Transcriptional Profiling and Key Enzyme Activity of Epichloë sinensis Isolated from Festuca sinensis in Response to Na2SeO3
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mycelia
2.2. Analysis of Enzymatic Activities
2.3. RNA Extraction
2.4. RNA Sequencing and Data Analysis
3. Results
3.1. Changes in Enzyme Activities from Mycelia of Epichloë sinensis in Response to Selenium Conditions
3.2. Transcriptome Sequencing of Epichloë sinensis
3.2.1. Quality Assessment and Reference Train Election
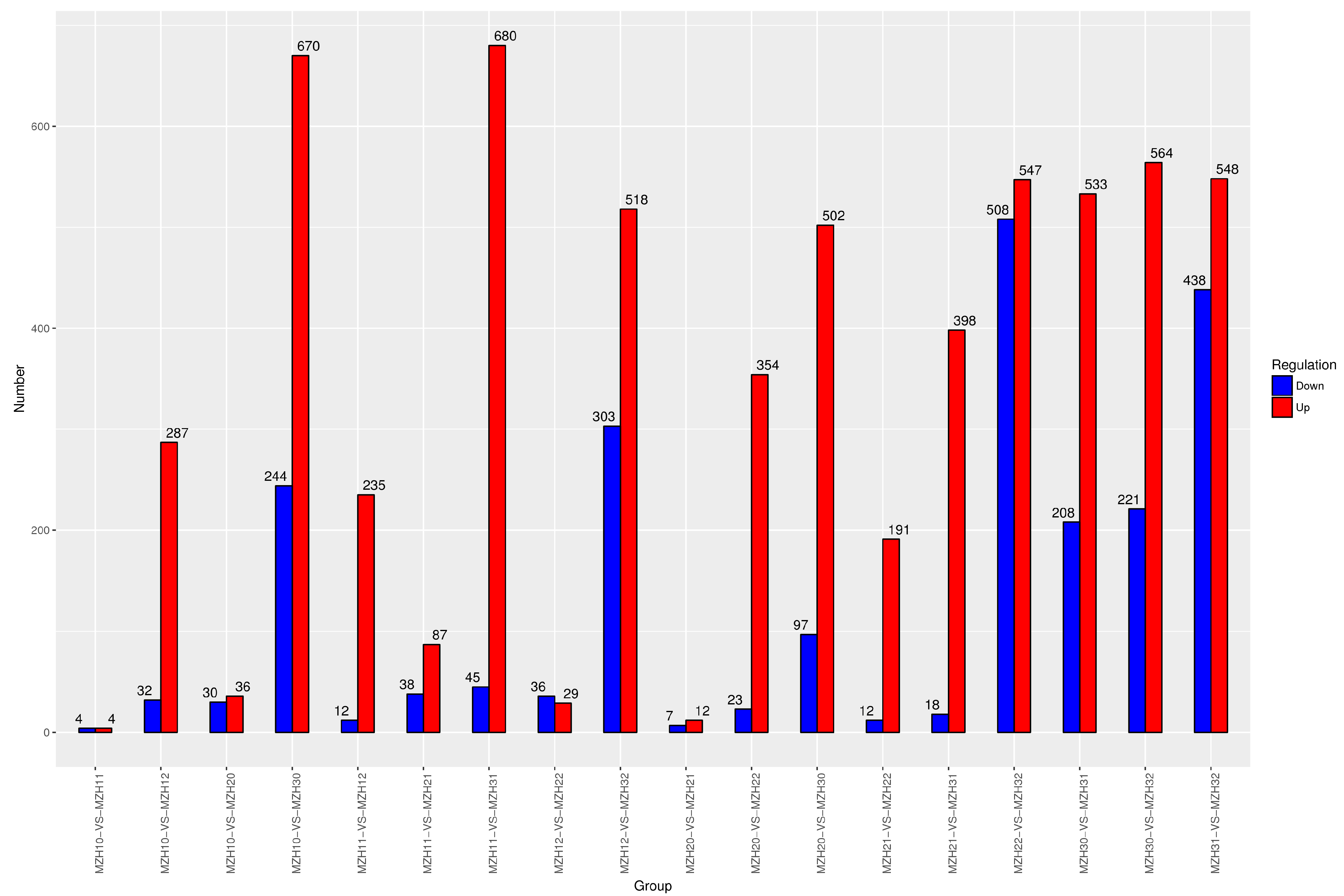
3.2.2. Analysis of Gene Expression and Differentially Expressed Genes (DEGs)
3.3. GO Annotation of Differentially Expressed Genes (DEGs)
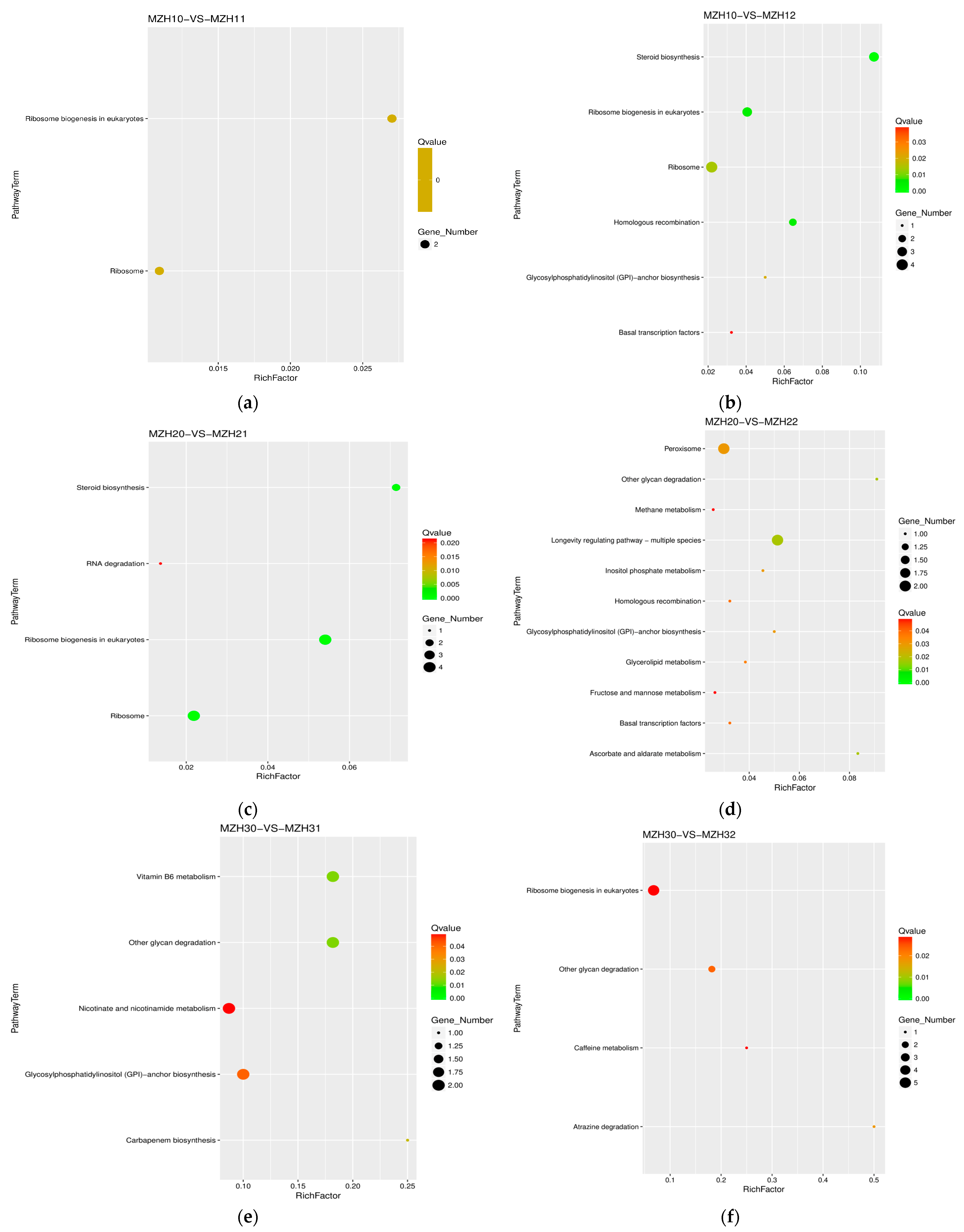
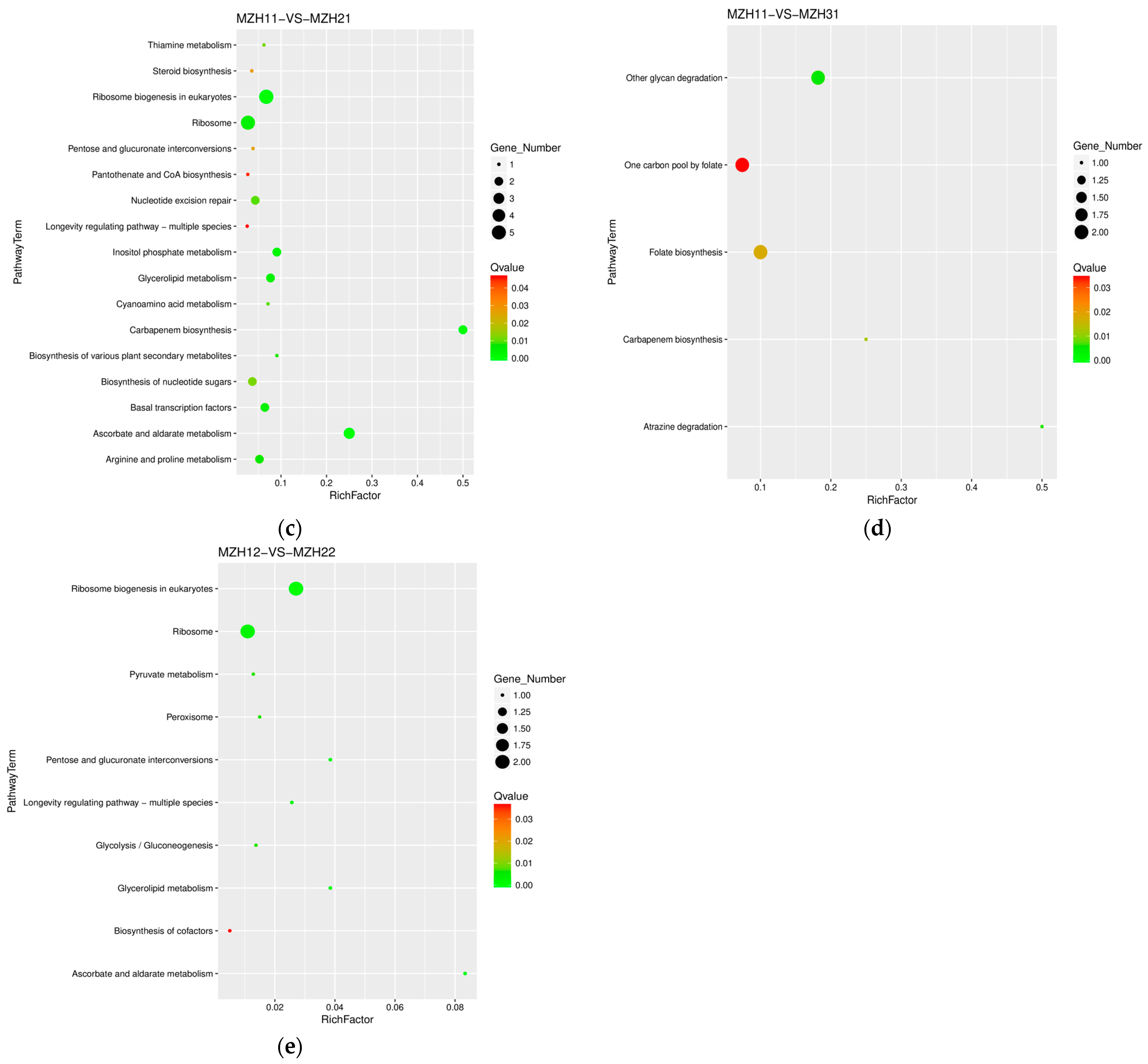
3.4. KEGG Annotation of Differentially Expressed Genes (DEGs)
3.5. Differentially Expressed Genes Involved in Ribosome and Peroxisome
3.6. Differentially Expressed Genes Involved in Carbohydrate Metabolism
3.7. Differentially Expressed Genes Involved in Glycan Biosynthesis and Metabolism
3.8. Differentially Expressed Genes Involved in Lipid Metabolism
3.9. Differentially Expressed Genes Involved in the Metabolism of Cofactors and Vitamins
3.10. Differentially Expressed Genes Involved in the Biosynthesis of Other Secondary Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winkel, L.H.E.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental selenium research: From microscopic processes to global under standing. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium-fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Mániková, D.; Vlasáková, D.; Loduhová, J.; Letavayová, L.; Vigašová, D.; Krascsenitsová, E.; Vlcková, V.; Brozmanová, J.; Chovanec, M. Investigations on the role of base excision repair and non-homologous end-joining pathways in sodium selenite-induced toxicity and mutagenicity in Saccharomyces cerevisiae. Mutagenesis 2009, 25, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Rensing, C.; Zheng, S.X. Microbial reduction and resistance to selenium: Mechanisms, applications and prospects. J. Hazard. Mater. 2022, 421, 126684. [Google Scholar] [CrossRef]
- Kessi, J.; Hanselmann, K.M. Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirill rubrum and Escherichia coli. J. Biol. Chem. 2004, 279, 50662–50669. [Google Scholar] [CrossRef]
- Kessi, J. Enzymic systems proposed to be involved in the dissimilatory reduction of selenite in the purple non-sulfur bacteria Rhodospirillum rubrum and Rhodobacter capsulatus. Microbiology 2006, 152, 731–743. [Google Scholar] [CrossRef]
- Eswayah, A.S.; Smith, T.J.; Gardiner, P.H.E. Microbial transformations of selenium species of relevance to bioremediation. Appl. Environ. Microbiol. 2016, 82, 4848–4859. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Kamnev, A.A. Proteins in microbial synthesis of selenium nanoparticles. Talanta 2017, 174, 539–547. [Google Scholar] [CrossRef]
- Ranjard, L.; Prigent-Combaret, C.; Nazaret, S.; Cournoyer, B. Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase. J. Bacteriol. 2002, 184, 3146–3149. [Google Scholar] [CrossRef]
- Ranjard, L.; Prigent-Combaret, C.; Favre-Bonté, S.; Monnez, C.; Nazaret, S.; Cournoyer, B. Characterization of a novel selenium methyltransferase from freshwater bacteria showing strong similarities with the calicheamicin methyltransferase. BBA—Gene Struct. Expr. 2004, 1679, 80–85. [Google Scholar] [CrossRef]
- Zhang, G.C.; Wang, D.H.; Wangg, D.H.; Wei, G.Y. The mechanism of improved intracellular organic selenium and glutathione contents in selenium-enriched Candida utilis by acid stress. Appl. Microbiol. Biotechnol. 2017, 101, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, V.; Hillestrøm, P.R.; Kápolna, E.; Larsen, E.H.; Olsson, L. Metabolic and bioprocess engineering for production of selenized yeast with increased content of seleno-methylselenocysteine. Metab. Eng. 2011, 13, 282–293. [Google Scholar] [CrossRef]
- Lacourciere, G.M.; Levine, R.L.; Stadtman, T.C. Direct detection of potential selenium delivery proteins by using an Escherichia coli strain unable to incorporate selenium from selenite into proteins. Proc. Natl. Acad. Sci. USA 2002, 99, 9150–9153. [Google Scholar] [CrossRef]
- Malkowski, M.G.; Quartley, E.; Friedman, A.E.; Babulski, J.; Kon, Y.; Wolfley, J.; Said, M.; Luft, J.R.; Phizicky, E.M.; DeTitta, G.T.; et al. Blocking S-adenosylmethionine synthesis in yeast allows selenomethionine incorporation and multiwavelength anomalous dispersion phasing. Proc. Natl. Acad. Sci. USA 2007, 104, 6678–6683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gladyshev, V.N. Comparative genomics of trace elements: Emerging dynamic view of trace element utilization and function. Chem. Rev. 2009, 109, 4828–4861. [Google Scholar] [CrossRef]
- Rosenfeld, C.E.; Kenyon, J.A.; James, B.R.; Santelli, C.M. Selenium (IV, VI) reduction and tolerance by fungi in an oxic environment. Geobiology 2017, 15, 441–452. [Google Scholar] [CrossRef]
- Staicu, L.C.; Ackerson, C.J.; Cornelis, P.; Ye, L.; Berendsen, R.L.; Hunter, W.J.; Noblitt, S.D.; Henry, C.S.; Cappa, J.J.; Montenieri, R.L.; et al. Pseudomonas moraviensis subsp. stanleyae: A bacterial endophyte capable of efficient selenite reduction to elemental selenium under aerobic conditions. J. Appl. Microbiol. 2015, 119, 400–410. [Google Scholar]
- Johnson, L.J.; de Bonth, C.; Briggs, L.R.; Caradus, J.R.; Finch, S.C.; Fleetwood, D.J.; Fletcher, L.R.; Hume, D.E.; Johnson, R.D.; Popay, A.J.; et al. The exploitation of epichloae endophytes for agricultural benefit. Fungal Divers. 2013, 60, 171–188. [Google Scholar] [CrossRef]
- Zamani, N.; Sabzalian, M.R.; Afyuni, M. Elevated atmospheric CO2 combined with Epichloë endophyte may improve growth and Cd phytoremediation potential of tall fescue (Festuca arundinacea L.). Environ. Sci. Pollut. Res. 2024, 31, 8164–8185. [Google Scholar] [CrossRef]
- Adeleke1, B.S.; Babalola, O.O. Roles of plant endosphere microbes in agriculture-a review. J. Plant Growth Regul. 2022, 41, 1411–1428. [Google Scholar] [CrossRef]
- Dwibedi, V.; Rath, S.K.; Joshi, M.; Kaur, R.; Kaur, G.; Singh, D.; Kaur, G.; Kaur, S. Microbial endophytes: Application towards sustainable agriculture and food security. Appl. Microbiol. Biotechnol. 2022, 106, 5359–5384. [Google Scholar] [CrossRef]
- Creamer, C.A.; Leewis, M.C.; Kracmarova-Farren, M.; Papik, J.; Kacur, S.; Freeman, J.; Uhlik, O.; Foster, A.L. A combined compost, dolomite, and endophyte addition is more effective than single amendments for improving phytorestoration of metal contaminated mine tailings. Plant Soil. 2024, 497, 219–240. [Google Scholar] [CrossRef]
- Nassar, A.R.A.; Eid, A.M.; Atta, H.M.E.; EI Naghy, W.S.; Fouda, A. Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci. Rep. 2023, 13, 9054. [Google Scholar] [CrossRef]
- Ni, X.C.; Tian, J.B.; Chen, C.M.; Huang, L.; Lei, J.; Yu, X.J.; Wang, X.G. Multiple exposures to high concentrations of selenate significantly improve selenate tolerability, red elemental selenium (Se0) and selenoprotein biosynthesis in Herbaspirillum camelliae WT00C. World J. Microbl Biotechnol. 2022, 38, 5. [Google Scholar] [CrossRef]
- Hussein, H.G.; El-Sayed, E.S.R.; Younis, N.A.; Hamdy, A.H.A.; Easa, S.M. Harnessing endophytic fungi for biosynthesis of selenium nanoparticles and exploring their bioactivities. AMB Expr. 2022, 12, 68. [Google Scholar] [CrossRef]
- Lindbloma, S.D.; Valdez-Barillas, J.R.; Fakra, S.C.; Marcus, M.A.; Wangelinec, A.L.; Pilon-Smits, E.A.H. Influence of microbial associations on selenium localization and speciation in roots of Astragalus and Stanleya hyperaccumulators. Environ. Exp. Bot. 2013, 88, 33–42. [Google Scholar] [CrossRef]
- Lindblom, S.D.; Wangeline, A.L.; Valdez Barillas, J.R.; Devibiss, B.; Fakra, S.C.; Pilon-Smits, E.A.H. Fungal endophyte Alternaria tenuissima can affect growth and selenium accumulation in its hyperaccumulator host Astragalus bisulcatus. Front. Plant Sci. 2018, 9, 1213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yin, Y.L.; Song, J.Q.; Li, S.X. Mixed sowing improves plant and soil bacterial community restoration in the degraded alpine meadow. Plant Soil. 2024, 49, 379–392. [Google Scholar] [CrossRef]
- Wang, Y.B.; Luo, Y.; Tian, P.; Peng, H.; Feng, J. Preliminary evaluation of the disease resistance of Festuca sinensis infected by Epichloë sinensis. J. Phytopathol. 2021, 169, 623–629. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Li, C.J.; Zhang, X.X.; Johnson, R.; Bao, G.S.; Yao, X.; Chai, Q. Effects of cold shocked Epichloë infected Festuca sinensis on ergot alkaloid accumulation. Fungal Ecol. 2015, 14, 99–104. [Google Scholar] [CrossRef]
- Xu, W.B.; Li, M.M.; Lin, W.H.; Nan, Z.B.; Tian, P. Effects of Epichloë sinensis endophyte and host ecotype on physiology of Festuca sinensis under different soil moisture conditions. Plants 2021, 10, 1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhou, Y.P.; Lin, W.H.; Li, M.M.; Wang, M.N.; Wang, Z.G.; Kuang, Y.; Tian, P. Effect of an Epichloë endophyte on adaptability to water stress in Festuca sinensis. Fungal Ecol. 2017, 30, 39–47. [Google Scholar] [CrossRef]
- Wang, M.N.; Tian, P.; Gao, M.; Li, M.M. The promotion of Festuca sinensis under heavy metal treatment mediated by Epichloë endophyte. Agronomy 2021, 11, 2049. [Google Scholar] [CrossRef]
- Yao, X.; Christensen, M.J.; Bao, G.S.; Zhang, C.P.; Li, X.Z.; Li, C.J.; Nan, Z.B. A toxic endophyte-infected grass helps reverse degradation and loss of biodiversity of over-grazed grasslands in northwest China. Sci. Rep. 2015, 5, 18527. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Xu, W.B.; Li, C.J.; Song, H.; Wang, M.N.; Schardl, C.L.; Nan, Z.B. Phylogenetic relationship and taxonomy of a hybrid Epichloë species symbiotic with Festuca sinensis. Mycol. Prog. 2020, 19, 1069–1081. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, P. Growth and characteristics of two different Epichloë sinensis strains under different cultures. Front. Microbiol. 2021, 12, 726935. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Xie, H.C.; Ma, X.L.; Ju, J.S.; Luo, Q.Y.; Qiao, F. Effect of sodium selenite concentration and culture time on extracellular and intracellular metabolite profiles of Epichloë sp. isolated from Festuca sinensis in liquid culture. Agriculture 2022, 12, 1423. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Jiao, L.; Ju, J.S.; Ma, X.L. Effect of sodium selenite on the metabolite profile of Epichloë sp. mycelia from Festuca sinensis in solid culture. Biol. Trace Elem. Res. 2022, 200, 4865–4879. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCUe, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Harris, M.A.; Clark, J.; Ireland, A.; Lomax, J.; Ashburner, M.; Foulger, R.; Eilbeck, K.; Lewis, S.; Marshall, B.; Mungall, C.; et al. The Gene Oncology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Mohamadhasani, F.; Rahimi, M. Growth response and mycoremediation of heavy metals by fungus Pleurotus sp. Sci. Rep. 2022, 12, 19947. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Xia, C.C.; Yang, F.; Xu, N.; Wu, Q.; Hu, Y.; Xia, L.S.; Wang, C.; Zhou, M.Z. Effect of selenium supplements on the antioxidant activity and nitrite degradation of lactic acid bacteria. World J. Microbiol. Biotechnol. 2019, 35, 61. [Google Scholar] [CrossRef]
- Kaur, T.; Bansal, M.P. Selenium enrichment and anti-oxidant status in baker’s yeast, Saccharomyces cerevisiae at different sodium selenite concentrations. Nutr. Hosp. 2006, 21, 704–708. [Google Scholar]
- Assunção, M.; Martins, L.L.; Mourato, M.P.; Baleiras-Couto, M.M. Effect of selenium on growth and antioxidant enzyme activities of wine related yeasts. World J. Microbiol. Biotechnol. 2015, 31, 1899–1906. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Yin, X.B.; Lin, Z.Q.; Bañuelos, G.S.; Yuan, L.X.; Liu, Y.; Li, M. Inhibitory effect of selenium against Penicillium expansum and its possible mechanisms of action. Curr. Microbiol. 2014, 69, 192–201. [Google Scholar] [CrossRef]
- Poluboyarinov, P.A.; Kuznetsova, A.V.; Moiseeva, I.Y.; Mikulyak, N.I.; Kaplun, A.P. Induction of antioxidant activity by selenium compounds in the Aspergillus niger mycelium. Russ. J. Bioorg. Chem. 2023, 49, 823–835. [Google Scholar] [CrossRef]
- Martínez, F.G.; Moreno-Martin, G.; Mozzi, F.; Madrid, Y.; Pescuma, M. Selenium stress response of the fruit origin strain Fructobacillus tropaeoli CRL 2034. Appl. Microbiol. Biotechnol. 2023, 107, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Bébien, M.; Lagniel, G.; Garin, J.; Touati, D.; Verméglio, A.; Labarre, J. Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J. Bacteriol. 2002, 184, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Roux, M.; Covés, J. The iron-containing superoxide dismutase of Ralstonia metallidurans CH34. FEMS Microbiol. Lett. 2002, 210, 129–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zalepkina, S.A.; Smirnova, V.F.; Borisovb, A.V.; Matsulevich, Z.V. Genomic profiling of the response of Aspergillus oryzae to the treatment with bis(2-pyridine-1-oxide) diselenide. Russ. J. Genet. 2019, 55, 301–308. [Google Scholar] [CrossRef]
- Juby, S.; Soumya, P.; Jayachandran, K.; Radhakrishnan, E.K. Morphological, metabolomic and genomic evidences on drought stress protective functioning of the endophyte Bacillus safensis Ni7. Curr. Microbiol. 2024, 81, 209. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, Y.; Feng, L. The mechanism of degradation of alizarin red by a white-rot fungus Trametes gibbosa. BMC Biotechnol. 2021, 21, 64. [Google Scholar] [CrossRef]
- Senabio, J.A.; de Campos Pereira, F.; Pietro-Souza, W.; Sousa, T.F.; Silva, G.F.; Soares, M.A. Enhanced mercury phytoremediation by Pseudomonodictys pantanalensis sp. nov. A73 and Westerdykella aquatica P71. Braz. J. Microbiol. 2023, 54, 949–964. [Google Scholar] [CrossRef]
- Kieliszek, M.; Bierla, K.; Jiménez-Lamana, J.; Kot, A.M.; Alcántara-Durán, J.; Piwowarek, K.; Błazejak, S.; Szpunar, J. Metabolic response of the yeast Candida utilis during enrichment in selenium. Int. J. Mol. Sci. 2020, 21, 5287. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhou, Y.; Yang, H.; Liao, Y.; Ma, T.; Wang, F. Enhanced selenocysteine biosynthesis for seleno-methylselenocysteine production in Bacillus subtilis. Appl. Microbiol. Biotechnol. 2023, 107, 2843–2854. [Google Scholar] [CrossRef]
- Wang, Z.X.; Zhou, X.Z.; Meng, H.M.; Liu, Y.J.; Zhou, Q.; Huang, B. Comparative transcriptomic analysis of the heat stress response in the filamentous fungus Metarhizium anisopliae using RNA-Seq. Appl. Microbiol. Biotechnol. 2014, 98, 5589–5597. [Google Scholar] [CrossRef]
- Li, X.L.; Yan, L.J.; Li, Q.; Tan, H.; Zhou, J.; Miao, R.Y.; Ye, L.; Peng, W.H.; Zhang, X.P.; Tan, W.; et al. Transcriptional profiling of Auricularia cornea in selenium accumulation. Sci. Rep. 2019, 9, 5641. [Google Scholar] [CrossRef]
- Yang, X.Y.; Dai, X.F.; Jin, H.N.; Lin, G.G.; Wang, Z.H.; Song, Y.; Zhang, W.; Man, C.X.; Jiang, Y.J. Physicochemical and transcriptomic responses of Lactobacillus brevis JLD715 to sodium selenite. J. Sci. Food Agr. 2021, 101, 4332–4341. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.B.; Kroll, K.; Vödisch, M.; Mazurie, A.; Kniemeyer, O.; Cramer, R.A. Transcriptomic and proteomic analyses of the Aspergillus fumigatus hypoxia response using an oxygencontrolled fermenter. BMC Genom. 2012, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Higgins, V.J.; Beckhouse, A.G.; Oliver, A.D.; Rogers, P.J.; Dawes, I.W. Yeast genome-wide expression analysis identifies a strong ergosterol and oxidative stress response during the initial stages of an industrial lager fermentation. Appl. Environ. Microbiol. 2003, 69, 4777–4787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freitas, D.F.; da Rocha, I.M.; Vieira-da-Motta, O.; Santos, C.P. The role of melanin in the biology and ecology of nematophagous fungi. J. Chem. Ecol. 2021, 47, 597–613. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Ma, X.X.; Jiang, S.J.; Hou, X.Y.; Li, P.; Cheng, Y.; Lv, J.; Li, S.R.; Ma, T.Y.; et al. Characterization and anti-infammatory effect of selenium-enriched probiotic Bacillus amyloliquefaciens C-1, a potential postbiotics. Sci. Rep. 2023, 13, 14302. [Google Scholar]
- Oide, S.; Krasnoff, S.B.; Gibson, D.M.; Turgeon, B.G. Intracellular siderophores are essential for ascomycete sexual development in heterothallic Cochliobolus heterostrophus and homothallic Gibberella zeae. Eukaryot. Cell 2007, 6, 1339–1353. [Google Scholar] [CrossRef]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef]
- Koulman, A.; Lee, V.T.; Fraser, K.; Johnson, L.; Arcus, V.; Lott, S.J.; Rasmussen, S. Identification of extracellular siderophores and a related peptide from the endophytic fungus Epichloë festucae in culture and endophyte-infected Lolium perenne. Phytochemistry 2012, 75, 128–139. [Google Scholar] [CrossRef]
- Rashmi, V.; ShylajaNaciyar, M.; Rajalakshmi, R.; D’Souza, S.F.; Prabaharan, D.; Uma, L. Siderophore mediated uranium sequestration by marine cyanobacterium Synechococcus elongatus BDU 130911. Bioresour. Technol. 2013, 130, 204–210. [Google Scholar] [CrossRef]
- Song, Y.; Wu, X.; Li, Z.; Ma, Q.Q.; Bao, R. Molecular mechanism of siderophore regulation by the Pseudomonas aeruginosa BfmRS two-component system in response to osmotic stress. Commun. Biol. 2024, 7, 295. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Merten, D.; Svatos, A.; Buechel, G.; Kothe, E. Siderophores mediate reduced and increased uptake of cadmium by Streptomyces tendae F4 and sunflower (Helianthus annuus), respectively. J. Appl. Microbiol. 2009, 107, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Kragl, C.; Schrettl, M.; Abt, B.; Sarg, B.; Lindner, H.H.; Haas, H. EstB-mediated hydrolysis of the siderophore triacetylfusarinine C optimizes iron uptake of Aspergillus fumigatus. Eukaryot. Cell 2007, 6, 1278–1285. [Google Scholar] [CrossRef]
- Wallner, A.; Blatzer, M.; Schrettl, M.; Sarg, B.; Lindner, H.; Haas, H. Ferricrocin, a siderophore involved in intra- and transcellular iron distribution in Aspergillus fumigates. Appl. Environ. Microbiol. 2009, 75, 4194–4196. [Google Scholar] [CrossRef] [PubMed]
- Eisendle, M.; Oberegger, H.; Zadra, I.; Haas, H. The intracellular siderophore ferricrocin is involved in iron storage, oxidative-stress resistance, germination, and sexual development in Aspergillus nidulans. Eukaryot. Cell 2006, 5, 1596–1603. [Google Scholar] [CrossRef]
- Hof, C.; Eisfeld, K.; Kai, W.; Antelo, L.; Foster, A.J.; Anke, H. Ferricrocin synthesis in Magnaporthe grisea and its role in pathogenicity in rice. Mol. Plant Pathol. 2007, 8, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Jirakkakul, J.; Wichienchote, N.; Likhitrattanapisal, S.; Supawadee Ingsriswang, S.; Yoocha, T.; Tangphatsornruang, S.; Wasuwan, R.; Cheevadhanarak, S.; Morakot Tanticharoen, M.; Amnuaykanjanasin, A. Iron homeostasis in the absence of ferricrocin and its consequences in fungal development and insect virulence in Beauveria bassiana. Sci. Rep. 2021, 11, 19624. [Google Scholar] [CrossRef]






| Enzyme Activity | Culture Time (h) | Se Concentration (mM) | ||
|---|---|---|---|---|
| 0 | 0.1 | 0.2 | ||
| Superoxide dismutase (U/g) | 2 | 91.62 ± 6.91 Cc | 255.9 ± 16.17 Bb | 869.53 ± 80.28 Ba |
| 12 | 351.61 ± 11.20 Bb | 962.85 ± 9.48 Aa | 990.39 ± 47.82 Aa | |
| 36 | 618.35 ± 31.47 Ab | 1013.25 ± 91.39 Aab | 1094.41 ± 97.61 Aa | |
| Glutathione reductase (nmol/min/g) | 2 | 786.13 ± 61.89 Cc | 1143.47 ± 61.89 Cb | 1750.93 ± 123.78 Ba |
| 12 | 2108.27 ± 163.75 Ab | 2394.13 ± 163.75 Ab | 3930.67 ± 327.50 Aa | |
| 36 | 1465.07 ± 163.75 Bb | 1929.6 ± 185.68 Ba | 2108.27 ± 163.75 Ba | |
| Glutathione S-transferase (nmol/min/g) | 2 | 81.31 ± 4.02 Cc | 148.22 ± 8.85 Cb | 172.85 ± 9.66 Ca |
| 12 | 152.87 ± 14.64 Bb | 432.12 ± 24.14 Ba | 446.06 ± 24.14 Ba | |
| 36 | 264.85 ± 24.14 Ab | 518.55 ± 50.87 Aa | 521.33 ± 26.56 Aa | |
| Cysteine synthetase (U/g) | 2 | 0.70 ± 0.04 Bb | 1.11 ± 0.07 Ba | 1.22 ± 0.23 Aa |
| 12 | 1.08 ± 0.02 Ab | 1.13 ± 0.03 Ba | 1.24 ± 0.08 Aa | |
| 36 | 1.14 ± 0.02 Ac | 1.25 ± 0.02 Ab | 1.38 ± 0.03 Aa | |
| Methionine synthetase (U/kg) | 2 | 9.20 ± 0.58 Cb | 21.00 ± 1.80 Ca | 21.55 ± 0.94 Ca |
| 12 | 17.35 ± 2.84 Bc | 95.18 ± 0.67 Bb | 113.30 ± 9.47 Ba | |
| 36 | 29.55 ± 3.44 Ac | 177.09 ± 7.80 Ab | 205.06 ± 2.59 Aa | |
| Selenocysteine methyltransferase (U/g) | 2 | 0.39 ± 0.01 Bb | 1.65 ± 0.14 Ba | 1.77 ± 0.42 Ba |
| 12 | 0.44 ± 0.03 ABc | 2.50 ± 0.12 Aa | 1.75 ± 0.31 Bb | |
| 36 | 0.59 ± 0.13 Ab | 2.61 ± 0.13 Aa | 3.08 ± 0.85 Aa | |
| Group | GO Analysis | KEGG Analysis | ||||
|---|---|---|---|---|---|---|
| GO Term | Up-DEGs | Down-DEGs | KEGG Pathway | Up-DEGs | Down-DEGs | |
| MZH10-VS-MZH11 | 9 | 9 | 0 | 2 | 0 | 4 |
| MZH10-VS-MZH12 | 33 | 28 | 30 | 6 | 6 | 8 |
| MZH11-VS-MZH12 | 31 | 31 | 0 | 12 | 12 | 3 |
| MZH20-VS-MZH21 | 40 | 34 | 18 | 4 | 9 | 2 |
| MZH20-VS-MZH22 | 24 | 38 | 1 | 11 | 13 | 0 |
| MZH21-VS-MZH22 | 30 | 28 | 9 | 11 | 9 | 12 |
| MZH30-VS-MZH31 | 30 | 38 | 20 | 5 | 5 | 4 |
| MZH30-VS-MZH32 | 22 | 34 | 18 | 4 | 2 | 7 |
| MZH31-VS-MZH32 | 66 | 238 | 81 | 2 | 65 | 7 |
| MZH10-VS-MZH20 | 31 | 23 | 12 | 11 | 6 | 9 |
| MZH10-VS-MZH30 | 59 | 100 | 52 | 2 | 4 | 11 |
| MZH20-VS-MZH30 | 33 | 55 | 11 | 1 | 19 | 2 |
| MZH11-VS-MZH21 | 38 | 22 | 21 | 17 | 21 | 13 |
| MZH11-VS-MZH31 | 24 | 45 | 2 | 5 | 8 | 0 |
| MZH21-VS-MZH31 | 20 | 26 | 4 | 13 | 16 | 8 |
| MZH12-VS-MZH22 | 5 | 2 | 3 | 10 | 4 | 8 |
| MZH12-VS-MZH32 | 27 | 29 | 31 | 0 | 0 | 0 |
| MZH22-VS-MZH32 | 70 | 57 | 144 | 2 | 35 | 7 |
| Group | Pathway ID | KEGG Name | Gene ID | Fold-Change |
|---|---|---|---|---|
| MZH10-VS-MZH11 | ko03010 ko03008 | ribosome ribosome biogenesis in eukaryotes | tig00002100G000020 | 0.44 |
| tig00002101G005230 | 0.46 | |||
| MZH10-VS-MZH12 | ko03010 | ribosome | tig00002100G000010 | 2.54 |
| tig00000015G000020 | 4.87 | |||
| tig00002101G005240 | 0.28 | |||
| tig00002100G000020 | 0.30 | |||
| ko00563 | glycosylphosphatidylinositol (GPI)-anchor biosynthesis | tig00000002G007450 | 4.50 | |
| ko00100 | steroid biosynthesis | tig00000002G007490 | 0.45 | |
| tig00000002G018450 | 0.47 | |||
| tig00000030G008490 | 0.42 | |||
| MZH20-VS-MZH21 | ko03010 ko03008 | ribosome ribosome biogenesis in eukaryotesRibosome | tig00002101G005240 | 7.22 |
| tig00002100G000020 | 6.21 | |||
| tig00002101G005250 | 5.42 | |||
| tig00002100G000010 | 3.09 | |||
| ko00100 | steroid biosynthesis | tig00000002G007490 | 0.42 | |
| tig00000030G008490 | 0.33 | |||
| MZH20-VS-MZH22 | ko04146 | peroxisome | tig00000001G030720 | 2.64 |
| tig00000107G004080 | 3.04 | |||
| ko00053 ko00562 | aldarate metabolism inositol phosphate metabolism | tig00000122G002730 | 2.21 | |
| ko00051 | fructose and mannose metabolism | tig00000099G005510 | 2.42 | |
| ko00563 | glycosylphosphatidylinositol (GPI)-anchor biosynthesis | tig00000002G007450 | 7.78 | |
| ko00511 | other glycan degradation | tig00000016G001160 | 2.73 | |
| ko00561 | glycerolipid metabolism | tig00000099G005510 | 2.42 | |
| MZH30-VS-MZH31 | ko00563 | glycosylphosphatidylinositol (GPI)-anchor biosynthesis | tig00000002G007450 | 322.50 |
| tig00000122G003550 | 0.47 | |||
| ko00511 | other glycan degradation | tig00000002G008510 | 2.66 | |
| tig00000016G001160 | 5.08 | |||
| ko00750 | vitamin B6 metabolism | tig00000016G004440 | 0.46 | |
| tig00000002G011990 | 0.50 | |||
| ko00760 | nicotinate and nicotinamide metabolism | tig00002100G002750 | 0.49 | |
| tig00000030G016720 | 2.26 | |||
| ko00332 | carbapenem biosynthesis | tig00000001G029780 | 2.20 | |
| MZH30-VS-MZH32 | ko03008 | ribosome biogenesis in eukaryotes | tig00002101G005240, | 0.31 |
| tig00002100G000020, | 0.37 | |||
| tig00002100G000010, | 0.35 | |||
| tig00002101G005230, | 0.49 | |||
| tig00000015G006270 | 0.48 | |||
| ko00511 | other glycan degradation | tig00000016G001160 | 6.27 | |
| tig00000001G036310 | 0.49 | |||
| ko00232 | caffeine metabolism | tig00000077G005930 | 2.05 | |
| MZH10-VS-MZH20 | ko03008 | ribosome biogenesis in eukaryotes | tig00002101G005240 | 0.32 |
| tig00000016G015310 | 0.45 | |||
| ko04146 | peroxisome | tig00000094G002550 | 2.08 | |
| ko00053 ko00562 | ascorbate and aldarate metabolism inositol phosphate metabolism | tig00000105G003930 | 0.27 | |
| tig00000122G002730 | 0.44 | |||
| ko00511 | other glycan degradation | tig00000001G036310 | 2.88 | |
| ko00561 | glycerolipid metabolism | tig00000077G000990 | 0.49 | |
| MZH10-VS-MZH30 | ko03008 | ribosome biogenesis in eukaryotes | tig00002101G004790 | 3.36 |
| tig00000105G002750 | 2.05 | |||
| tig00002100G000010 | 0.23 | |||
| tig00002100G000020 | 0.31 | |||
| tig00002101G005250 | 0.40 | |||
| tig00002101G005240 | 0.47 | |||
| tig00000030G011540 | 0.42 | |||
| tig00000016G003870 | 0.47 | |||
| tig00000002G011500 | 0.45 | |||
| tig00000002G011250 | 0.49 | |||
| MZH11-VS-MZH21 | ko03008 ko03010 | ribosome biogenesis in eukaryotes ribosome | tig00002100G000020 | 7.11 |
| tig00002101G005250 | 6.53 | |||
| tig00002101G005230 | 3.65 | |||
| tig00002101G005240 | 3.58 | |||
| tig00002100G000010 | 2.83 | |||
| ko00053 ko00562 | ascorbate and aldarate metabolism inositol phosphate metabolism | tig00000105G003930 | 0.44 | |
| tig00000122G002730 | 0.50 | |||
| ko00100 | steroid biosynthesis | tig00000030G008490 | 0.42 | |
| ko00561 | glycerolipid metabolism | tig00000094G003040 | 0.50 | |
| tig00002100G000060 | 2.40 | |||
| ko00332 | carbapenem biosynthesis | tig00000001G038060 | 2.18 | |
| tig00000001G029780 | 0.45 | |||
| MZH11-VS-MZH31 | ko00511 | other glycan degradation | tig00000002G008510 | 2.77 |
| tig00000016G001160 | 3.21 | |||
| ko00332 | carbapenem biosynthesis | tig00000001G038060 | 2.75 | |
| MZH12-VS-MZH22 | ko03008 | ribosome biogenesis in eukaryotes | tig00002101G005250 | 2.03 |
| tig00002100G000010 | 0.47 | |||
| ko04146 | peroxisome | tig00000001G030720 | 2.31 | |
| ko00561 | glycerolipid metabolism | tig00000122G003720 | 0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Liu, Y.; Ma, Y.; Qiao, F.; Xie, H.; Luo, Q. Transcriptional Profiling and Key Enzyme Activity of Epichloë sinensis Isolated from Festuca sinensis in Response to Na2SeO3. Fermentation 2024, 10, 445. https://doi.org/10.3390/fermentation10090445
Zhou L, Liu Y, Ma Y, Qiao F, Xie H, Luo Q. Transcriptional Profiling and Key Enzyme Activity of Epichloë sinensis Isolated from Festuca sinensis in Response to Na2SeO3. Fermentation. 2024; 10(9):445. https://doi.org/10.3390/fermentation10090445
Chicago/Turabian StyleZhou, Lianyu, Yu Liu, Yun Ma, Feng Qiao, Huichun Xie, and Qiaoyu Luo. 2024. "Transcriptional Profiling and Key Enzyme Activity of Epichloë sinensis Isolated from Festuca sinensis in Response to Na2SeO3" Fermentation 10, no. 9: 445. https://doi.org/10.3390/fermentation10090445
APA StyleZhou, L., Liu, Y., Ma, Y., Qiao, F., Xie, H., & Luo, Q. (2024). Transcriptional Profiling and Key Enzyme Activity of Epichloë sinensis Isolated from Festuca sinensis in Response to Na2SeO3. Fermentation, 10(9), 445. https://doi.org/10.3390/fermentation10090445




