The Most Promising Next-Generation Probiotic Candidates—Impact on Human Health and Potential Application in Food Technology
Abstract
1. Introduction
2. Gut Microbiota
2.1. The Role of Food in Modulating the Gut Microbiota
2.2. The New Generation of Probiotics and Postbiotics
2.2.1. Akkermansia muciniphila
2.2.2. Faecalibacterium prausnitzii
2.2.3. Bacteroides thetaiotaomicron
2.2.4. Christensenella minuta
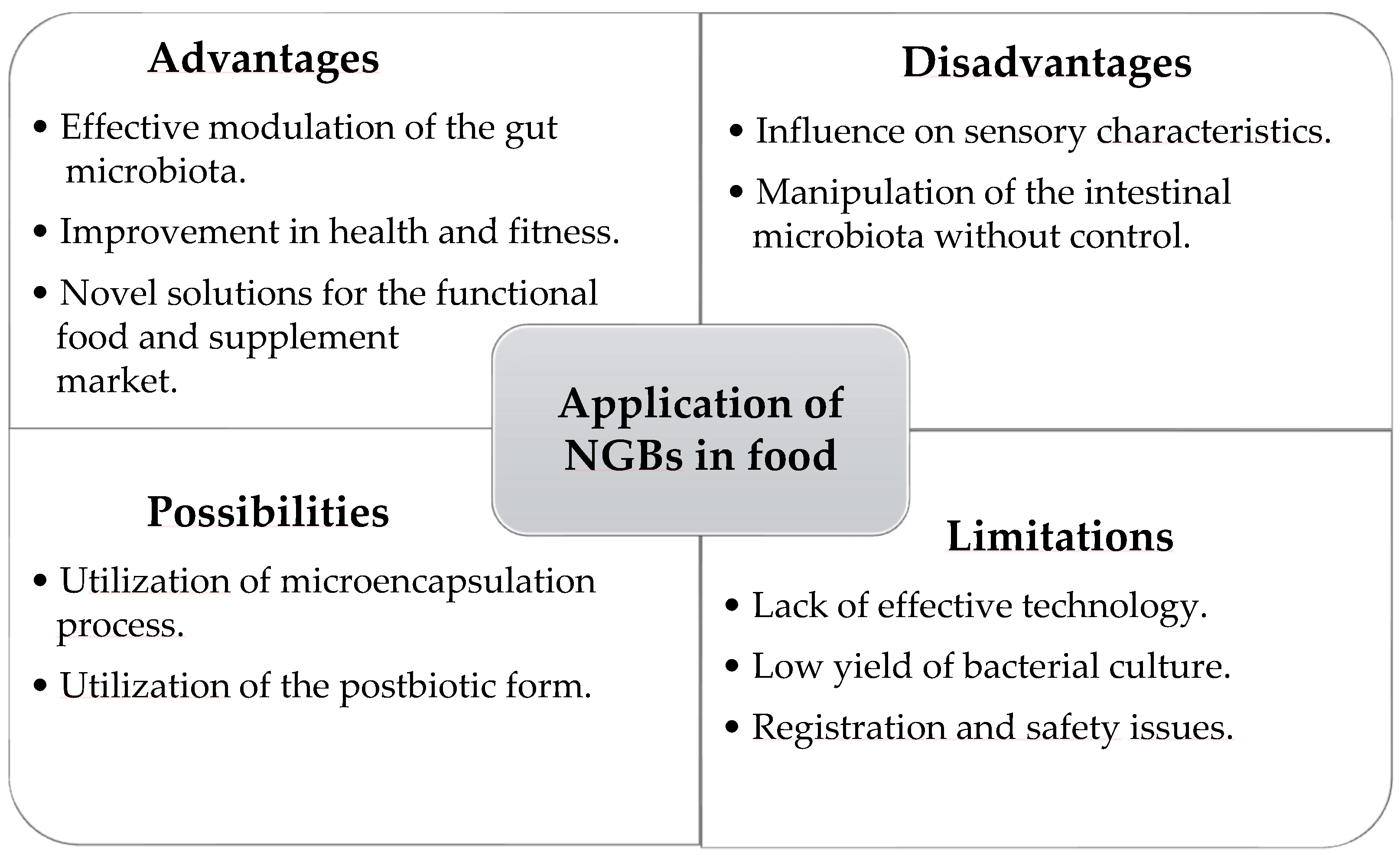
3. A Perspective on the Potential Use of These Types of Bacteria in Food Technology
4. Limitations and Prospects for the Use of Next-Generation Probiotics in Food Supplementation and Technology
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Gille, D.; Schmid, A.; Walther, B.; Vergères, G. Fermented Food and Non-Communicable Chronic Diseases: A Review. Nutrients 2018, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Grunert, K.G.; do Canto, N.R.; Liu, R.; Salnikova, E. Well-being as a global food trend: Health, sustainability and authenticity. Dan. Food Innov. Pap. 2019. [Google Scholar]
- Zwierczyk, U.; Sowada, C.; Duplaga, M. Eating Choices—The Roles of Motivation and Health Literacy: A Cross-Sectional Study. Nutrients 2022, 14, 4026. [Google Scholar] [CrossRef] [PubMed]
- Baz, S.S.B.; Malibarey, W.M.; Alsalmi, H.A.; Alzaydi, M.D.; Alqahtani, A.M.; Alghamdi, R.Y.; Sr, S.S.B.B.; Ii, W.M.M.; Sr, H.A.; Alzaydi, M.D.; et al. The Impact of a Healthy Lifestyle on Psychological Well-Being Among Saudi Adolescent Girls Attending Secondary Schools in Taif City, Saudi Arabia. Cureus 2023, 15, e50189. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Kovačević, D.B.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Develop-ment, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Küçükgöz, K.; Trząskowska, M. Nondairy Probiotic Products: Functional Foods That Require More Attention. Nutrients 2022, 14, 753. [Google Scholar] [CrossRef]
- Goetzke, B.; Nitzko, S.; Spiller, A. Consumption of Organic and Functional Food. A Matter of Well-Being and Health? Appetite 2014, 77, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Functional and Natural Health Food: Market Value Forecast Worldwide 2023–2033. Available online: https://www.statista.com/statistics/502267/global-health-and-wellness-food-market-value/ (accessed on 8 June 2024).
- Shiby, V.K.; Mishra, H.N. Fermented Milks and Milk Products as Functional Foods—A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Granato, D.; Branco, G.F.; Cruz, A.G.; de Faria, J.A.F.; Shah, N.P. Probiotic Dairy Products as Functional Foods. Compr. Rev. Food Sci. Food Saf. 2010, 9, 455–470. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus Beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Ghelardi, E.; Mazzantini, D.; Celandroni, F.; Calvigioni, M.; Panattoni, A.; Lupetti, A.; Bois De Fer, B.; Perez, M. Analysis of the Microbial Content of Probiotic Products Commercialized Worldwide and Survivability in Conditions Mimicking the Human Gut Environment. Front. Microbiol. 2023, 14, 1127321. [Google Scholar] [CrossRef] [PubMed]
- Aponte, M.; Murru, N.; Shoukat, M. Therapeutic, Prophylactic, and Functional Use of Probiotics: A Current Perspective. Front. Microbiol. 2020, 11, 562048. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Negrete-Romero, B.; Valencia-Olivares, C.; Baños-Dossetti, G.A.; Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Nutritional Contributions and Health Associations of Traditional Fermented Foods. Fermentation 2021, 7, 289. [Google Scholar] [CrossRef]
- Kruk, M.; Lalowski, P.; Hoffmann, M.; Trząskowska, M.; Jaworska, D. Probiotic Bacteria Survival and Shelf Life of High Fibre Plant Snack—Model Study. Plant Foods Hum. Nutr. 2024. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Butani, K.; Kumar, A.; Singh, S.; Prajapati, B.G. Effects of Fermented Food Consumption on Non-Communicable Diseases. Foods 2023, 12, 687. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Vera-Santander, V.E.; Hernández-Figueroa, R.H.; Jiménez-Munguía, M.T.; Mani-López, E.; López-Malo, A. Health Benefits of Consuming Foods with Bacterial Probiotics, Postbiotics, and Their Metabolites: A Review. Molecules 2023, 28, 1230. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sabharwal, V.; Kaushik, P.; Joshi, A.; Aayushi, A.; Suri, M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022, 6, 887642. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Das, T.K.; Pradhan, S.; Chakrabarti, S.; Mondal, K.C.; Ghosh, K. Current Status of Probiotic and Related Health Benefits. Appl. Food Res. 2022, 2, 100185. [Google Scholar] [CrossRef]
- Borkent, J.; Ioannou, M.; Laman, J.D.; Haarman, B.C.M.; Sommer, I.E.C. Role of the Gut Microbiome in Three Major Psychiatric Disorders. Psychol. Med. 2022, 52, 1222–1242. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–Gut–Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Sig. Transduct. Target Ther. 2024, 9, 1–53. [Google Scholar] [CrossRef]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-Generation Probiotics: The Upcoming Biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef]
- Hasnain, M.A.; Kang, D.-K.; Moon, G.-S. Research Trends of next Generation Probiotics. Food Sci. Biotechnol. 2024, 33, 2111–2121. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The Role of the Gut Microbiome in the Healthy Adult Status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wrigley-Carr, H.E.; van Dorst, J.M.; Ooi, C.Y. Intestinal Dysbiosis and Inflammation in Cystic Fibrosis Impacts Gut and Multi-Organ Axes. Med. Microecol. 2022, 13, 100057. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Sig. Transduct. Target Ther. 2022, 7, 1–28. [Google Scholar] [CrossRef]
- Vernocchi, P.; Del Chierico, F.; Putignani, L. Gut Microbiota Metabolism and Interaction with Food Components. Int. J. Mol. Sci. 2020, 21, 3688. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ijssennagger, N.; van der Meer, R.; van Mil, S.W.C. Sulfide as a Mucus Barrier-Breaker in Inflammatory Bowel Dis-ease? Trends Mol. Med. 2016, 22, 190–199. [Google Scholar] [CrossRef]
- Kaliannan, K.; Wang, B.; Li, X.-Y.; Kim, K.-J.; Kang, J.X. A Host-Microbiome Interaction Mediates the Opposing Effects of Omega-6 and Omega-3 Fatty Acids on Metabolic Endotoxemia. Sci. Rep. 2015, 5, 11276. [Google Scholar] [CrossRef]
- Dahl, W.J.; Rivero Mendoza, D.; Lambert, J.M. Chapter Eight—Diet, Nutrients and the Microbiome. In Progress in Molecular Biology and Translational Science; The Microbiome in Health and Disease; Sun, J., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 171, pp. 237–263. [Google Scholar]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Wein-berger, A.; et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef]
- Reid, G. Probiotics: Definition, Scope and Mechanisms of Action. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 17–25. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging Health Concepts in the Probiotics Field: Streamlining the Definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Zimmermann, A.K.; Ouwehand, A.C. Contemporary Meta-Analysis of Short-Term Probiotic Consumption on Gastrointestinal Transit. World J. Gastroenterol. 2016, 22, 5122–5131. [Google Scholar] [CrossRef]
- Ng, Q.X.; Lim, Y.L.; Yaow, C.Y.L.; Ng, W.K.; Thumboo, J.; Liew, T.M. Effect of Probiotic Supplementation on Gut Microbiota in Patients with Major Depressive Disorders: A Systematic Review. Nutrients 2023, 15, 1351. [Google Scholar] [CrossRef] [PubMed]
- Klingbeil, E.; de La Serre, C.B. Microbiota Modulation by Eating Patterns and Diet Composition: Impact on Food In-take. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1254–R1260. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Scott, S.M.; Whelan, K. Probiotics and Constipation: Mechanisms of Action, Evidence for Effectiveness and Utilisation by Patients and Healthcare Professionals. Proc. Nutr. Soc. 2020, 79, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Lin, T.-L.; Tsai, Y.-L.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Next Generation Probiotics in Disease Amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Saarela, M.H. Safety Aspects of next Generation Probiotics. Curr. Opin. Food Sci. 2019, 30, 8–13. [Google Scholar] [CrossRef]
- Kubasova, T.; Seidlerova, Z.; Rychlik, I. Ecological Adaptations of Gut Microbiota Members and Their Consequences for Use as a New Generation of Probiotics. Int. J. Mol. Sci. 2021, 22, 5471. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics in Medicine: A Long Debate. Front. Immunol. 2020, 11, 2192. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia Muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef]
- Hagi, T.; Belzer, C. The Interaction of Akkermansia Muciniphila with Host-Derived Substances, Bacteria and Diets. Appl. Microbiol. Biotechnol. 2021, 105, 4833–4841. [Google Scholar] [CrossRef] [PubMed]
- van der Ark, K.C.H.; Aalvink, S.; Suarez-Diez, M.; Schaap, P.J.; de Vos, W.M.; Belzer, C. Model-Driven Design of a Minimal Medium for Akkermansia Muciniphila Confirms Mucus Adaptation. Microb. Biotechnol. 2018, 11, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H.; Zhang, F. Akkermansia Muciniphila Is a Promising Probiotic. Microb. Biotechnol. 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Abuqwider, J.N.; Mauriello, G.; Altamimi, M. Akkermansia Muciniphila, a New Generation of Beneficial Microbiota in Modulating Obesity: A Systematic Review. Microorganisms 2021, 9, 1098. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia Muciniphila: Paradigm for next-Generation Beneficial Microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and Its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef]
- Miranda, V.C.; Souza, R.O.; Quintanilha, M.F.; Gallotti, B.; Assis, H.C.; Faria, A.M.C.; Nicoli, J.R.; Cara, D.C.; Mar-tins, F.S. A Next-Generation Bacteria (Akkermansia Muciniphila BAA-835) Presents Probiotic Potential Against Ovalbumin-Induced Food Allergy in Mice. Probiotics Antimicro. Prot. 2024, 16, 737–751. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Pasteurised Akkermansia Muciniphila as a Novel Food Pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06780. [Google Scholar] [CrossRef]
- Parrish, A.; Boudaud, M.; Grant, E.T.; Willieme, S.; Neumann, M.; Wolter, M.; Craig, S.Z.; De Sciscio, A.; Cosma, A.; Hunewald, O.; et al. Akkermansia Muciniphila Exacerbates Food Allergy in Fibre-Deprived Mice. Nat. Microbiol. 2023, 8, 1863–1879. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Machado, D.; Barbosa, J.C.; Domingos, M.; Almeida, D.; Andrade, J.C.; Freitas, A.C.; Gomes, A.M. Revealing Antimi-crobial Resistance Profile of the Novel Probiotic Candidate Faecalibacterium Prausnitzii DSM 17677. Int. J. Food Microbiol. 2022, 363, 109501. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhao, S.; Li, Y. Faecalibacterium Prausnitzii: A Next-Generation Probiotic in Gut Disease Improvement. Can. J. Infect. Dis. Med. Microbiol. 2021, 6666114. [Google Scholar] [CrossRef]
- Maioli, T.U.; Borras-Nogues, E.; Torres, L.; Barbosa, S.C.; Martins, V.D.; Langella, P.; Azevedo, V.A.; Chatel, J.-M. Possible Benefits of Faecalibacterium Prausnitzii for Obesity-Associated Gut Disorders. Front. Pharmacol. 2021, 12, 740636. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional Characterization of Novel Faecalibacterium Prausnitzii Strains Isolated from Healthy Volunteers: A Step Forward in the Use of F. Prausnitzii as a Next-Generation Probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef]
- Ferreira-Halder, C.V.; de Faria, A.V.S.; Andrade, S.S. Action and Function of Faecalibacterium Prausnitzii in Health and Disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef]
- Khan, M.T.; van Dijl, J.M.; Harmsen, H.J.M. Antioxidants Keep the Potentially Probiotic but Highly Oxygen-Sensitive Human Gut Bacterium Faecalibacterium Prausnitzii Alive at Ambient Air. PLoS ONE 2014, 9, e96097. [Google Scholar] [CrossRef]
- Ryan, D.; Jenniches, L.; Reichardt, S.; Barquist, L.; Westermann, A.J. A High-Resolution Transcriptome Map Identifies Small RNA Regulation of Metabolism in the Gut Microbe Bacteroides Thetaiotaomicron. Nat. Commun. 2020, 11, 3557. [Google Scholar] [CrossRef]
- Ye, M.; Yu, J.; Shi, X.; Zhu, J.; Gao, X.; Liu, W. Polysaccharides Catabolism by the Human Gut Bacterium -Bacteroides Thetaiotaomicron: Advances and Perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 3569–3588. [Google Scholar] [CrossRef]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A Genomic View of the Human-Bacteroides Thetaiotaomicron Symbiosis. Science 2003, 299, 2074–2076. [Google Scholar] [CrossRef]
- Xu, J.; Chiang, H.C.; Bjursell, M.K.; Gordon, J.I. Message from a Human Gut Symbiont: Sensitivity Is a Prerequisite for Sharing. Trends Microbiol. 2004, 12, 21–28. [Google Scholar] [CrossRef]
- Comstock, L.E.; Coyne, M.J. Bacteroides Thetaiotaomicron: A Dynamic, Niche-Adapted Human Symbiont. BioEssays 2003, 25, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, L.; Miquel, S.; Noordine, M.-L.; Bouet, S.; Chevalier-Curt, M.J.; Robert, V.; Philippe, C.; Bridonneau, C.; Cherbuy, C.; Robbe-Masselot, C.; et al. Bacteroides Thetaiotaomicron and Faecalibacterium Prausnitziiinfluence the Production of Mucus Glycans and the Development of Goblet Cells in the Colonic Epithelium of a Gnotobiotic Model Rodent. BMC Biol. 2013, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, J.; Zhang, H.; Lee, Y.-K.; Zhai, Q.; Chen, W. Roles of Intestinal Bacteroides in Human Health and Diseases. Crit. Rev. Food Sci. Nutr. 2021, 61, 3518–3536. [Google Scholar] [CrossRef] [PubMed]
- Durant, L.; Stentz, R.; Noble, A.; Brooks, J.; Gicheva, N.; Reddi, D.; O’Connor, M.J.; Hoyles, L.; McCartney, A.L.; Man, R.; et al. Bacteroides Thetaiotaomicron-Derived Outer Membrane Vesicles Promote Regulatory Dendritic Cell Responses in Health but Not in Inflammatory Bowel Disease. Microbiome 2020, 8, 88. [Google Scholar] [CrossRef]
- Fonseca, S.; Carvalho, A.L.; Miquel-Clopés, A.; Jones, E.J.; Juodeikis, R.; Stentz, R.; Carding, S.R. Extracellular Vesicles Produced by the Human Gut Commensal Bacterium Bacteroides Thetaiotaomicron Elicit Anti-Inflammatory Responses from Innate Immune Cells. Front. Microbiol. 2022, 13, 1050271. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kang, Y.; Huang, Y.; Xiao, Y.; Song, L.; Lu, S.; Ren, Z. A Strain of Bacteroides Thetaiotaomicron Attenuates Colonization of Clostridioides Difficile and Affects Intestinal Microbiota and Bile Acids Profile in a Mouse Model. Biomed. Pharmacother. 2021, 137, 111290. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.; Nakayama-Imaohji, H.; Hashimoto, M.; Tada, A.; Yamasaki, H.; Nagao, T.; Kuwahara, T. The Human Gut Microbe Bacteroides Thetaiotaomicron Suppresses Toxin Release from Clostridium Difficile by Inhibiting Autolysis. Antibiotics 2021, 10, 187. [Google Scholar] [CrossRef]
- Böger, M.; Hekelaar, J.; van Leeuwen, S.S.; Dijkhuizen, L.; Lammerts van Bueren, A. Structural and Functional Char-acterization of a Family GH53 β-1,4-Galactanase from Bacteroides Thetaiotaomicron That Facilitates Degradation of Prebiotic Galactooligosaccharides. J. Struct. Biol. 2019, 205, 1–10. [Google Scholar] [CrossRef]
- Lammerts van Bueren, A.; Mulder, M.; van Leeuwen, S.; Dijkhuizen, L. Prebiotic Galactooligosaccharides Activate Mucin and Pectic Galactan Utilization Pathways in the Human Gut Symbiont Bacteroides Thetaiotaomicron. Sci. Rep. 2017, 7, 40478. [Google Scholar] [CrossRef]
- Fernandez-Julia, P.J.; Munoz-Munoz, J.; van Sinderen, D. A Comprehensive Review on the Impact of β-Glucan Metabolism by Bacteroides and Bifidobacterium Species as Members of the Gut Microbiota. Int. J. Biol. Macromol. 2021, 181, 877–889. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Guo, X.; Guo, C.; Wang, Y.; Du, Z.; Zhang, Z.; Xie, C.; Ding, K. Discrete Genetic Loci in Human Gut Bacteroides Thetaiotaomicron Confer Pectin Metabolism. Carbohydr. Polym. 2021, 272, 118534. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, C.; Sifuentes-Dominguez, L.; Zarek, C.M.; Propheter, D.C.; Kuang, Z.; Wang, Y.; Pendse, M.; Ruhn, K.A.; Hassell, B.; et al. Small Proline-Rich Protein 2A Is a Gut Bactericidal Protein Deployed during Helminth Infection. Science 2021, 374, eabe6723. [Google Scholar] [CrossRef]
- Modasia, A.; Parker, A.; Jones, E.; Stentz, R.; Brion, A.; Goldson, A.; Defernez, M.; Wileman, T.; Ashley Blackshaw, L.; Carding, S.R. Regulation of Enteroendocrine Cell Networks by the Major Human Gut Symbiont Bacteroides Thetaiotaomicron. Front. Microbiol. 2020, 11, 575595. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.; Sanderson, I.R.; Muhammed, R.; Allen, S.; Tzivinikos, C.; Henderson, P.; Gervais, L.; Jeffery, I.B.; Mullins, D.P.; O’Herlihy, E.A.; et al. A Double-Blind, Placebo-Controlled Trial to Assess Safety and Tolerability of (Thetanix) Bacteroides Thetaiotaomicron in Adolescent Crohn’s Disease. Clin. Transl. Gastroenterol. 2021, 12, e00287. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Hayashi, N.; Lu, T.K. Engineering the Human Gut Commensal Bacteroides Thetaiotaomicron with Synthetic Biology. Curr. Opin. Chem. Biol. 2022, 70, 102178. [Google Scholar] [CrossRef]
- Curtis, M.M.; Hu, Z.; Klimko, C.; Narayanan, S.; Deberardinis, R.; Sperandio, V. The Gut Commensal Bacteroides Thetaiotaomicron Exacerbates Enteric Infection through Modification of the Metabolic Landscape. Cell Host Microbe 2014, 16, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Oba, S.; Sunagawa, T.; Tanihiro, R.; Awashima, K.; Sugiyama, H.; Odani, T.; Nakamura, Y.; Kondo, A.; Sasaki, D.; Sasaki, K. Prebiotic Effects of Yeast Mannan, Which Selectively Promotes Bacteroides Thetaiotaomicron and Bacteroides Ovatus in a Human Colonic Microbiota Model. Sci. Rep. 2020, 10, 17351. [Google Scholar] [CrossRef]
- Ignatyeva, O.; Tolyneva, D.; Kovalyov, A.; Matkava, L.; Terekhov, M.; Kashtanova, D.; Zagainova, A.; Ivanov, M.; Yudin, V.; Makarov, V.; et al. Christensenella Minuta, a New Candidate next-Generation Probiotic: Current Evidence and Future Trajectories. Front. Microbiol. 2024, 14, 1241259. [Google Scholar] [CrossRef]
- Ang, W.-S.; Law, J.W.-F.; Letchumanan, V.; Hong, K.W.; Wong, S.H.; Ab Mutalib, N.S.; Chan, K.-G.; Lee, L.-H.; Tan, L.T.-H. A Keystone Gut Bacterium Christensenella Minuta—A Potential Biotherapeutic Agent for Obesity and Associated Metabolic Diseases. Foods 2023, 12, 2485. [Google Scholar] [CrossRef]
- Pető, Á.; Kósa, D.; Szilvássy, Z.; Fehér, P.; Ujhelyi, Z.; Kovács, G.; Német, I.; Pócsi, I.; Bácskay, I. Scientific and Pharmaceutical Aspects of Christensenella Minuta, a Promising Next-Generation Probiotic. Fermentation 2023, 9, 767. [Google Scholar] [CrossRef]
- Xu, C.; Jiang, H.; Feng, L.-J.; Jiang, M.-Z.; Wang, Y.-L.; Liu, S.-J. Christensenella Minuta Interacts with Multiple Gut Bacteria. Front. Microbiol. 2024, 15, 1301073. [Google Scholar] [CrossRef] [PubMed]
- Mazier, W.; Le Corf, K.; Martinez, C.; Tudela, H.; Kissi, D.; Kropp, C.; Coubard, C.; Soto, M.; Elustondo, F.; Rawadi, G.; et al. A New Strain of Christensenella Minuta as a Potential Biotherapy for Obesity and Associated Metabolic Diseases. Cells 2021, 10, 823. [Google Scholar] [CrossRef]
- Liu, C.; Du, M.-X.; Xie, L.-S.; Wang, W.-Z.; Chen, B.-S.; Yun, C.-Y.; Sun, X.-W.; Luo, X.; Jiang, Y.; Wang, K.; et al. Gut Commensal Christensenella Minuta Modulates Host Metabolism via Acylated Secondary Bile Acids. Nat. Microbiol. 2024, 9, 434–450. [Google Scholar] [CrossRef]
- Kropp, C.; Le Corf, K.; Relizani, K.; Tambosco, K.; Martinez, C.; Chain, F.; Rawadi, G.; Langella, P.; Claus, S.P.; Mar-tin, R. The Keystone Commensal Bacterium Christensenella Minuta DSM 22607 Displays Anti-Inflammatory Properties Both in Vitro and in Vivo. Sci. Rep. 2021, 11, 11494. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Zheng, S.; Zheng, W.; Shi, C.; Ning, K.; Zhang, Q.; Xie, Y.; Xiang, H.; Xie, Q. Christensenella Regulated by Huang-Qi-Ling-Hua-San Is a Key Factor by Which to Improve Type 2 Diabetes. Front. Microbiol. 2022, 13, 1022403. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-W.; Huang, H.-J.; Wang, X.-M.; Wei, R.-Q.; Niu, H.-Y.; Chen, H.-Y.; Luo, M.; Abdugheni, R.; Wang, Y.-L.; Liu, F.-L.; et al. Christensenella Strain Resources, Genomic/Metabolomic Profiling, and Association with Host at Species Lev-el. Gut Microbes 2024, 16, 2347725. [Google Scholar] [CrossRef] [PubMed]
- Relizani, K.; Le Corf, K.; Kropp, C.; Martin-Rosique, R.; Kissi, D.; Déjean, G.; Bruno, L.; Martinez, C.; Rawadi, G.; Elustondo, F.; et al. Selection of a Novel Strain of Christensenella Minuta as a Future Biotherapy for Crohn’s Disease. Sci. Rep. 2022, 12, 6017. [Google Scholar] [CrossRef]
- Akbuğa-Schön, T.; Suzuki, T.A.; Jakob, D.; Vu, D.L.; Waters, J.L.; Ley, R.E. The Keystone Gut Species Christensenella Minuta Boosts Gut Microbial Biomass and Voluntary Physical Activity in Mice. mBio 2023, 15, e02836-23. [Google Scholar] [CrossRef]
- Kumari, M.; Singh, P.; Nataraj, B.H.; Kokkiligadda, A.; Naithani, H.; Ali, S.A.; Behare, P.V.; Nagpal, R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res. Int. 2021, 150, 110716. [Google Scholar] [CrossRef]
- Aeron, G.; Morya, S. Immobilization and Microencapsulationr. J. Adv. Res. Biotechnol. 2017, 2, 3520033. [Google Scholar]
- Kowalska, E.; Ziarno, M.; Ekielski, A.; Żelaziński, T. Materials Used for the Microencapsulation of Probiotic Bacteria in the Food Industry. Molecules 2022, 27, 3321. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Q.; Ferguson, L.R.; Shu, Q.; Weir, I.; Garg, S. Development of a Novel Probiotic Delivery System Based on Microencapsulation with Protectants. Appl. Microbiol. Biotechnol. 2012, 93, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Panjanapongchai, S.; Chavapradit, C.; Anal, A.K. Microencapsulation of Probiotics and Its Potential Industrial Ap-plications. In Probiotics, Prebiotics and Synbiotics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 213–232. ISBN 978-1-119-70216-0. [Google Scholar]
- Chen, Y.; Meenu, M.; Baojun, X. A Narrative Review on Microencapsulation of Obligate Anaerobe Probiotics Bifidobacterium, Akkermansia Muciniphila, and Faecalibacterium Prausnitzii. Food Rev. Int. 2022, 38, 373–402. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in Microencapsulation of Probiotics: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Druart, C.; Plovier, H.; Van Hul, M.; Brient, A.; Phipps, K.R.; de Vos, W.M.; Cani, P.D. Toxicological Safety Evalua-tion of Pasteurized Akkermansia Muciniphila. J. Appl. Toxicol. 2021, 41, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Dwibedi, C.; Sundh, D.; Pradhan, M.; Kraft, J.D.; Caesar, R.; Tremaroli, V.; Lorentzon, M.; Bäckhed, F. Synergy and Oxygen Adaptation for Development of Next-Generation Probiotics. Nature 2023, 620, 381–385. [Google Scholar] [CrossRef]
- Noyola, A.; Morgan-Sagastume, J.M.; López-Hernández, J.E. Treatment of Biogas Produced in Anaerobic Reactors for Domestic Wastewater: Odor Control and Energy/Resource Recovery. Rev. Environ. Sci. Biotechnol. 2006, 5, 93–114. [Google Scholar] [CrossRef]
- Hwang, O.H.; Cho, S.B.; Han, D.W.; Lee, S.R.; Kwag, J.H.; Park, S.K. Effect of Storage Period on the Changes of Odorous Compound Concentrations and Bacterial Ecology for Identifying the Cause of Odor Production from Pig Slurry. PLoS ONE 2016, 11, e0162714. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Mazier, W.; Frederic, E.; Rinaldi, L.; Rasmussen, S.; Rawadi, G. Safety evaluation of Christensenella minuta as a novel microbiome-based biotherapy to treat obesity. Obesity 2021, 29, 158. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalowski, P.; Zielińska, D. The Most Promising Next-Generation Probiotic Candidates—Impact on Human Health and Potential Application in Food Technology. Fermentation 2024, 10, 444. https://doi.org/10.3390/fermentation10090444
Lalowski P, Zielińska D. The Most Promising Next-Generation Probiotic Candidates—Impact on Human Health and Potential Application in Food Technology. Fermentation. 2024; 10(9):444. https://doi.org/10.3390/fermentation10090444
Chicago/Turabian StyleLalowski, Piotr, and Dorota Zielińska. 2024. "The Most Promising Next-Generation Probiotic Candidates—Impact on Human Health and Potential Application in Food Technology" Fermentation 10, no. 9: 444. https://doi.org/10.3390/fermentation10090444
APA StyleLalowski, P., & Zielińska, D. (2024). The Most Promising Next-Generation Probiotic Candidates—Impact on Human Health and Potential Application in Food Technology. Fermentation, 10(9), 444. https://doi.org/10.3390/fermentation10090444






