | 22.59 ± 1.48 (a) | 3.17 ± 0.32 (c) | 0.41 ± 0.03 (b) | 2.53 ± 1.74 (c) |
| PF-H (t24) | 17.99 ± 1.10 (d,b) | 0.52 ± 0.22 (d) | 0.24 ± 0.02 (c) | 16.20 ± 1.28 (b) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin, A.W.; Gautheron, O.; Galle, S. Thermal Treatment and Fermentation of Legume Flours with Leuconostoc citreum TR116 for the Development of Spreadable Meat Alternatives. Fermentation 2024, 10, 412. https://doi.org/10.3390/fermentation10080412
Sahin AW, Gautheron O, Galle S. Thermal Treatment and Fermentation of Legume Flours with Leuconostoc citreum TR116 for the Development of Spreadable Meat Alternatives. Fermentation. 2024; 10(8):412. https://doi.org/10.3390/fermentation10080412
Chicago/Turabian StyleSahin, Aylin W., Ophélie Gautheron, and Sandra Galle. 2024. "Thermal Treatment and Fermentation of Legume Flours with Leuconostoc citreum TR116 for the Development of Spreadable Meat Alternatives" Fermentation 10, no. 8: 412. https://doi.org/10.3390/fermentation10080412
APA StyleSahin, A. W., Gautheron, O., & Galle, S. (2024). Thermal Treatment and Fermentation of Legume Flours with Leuconostoc citreum TR116 for the Development of Spreadable Meat Alternatives. Fermentation, 10(8), 412. https://doi.org/10.3390/fermentation10080412






