Naturally Fermented Gordal and Manzanilla Green Table Olives: Effect of Single Yeast Starters on Fermentation and Final Characteristics of the Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Yeast Strains
2.1.1. Growth and Volatile Profile of Yeasts in OCM
2.1.2. Enzymatic Activities of Yeasts
2.2. Olive Processing
2.2.1. Physicochemical Characteristics
2.2.2. Major Fermentation End-Products
2.2.3. Volatile Compounds
2.2.4. Phenolic Compounds
2.2.5. Determination of Instrumental Color and Firmness
2.2.6. Sensory Analysis
2.2.7. Evaluation of Olive Bitterness by Total Phenol Content Analysis
2.2.8. Microbiological Analyses
2.3. Statistical Analyses
3. Results and Discussion
3.1. Selection of Yeast Strains to Be Used as Starters
3.2. Natural-Style Green Olive Fermentations
3.2.1. Evolution of Microbial Counts and Physicochemical Parameters during Fermentations
3.2.2. Fermentation End-Products
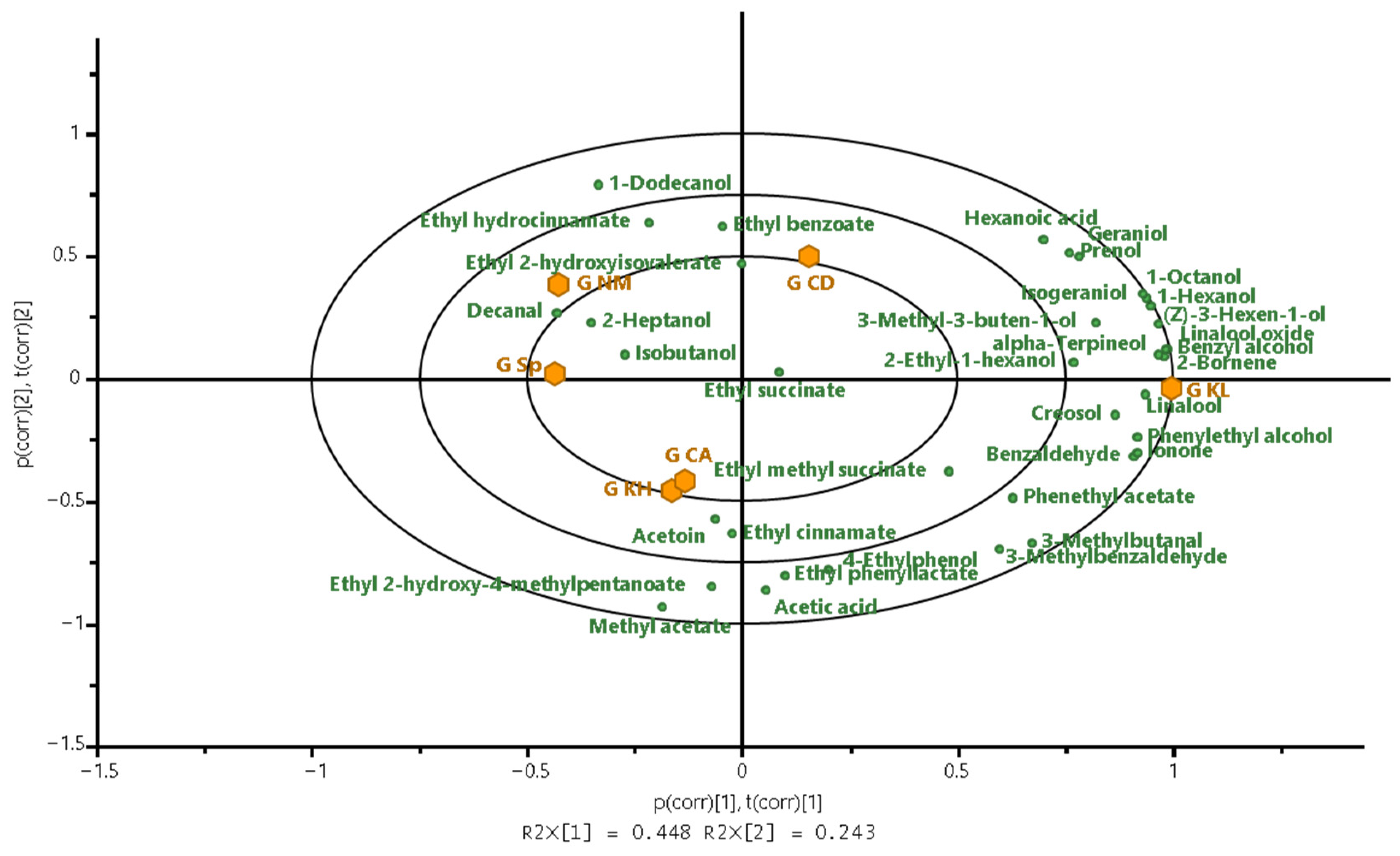
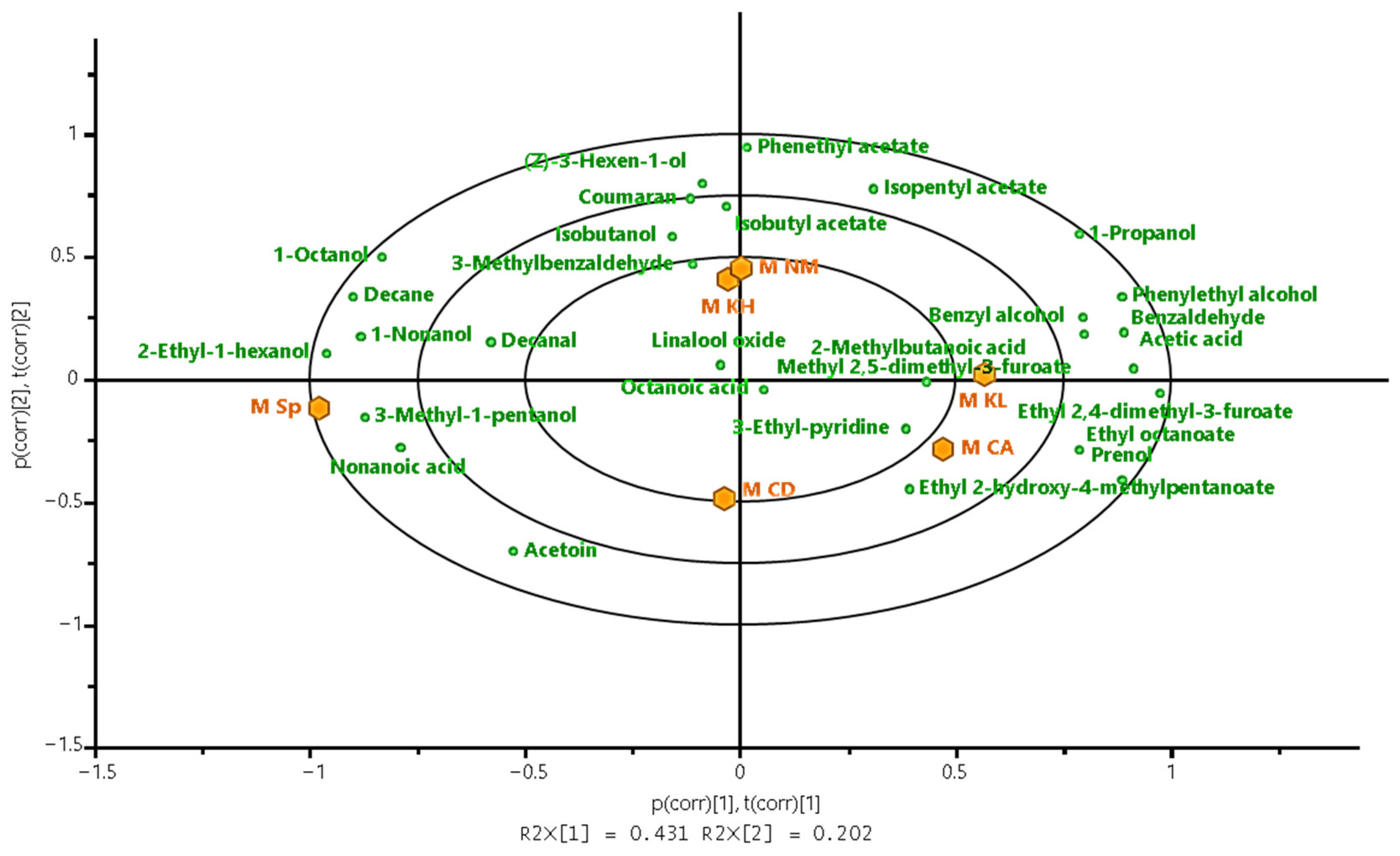
3.2.3. Volatile Compounds
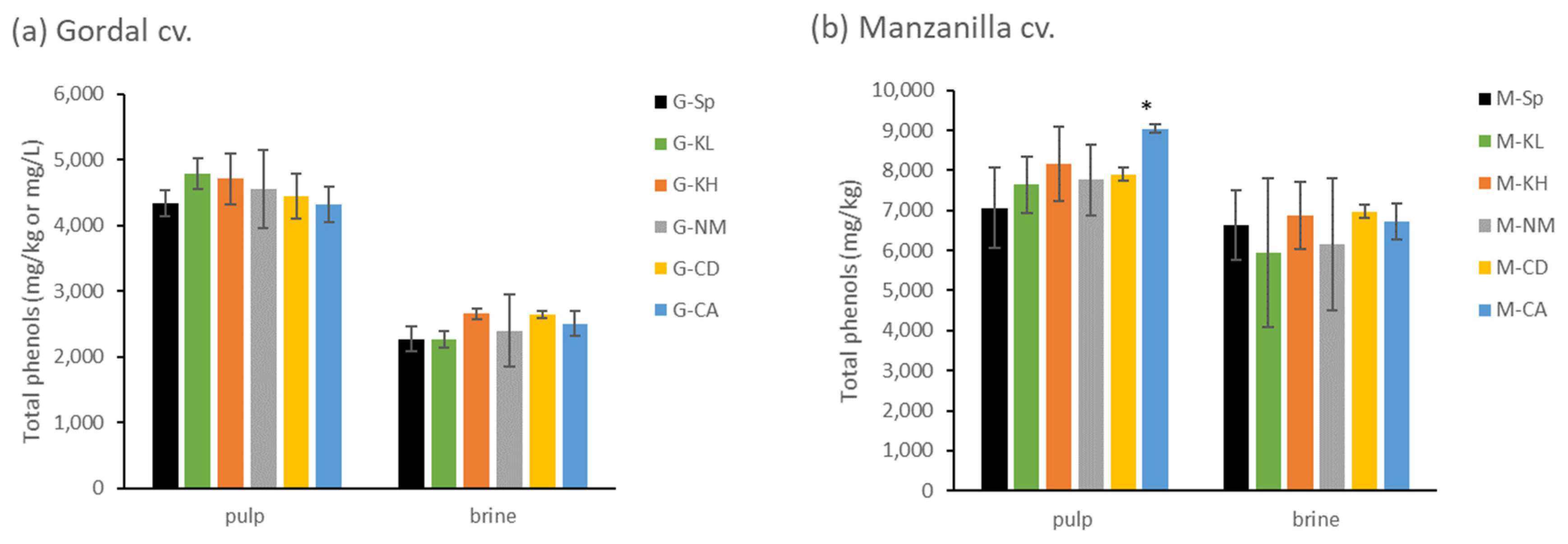
3.2.4. Phenolic Compounds
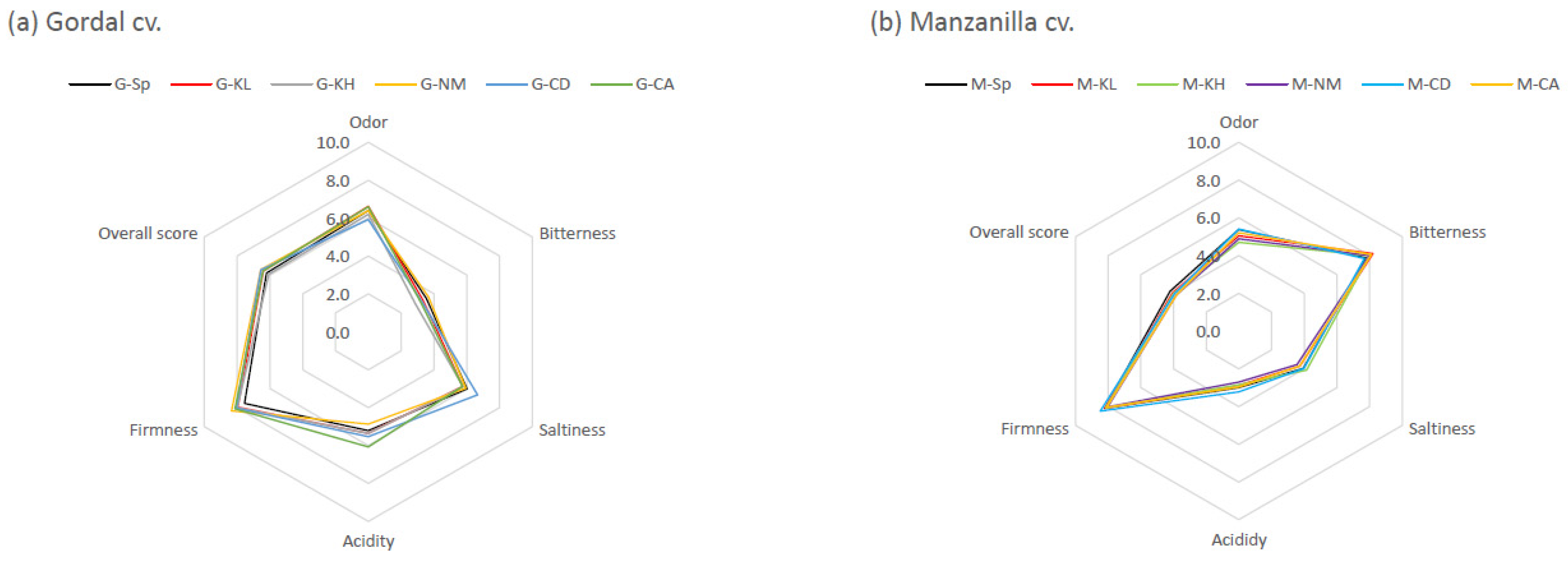
3.2.5. Sensory Characteristics of Final Products
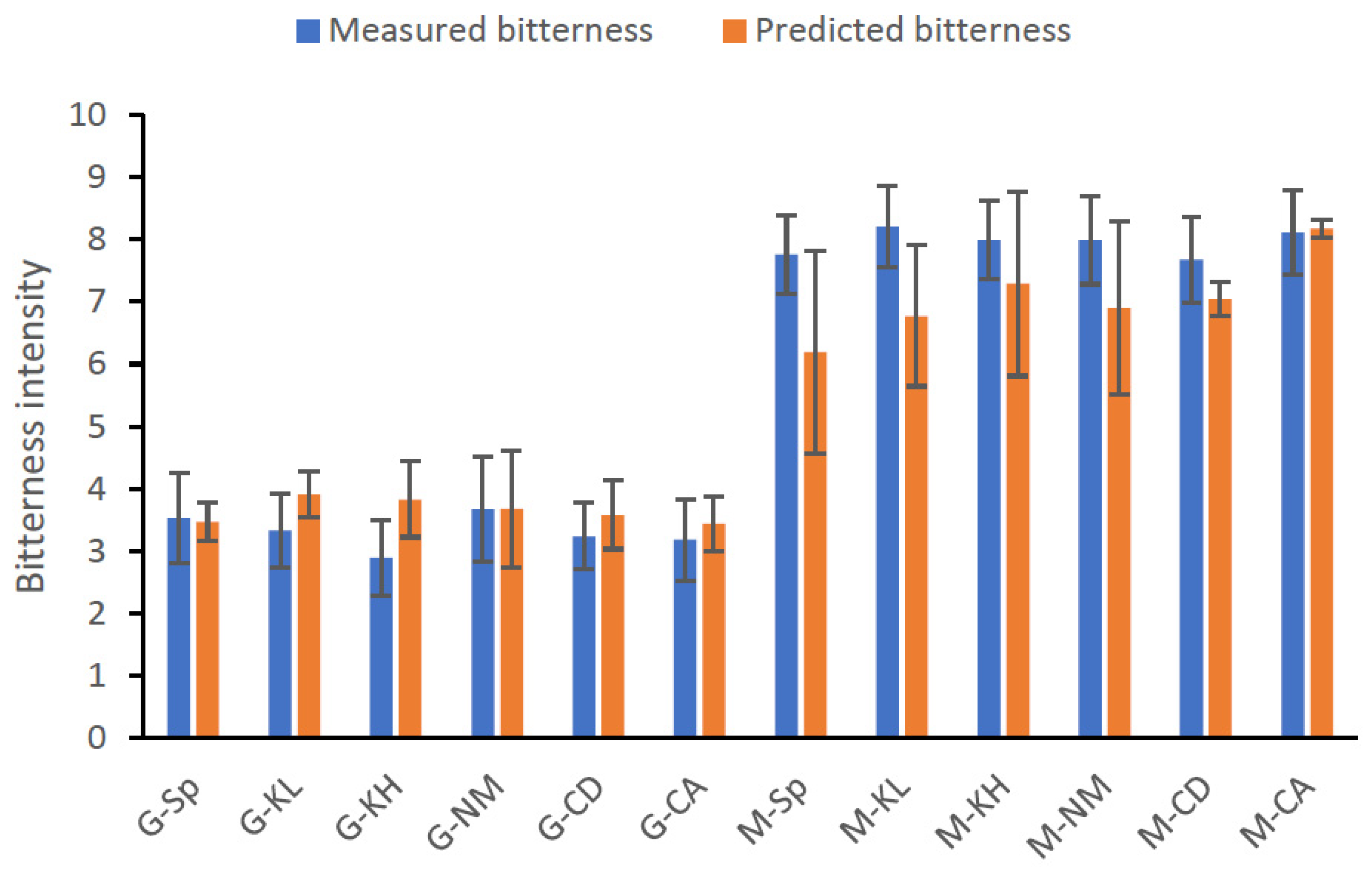
3.2.6. Evaluation of Olive Bitterness by Total Phenol Content Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garrido-Fernández, A.; Fernández-Díez, M.J.; Adams, R.M. Table Olives: Production and Processing; Chapman & Hall: London, UK, 1997; pp. 10–21. [Google Scholar]
- Chytiri, A.; Tasioula-Margari, M.; Bleve, G.; Kontogianni, V.G.; Kallimanis, A.; Kontominas, M.G. Effect of different inoculation strategies of selected yeast and LAB cultures on Conservolea and Kalamàta table olives considering phenol content, texture, and sensory attributes. J. Sci. Food Agric. 2020, 100, 926–935. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Sánchez, A.H.; López-López, A.; Cortés-Delgado, A.; Montaño, A. Microbial and chemical characterization of natural-style green table olives from the Gordal, Hojiblanca and Manzanilla cultivars. Foods 2023, 12, 2386. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Durante, M.; Ramires, F.A.; Grieco, F.; Tommasi, L.; Perbellini, E.; Falco, V.; Tasioula-Margari, M.; Logrieco, A.F.; Mita, G.; et al. New process for production of fermented black table olives using selected autochthonous microbial resources. Front. Microbiol. 2015, 6, 1007. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Anglana, C.; Crupi, P.; Virtuosi, I.; Fiume, P.; Di Terlizzi, B.; Moselhy, N.; Attay, H.A.; Pati, S.; Logrieco, A.F.; et al. Efficacy of yeast starters to drive and improve Picual, Manzanilla and Kalamàta table olive fermentation. J. Sci. Food Agric. 2019, 99, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Montaño, A.; Cortés-Delgado, A.; Sánchez, A.H.; Ruiz-Barba, J.L. Production of volatile compounds by wild-type yeasts in a natural olive-derived culture medium. Food Microbiol. 2021, 98, 103788. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Sánchez, A.H.; López-López, A.; Cortés-Delgado, A.; Montaño, A. Microbial community and volatilome changes in brines along the spontaneous fermentation of Spanish-style and natural-style green table olives (Manzanilla cultivar). Food Microbiol. 2023, 113, 104286. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Cortés-Delgado, A.; Sánchez, A.H.; López-López, A.; Montaño, A. Impact of selected wild yeast starters on the volatilome and phenolic content of Gordal, Manzanilla and Hojiblanca naturally fermented green olives. LWT-Food Sci. Technol. 2024, 195, 115811. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Evaluation of a single and combined inoculation of a Lactobacillus pentosus starter for processing cv. Arbequina natural green olives. Food Microbiol. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Marsilio, V.; Seghetti, L.; Iannucci, E.; Russi, F.; Lanza, B.; Felicioni, M. Use of a lactic acid bacteria starter culture during green olive (Olea europaea L. cv Ascolana tenera) processing. J. Sci. Food Agric. 2005, 85, 1084–1090. [Google Scholar] [CrossRef]
- Pino, A.; De Angelis, M.; Todero, A.; Van Hoorde, K.; Randazzo, C.L.; Caggia, C. Fermentation of Nocellara Etnea table olives by functional starter cultures at different low salt concentrations. Front. Microbiol. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Servili, M.; Settanni, L.; Veneziani, G.; Esposto, S.; Massitti, O.; Taticchi, A.; Urbani, S.; Montedoro, G.F.; Corsetti, A. The use of Lactobacillus pentosus 1MO to shorten the debittering process time of black table olives (cv. Itrana and Leccino): A pilot-scale application. J. Agric. Food Chem. 2006, 54, 3869–3875. [Google Scholar] [CrossRef]
- Lanza, B.; Di Marco, S.; Bacceli, M.; Di Serio, M.G.; Di Loreto, G.; Cellini, M.; Simone, N. Lactiplantibacillus plantarum used as single, multiple, and mixed starter combined with Candidad boidinii for table olive fermentations: Chemical, textural, and sensorial characterization of final products. Fermentation 2021, 7, 239. [Google Scholar] [CrossRef]
- De Angelis, M.; Campanella, D.; Cosmai, L.; Summo, C.; Rizzello, C.G.; Caponio, F. Microbiota and metabolome of un-started and started Greek-type fermentation of Bella di Cerignola table olives. Food Microbiol. 2015, 52, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Goulas, V.; Xenofontos, E.; Vouras, C.; Nikoloudakis, N.; Tsaltas, D. Benefits of the use of lactic acid bacteria starter in green cracked Cypriot table olives fermentation. Foods 2020, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Kamilari, E.; Tsaltas, D. Evolution of bacterial communities, physicochemical changes and sensorial attributes of natural whole and cracked Picual table olives during spontaneous and inoculated fermentation. Front. Microbiol. 2020, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.; Brenes, M.; de Castro, A.; Romero, C.; Medina, E. Oleuropein hydrolysis by lactic acid bacteria in natural green olives. LWT-Food Sci. Technol. 2017, 78, 165–171. [Google Scholar] [CrossRef]
- Beltrán, G.; Ruano, M.T.; Jiménez, A.; Uceda, M.; Aguilera, M.P. Evaluation of virgin olive oil bitterness by total phenol content analysis. Eur. J. Lipid Sci. Technol. 2007, 109, 193–197. [Google Scholar] [CrossRef]
- Favati, F.; Condelli, N.; Galgano, F.; Caruso, M.C. Extra virgin olive oil bitterness evaluation by sensory and chemical analyses. Food Chem. 2013, 139, 949–954. [Google Scholar] [CrossRef]
- Parafati, L.; Palmeri, R.; Pitino, I.; Restuccia, C. Killer yeasts isolated from olive brines: Technological and probiotic aptitudes. Food Microbiol. 2022, 103, 103950. [Google Scholar] [CrossRef]
- Sánchez, A.H.; de Castro, A.; Rejano, L.; Montaño, A. Comparative study on chemical changes in olive juice and brine during green olive fermentation. J. Agric. Food Chem. 2000, 48, 5975–5980. [Google Scholar] [CrossRef]
- Arroyo-López, F.N.; Romero-Gil, V.; Bautista-Gallego, J.; Rodríguez-Gómez, F.; Jiménez-Díaz, R.; García-García, P.; Querol, A.; Garrido-Fernández, A. Potential benefits of the application of yeast starters in table olive processing. Front. Microbiol. 2012, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.-J.E. Table olive fermentation using starter cultures with multifunctional potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef]
- Golomb, B.L.; Morales, V.; Jung, A.; Yau, B.; Boundy-Mills, K.L.; Marco, M.L. Effects of pectinolytic yeast on the microbial composition and spoilage of olive fermentations. Food Microbiol. 2013, 33, 97–106. [Google Scholar] [CrossRef]
- Ciafardini, G.; Zullo, B.A. Use of selected yeast starter cultures in industrial-scale processing of brined Taggiasca black table olives. Food Microbiol. 2019, 84, 103250. [Google Scholar] [CrossRef]
- Bonatsou, S.; Panagou, E. Fermentation of cv. Kalamata natural black olives with potential multifunctional yeast starters. Foods 2022, 11, 3106. [Google Scholar] [CrossRef] [PubMed]
- IOC (International Olive Council). Trade Standard Applying to Table Olives; IOC: Madrid, Spain, 2004. [Google Scholar]
- Benítez-Cabello, A.; Delgado, A.M.; Quintas, C. Main challenges expected from the impact of climate change on microbial biodiversity of table olives: Current status and trends. Foods 2023, 12, 3712. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Feng, S.; Chen, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Seasonal variations in the chemical composition of Liangshan olive leaves and their antioxidant and anticancer activities. Foods 2019, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.; Tsaltas, D. Current status, recent advances, and main challenges on table olive fermentation: The present meets the future. Front. Microbiol. 2022, 12, 797295. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Ascorbic acid is the only bioactive that is better preserved by high hydrostatic pressure than by thermal treatment of a vegetable beverage. J. Agric. Food Chem. 2010, 58, 10070–10075. [Google Scholar] [CrossRef]
- Sánchez, A.H.; García-García, P.; Fernández, A.G. Spanish-style green table olive shelf-life. Int. J. Food Sci. Technol. 2013, 48, 1559–1568. [Google Scholar] [CrossRef]
- Parker, J.K. Introduction to aroma compounds in foods. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 1; pp. 3–23. [Google Scholar]

| Gordal olives | |||||||||
| Yeasts | LAB | ||||||||
| Treatments | 30 days | 60 days | 180 days | 30 days | 60 days | 180 days | |||
| G-Sp | 5.77 ± 0.14 a | 5.95 ± 0.49 | 4.17 ± 0.77 ab | 6.15 ± 0.14 | 5.93 ± 0.12 | 5.80 ± 0.66 bc | |||
| G-KL | 6.59 ± 0.07 b | 5.94 ± 0.30 | 4.47 ± 0.56 ab | 6.29 ± 0.02 | 5.35 ± 0.52 | 6.21 ± 0.54 c | |||
| G-KH | 7.08 ± 0.18 c | 5.72 ± 0.18 | 5.05 ± 0.13 b | 6.10 ± 0.10 | 5.77 ± 0.16 | 3.70 ± 0.55 a | |||
| G-NM | 6.96 ± 0.16 bc | 6.18 ± 0.45 | 4.88 ± 0.01 b | 6.15 ± 0.08 | 5.84 ± 0.04 | 4.64 ± 0.48 ab | |||
| G-CD | 6.09 ± 1.01 abc | 6.26 ± 0.39 | 4.89 ± 0.37 b | 6.27 ± 0.08 | 5.60 ± 0.25 | 5.57 ± 0.04 bc | |||
| G-CA | 7.21 ± 0.31 bc | 6.37 ± 0.40 | 3.68 ± 0.13 a | 6.34 ± 0.08 | 5.70 ± 0.13 | 5.24 ± 0.65 abc | |||
| Manzanilla olives | |||||||||
| Yeasts | LAB | ||||||||
| Treatments | 30 days | 90 days | 250 days | 30 days | 90 days | 250 days | |||
| M-Sp | 5.45 ± 0.04 a | 5.49 ± 0.01 | 4.83 ± 0.27 ab | ND | ND | ND | |||
| M-KL | 5.44 ± 0.02 a | 5.61 ± 0.26 | 4.36 ± 0.54 ab | ND | ND | ND | |||
| M-KH | 5.47 ± 0.01 a | 5.41 ± 0.05 | 5.03 ± 0.12 b | ND | ND | ND | |||
| M-NM | 5.81 ± 0.05 b | 5.29 ± 0.11 | 4.37 ± 0.28 ab | ND | ND | ND | |||
| M-CD | 5.63 ± 0.20 ab | 5.26 ± 0.21 | 4.56 ± 0.06 a | ND | ND | ND | |||
| M-CA | 5.51 ± 0.11 ab | 5.59 ± 0.11 | 4.50 ± 0.48 ab | ND | ND | ND | |||
| Sample | Oleuropein b | Hydroxytyrosol b | Tyrosol b | Verbascoside b | Hydroxytyrosol 4-Glucoside c |
|---|---|---|---|---|---|
| Uninoculated control | 6677 ± 32 | 73 ± 1 | 26 ± 1 | 21 ± 1 | 8.68 ± 0.03 |
| N. molendinolei IG9 | 6678 ± 61 | 117 ± 1 * | 24 ± 1 | 14 ± 2 | 6.92 ± 0.00 * |
| C. diddensiae IG12 | 6372 ± 209 | 74 ± 1 | 24 ± 3 | 13 ± 3 | 8.70 ± 0.27 |
| K. humilis AG5 | 6862 ± 233 | 71 ± 2 | 28 ± 2 | 25 ± 0 | 9.16 ± 0.37 |
| C. adriatica L30 | 7052 ± 259 | 118 ± 5 * | 33 ± 0 * | 35 ± 20 | 7.88 ± 0.05 * |
| K. lactis L39 | 7012 ± 45 | 75 ± 0 | 20 ± 1 * | 24 ± 0 | 9.46 ± 0.01 * |
| Gordal cv. | |||||||
| G-Sp | G-KL | G-KH | G-NM | G-CD | G-CA | Mean comparisons (ANOVA) b | |
| L* | 53.1 ± 2.2 | 53.4 ± 1.3 | 54.5 ± 1.2 | 54.0 ± 0.4 | 51.8 ± 2.1 | 54.4 ± 0.8 | NS |
| a* | 7.0 ± 0.4 | 6.6 ± 0.2 | 6.9 ± 0.3 | 6.4 ± 0.4 | 7.1 ± 0.1 | 6.9 ± 0.8 | NS |
| b* | 41.2 ± 2.2 | 40.2 ± 1.9 | 42.4 ± 1.5 | 40.8 ± 0.7 | 39.5 ± 1.8 | 41.8 ± 0.5 | NS |
| C* | 41.8 ± 2.1 | 40.7 ± 1.9 | 42.9 ± 1.4 | 41.3 ± 0.8 | 40.1 ± 1.7 | 42.4 ± 0.7 | NS |
| h° | 80.4 ± 1.1 | 80.7 ± 0.2 | 80.7 ± 0.7 | 81.1 ± 0.4 | 79.7 ± 0.3 | 80.7 ± 1.0 | NS |
| ΔE* | - | 2 ± 1 | 2 ± 2 | 1 ± 1 | 2 ± 2 | 2 ± 0 | NS |
| Instrumental firmness | 39.7 ± 2.4 | 37.8 ± 2.6 | 42.3 ± 0.5 | 43.0 ± 3.3 | 43.6 ± 5.8 | 41.8 ± 2.4 | NS |
| Manzanilla cv. | |||||||
| M-Sp | M-KL | M-KH | M-NM | M-CD | M-CA | Mean comparisons (ANOVA) | |
| L* | 43.8 ± 0.8 | 44.1 ± 1.3 | 44.3 ± 0.4 | 45.3 ± 0.8 | 43.0 ± 2.5 | 42.7 ± 0.6 | NS |
| a* | 6.7 ± 0.6 | 7.3 ± 0.4 | 7.2 ± 0.3 | 7.7 ± 0.2 | 6.9 ± 0.3 | 6.7 ± 0.4 | NS |
| b* | 23.6 ± 2.2 | 25.2 ± 1.9 | 24.0 ± 1.0 | 26.8 ± 0.5 | 25.0 ± 2.6 | 23.5 ± 0.7 | NS |
| C* | 24.6 ± 2.3 | 26.3 ± 2.0 | 25.0 ± 1.0 | 27.9 ± 0.5 | 26.0 ± 2.4 | 24.5 ± 0.6 | NS |
| h° | 74.2 ± 0.0 | 73.9 ± 0.3 | 73.4 ± 0.1 | 74.0 ± 0.0 | 74.5 ± 2.3 | 74.0 ± 1.3 | NS |
| ΔE* | - | 2 ± 2 | 1 ± 1 | 4 ± 1 | 3 ± 1 | 1 ± 1 | NS |
| Instrumental firmness | 61.0 ± 0.6 | 61.2 ± 2.4 | 61.3 ± 5.9 | 61.4 ± 0.2 | 59.0 ± 0.3 | 61.7 ± 0.5 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Barba, J.L.; Cortés-Delgado, A.; Sánchez, A.H.; López-López, A.; Montaño, A. Naturally Fermented Gordal and Manzanilla Green Table Olives: Effect of Single Yeast Starters on Fermentation and Final Characteristics of the Products. Fermentation 2024, 10, 439. https://doi.org/10.3390/fermentation10090439
Ruiz-Barba JL, Cortés-Delgado A, Sánchez AH, López-López A, Montaño A. Naturally Fermented Gordal and Manzanilla Green Table Olives: Effect of Single Yeast Starters on Fermentation and Final Characteristics of the Products. Fermentation. 2024; 10(9):439. https://doi.org/10.3390/fermentation10090439
Chicago/Turabian StyleRuiz-Barba, José Luis, Amparo Cortés-Delgado, Antonio Higinio Sánchez, Antonio López-López, and Alfredo Montaño. 2024. "Naturally Fermented Gordal and Manzanilla Green Table Olives: Effect of Single Yeast Starters on Fermentation and Final Characteristics of the Products" Fermentation 10, no. 9: 439. https://doi.org/10.3390/fermentation10090439
APA StyleRuiz-Barba, J. L., Cortés-Delgado, A., Sánchez, A. H., López-López, A., & Montaño, A. (2024). Naturally Fermented Gordal and Manzanilla Green Table Olives: Effect of Single Yeast Starters on Fermentation and Final Characteristics of the Products. Fermentation, 10(9), 439. https://doi.org/10.3390/fermentation10090439






