Influence of Molasses and Caesalpinia spinosa Meal Inoculums on Biogas Production from Cattle Manure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anaerobic Digestion Substrates
2.1.1. Caesalpinia Spinosa Meal
2.1.2. Molasses
2.1.3. Cattle Manure
2.2. Experimental System
2.3. Implementation of the Experimental System
2.4. Biogas Production Operating Systems
2.5. Measurement of Base Parameters
2.5.1. pH
2.5.2. Temperature
2.5.3. Total Solids
- ST: total solids concentration (%),
- M1: fresh weight of the sample (g),
- M2: dry weight of the sample at 65 °C (g).
2.5.4. Measurement of Biogas Volume and Methane Content
2.6. Statistical Analysis
3. Results
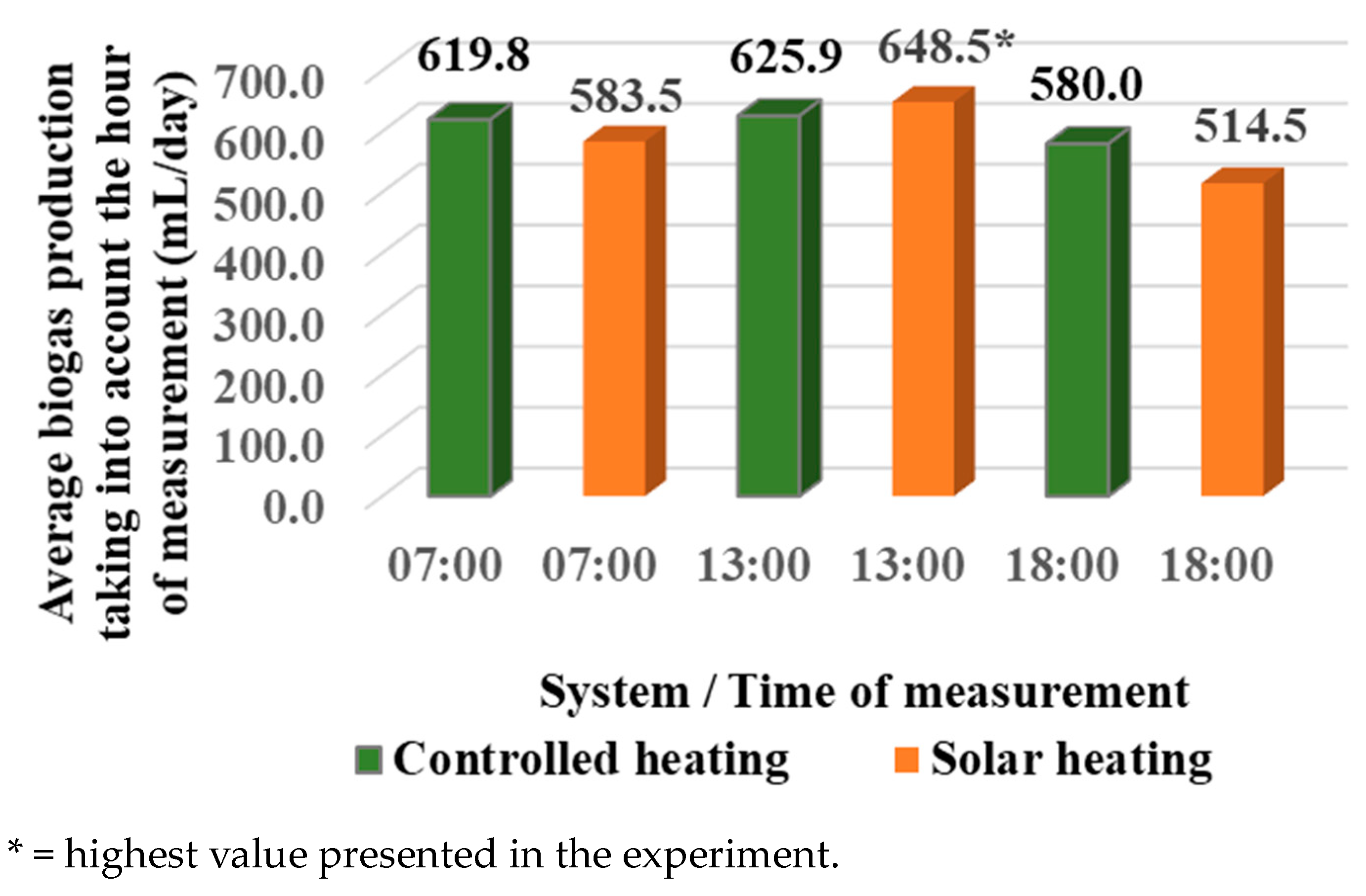
3.1. Evaluation of the Biogas Production of the Systems
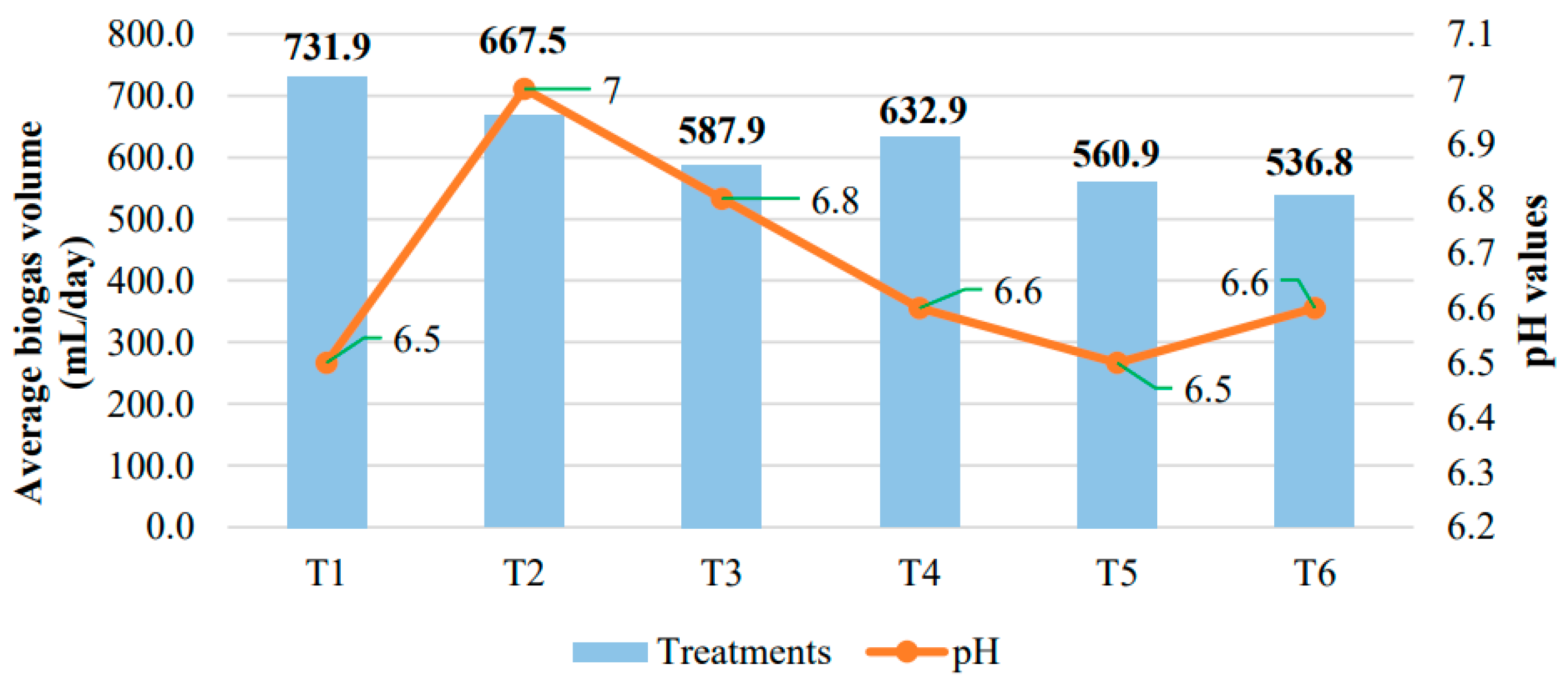
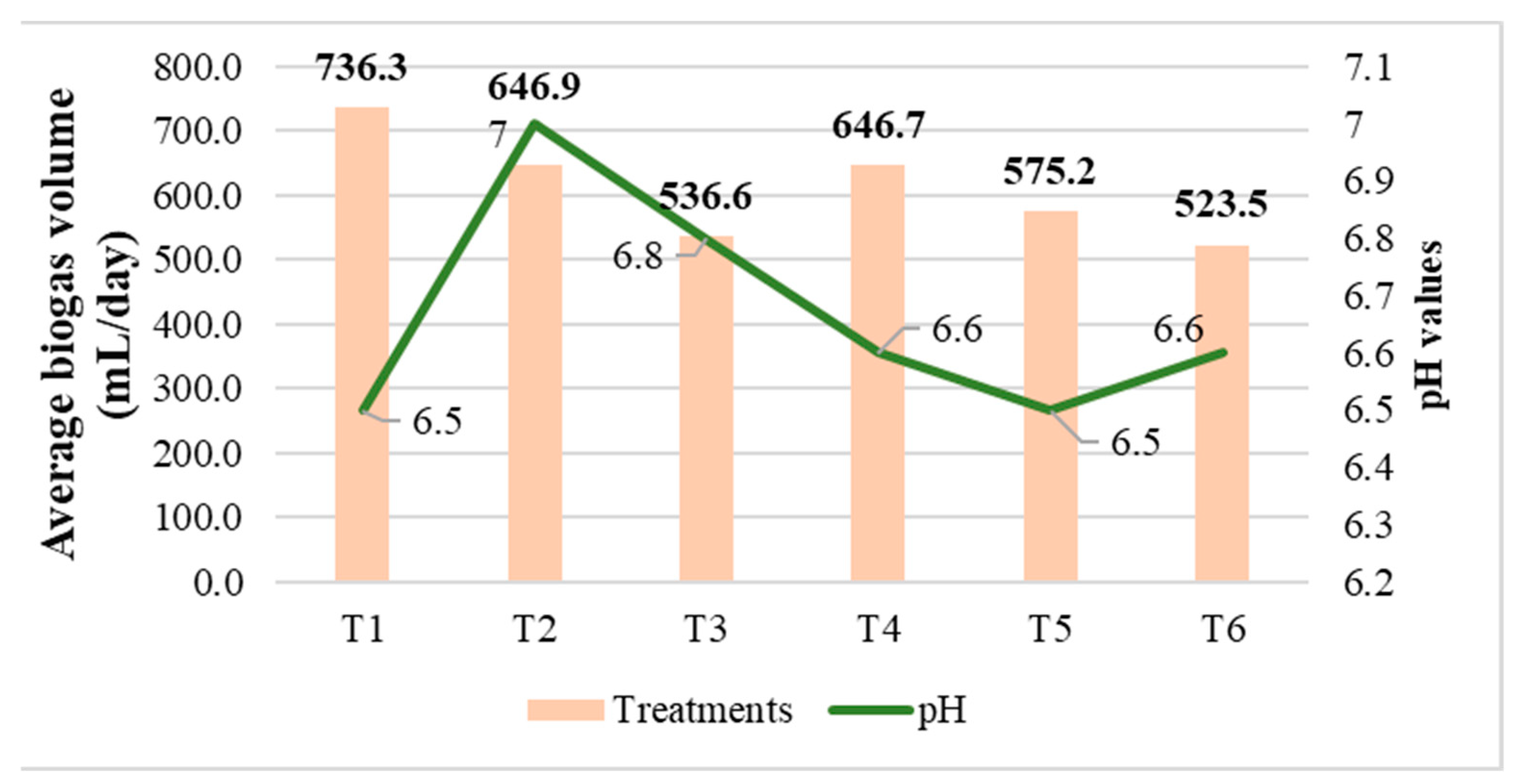
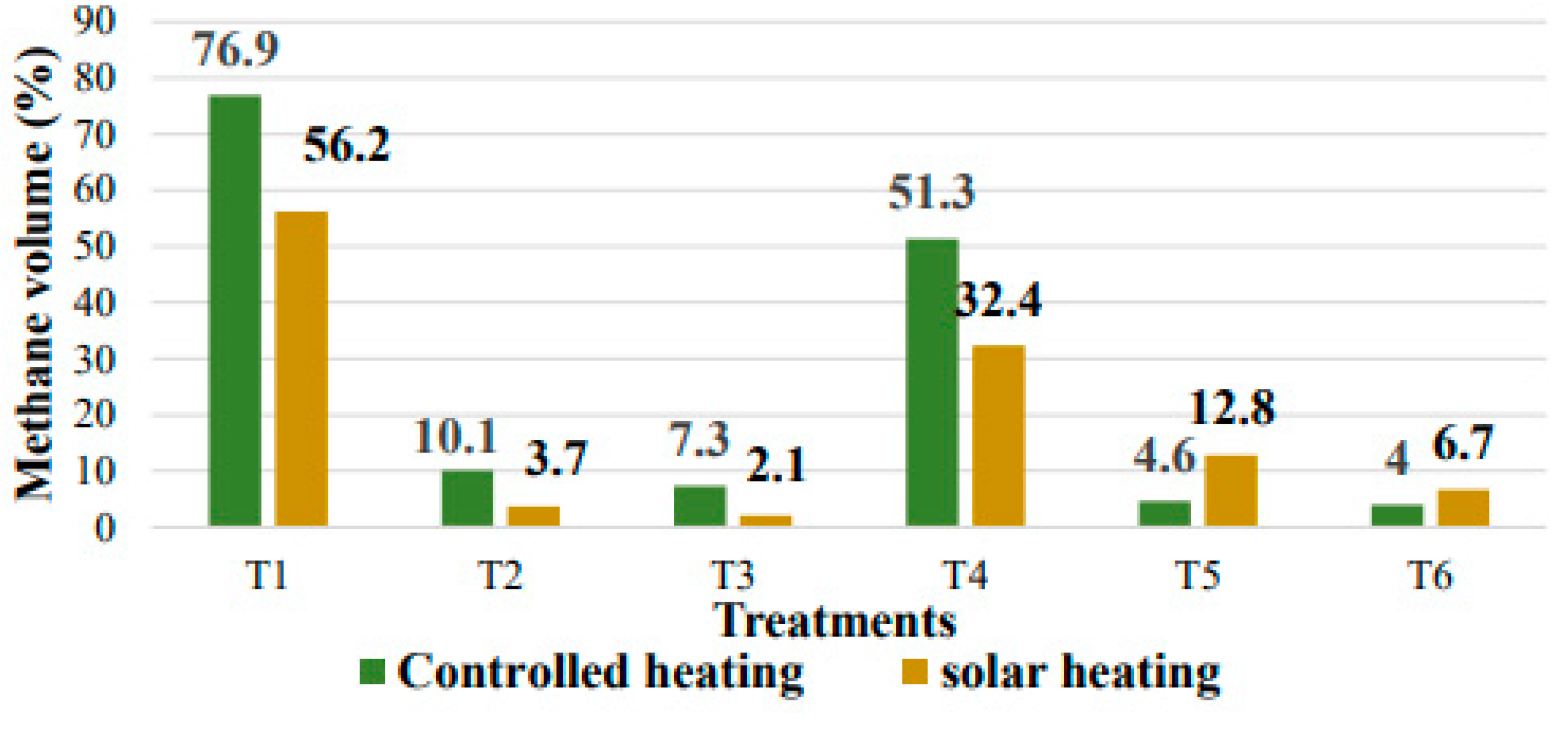
3.2. Evaluation of the Influence of Inoculums on Biogas Production by System and Treatment
3.2.1. Biogas Production with a Controlled Heating System
3.2.2. Biogas Production with Solar Heating System (Under Polycarbonate Parabolic Trough)
3.3. Production Start Time and Total Biogas Quantification
- Start of biogas production: Start-up occurred 14 h after starting up the biodigesters, which were loaded at 17:00 h and measured the next day at 07:00 h. This process was applied for both systems.
- Biogas production: During the 43-day evaluation, the total volume of biogas produced by the controlled heating system (T1) was 31,736 L, measured at 07:00 h (Figure 9). In contrast, the system with solar heating under a parabolic trough produced 32,933 L of biogas at T1, measured at 13:00 h (Figure 10), resulting in a difference of 1197 L in favor of the solar system. Small-scale biogas technology can replace fossil fuels in homes, allowing the use of environmentally friendly gas.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makul, N.; Fediuk, R.; Amran, M.; Al-Akwaa, M.; Pralat, K.; Nemova, D.; Petropavlovskii, K.; Novichenkova, T.; Petropavlovskaya, V.; Sulman, M. Utilization of Biomass to Ash: An Overview of the Potential Resources for Alternative Energy. Materials 2021, 14, 6482. [Google Scholar] [CrossRef] [PubMed]
- Olabi, A.G. 100% sustainable energy. Energy 2014, 77, 1–5. [Google Scholar] [CrossRef]
- Armeanu, D.; Vintilă, G.; Gherghina, Ş. Does Renewable Energy Drive Sustainable Economic Growth? Multivariate Panel Data Evidence for EU-28 Countries. Energies 2017, 10, 381. [Google Scholar] [CrossRef]
- Kriaučiūnas, D.; Žvirblis, T.; Kilikevičienė, K.; Kilikevičius, A.; Matijošius, J.; Rimkus, A.; Vainorius, D. Impact of Simulated Biogas Compositions (CH4 and CO2) on Vibration, Sound Pressure and Performance of a Spark Ignition Engine. Energies 2021, 14, 7037. [Google Scholar] [CrossRef]
- Kriaučiūnas, D.; Pukalskas, S.; Rimkus, A.; Barta, D. Analysis of the Influence of CO2 Concentration on a Spark Ignition Engine Fueled with Biogas. Appl. Sci. 2021, 11, 6379. [Google Scholar] [CrossRef]
- Castro-Rivera, R.; Solís-Oba, M.M.; Chicatto Gasperin, V.; Solís-Oba, A. Producción de biogás mediante codigestión de estiércol bovino y residuos de cosecha de tomate (Solanum lycopersicum L.). Rev. Int. Contam. Ambient. 2020, 6, 529–539. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Müller, B.; Schnürer, A. Importance of inoculum source and initial community structure for biogas production from agricultural substrates. Bioresour. Technol. 2017, 245, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Hansson, M.; Schnürer, A. Improved biogas production from whole stillage by co-digestion with cattle manure. Bioresour. Technol. 2012, 114, 314–319. [Google Scholar] [CrossRef]
- Guimarães, C.; Maia, D.; Serra, E. Construction of Biodigesters to Optimize the Production of Biogas from Anaerobic Co-Digestion of Food Waste and Sewage. Energies 2018, 11, 870. [Google Scholar] [CrossRef]
- Koch, K.; Lippert, T.; Drewes, J.E. The role of inoculum’s origin on the methane yield of different substrates in biochemical methane potential (BMP) tests. Bioresour. Technol. 2017, 243, 457–463. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Jiang, H. Microbial production of value-added bioproducts and enzymes from molasses, a by-product of sugar industry. Food Chem. 2021, 346, 128860. [Google Scholar] [CrossRef]
- Romaniuk, W.; Rogovskii, I.; Polishchuk, V.; Titova, L.; Borek, K.; Shvorov, S.; Roman, K.; Solomka, O.; Tarasenko, S.; Didur, V.; et al. Study of Technological Process of Fermentation of Molasses Vinasse in Biogas Plants. Processes 2022, 10, 2011. [Google Scholar] [CrossRef]
- Park, M.J.; Jo, J.H.; Park, D.; Lee, D.S.; Park, J.M. Comprehensive study on a two-stage anaerobic digestion process for the sequential production of hydrogen and methane from cost-effective molasses. Int. J. Hydrogen Energy 2010, 35, 6194–6202. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y. Coproduction of hydrogen and methane in a CSTR-IC two-stage anaerobic digestion system from molasses wastewater. Water Sci. Technol. 2019, 79, 270–277. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of methane production in anaerobic digestion process: A review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Lawal, A.A.; Dzivama, A.U.; Wasinda, M.K. Effect of inoculum to substrate ratio on biogas production of sheep paunch manure. Res. Agric. Eng. 2016, 62, 8–14. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, R.; Liu, F.; Yong, X.; Wu, X.; Zheng, T.; Jiang, M.; Jia, H. Biogas production and microbial community shift through neutral pH control during the anaerobic digestion of pig manure. Bioresour. Technol. 2016, 217, 44–49. [Google Scholar] [CrossRef]
- Schnürer, A. Biogas Production: Microbiology and Technology. In Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Wang, S.; Ma, F.; Ma, W.; Wang, P.; Zhao, G.; Lu, X. Influence of Temperature on Biogas Production Efficiency and Microbial Community in a Two-Phase Anaerobic Digestion System. Water 2019, 11, 133. [Google Scholar] [CrossRef]
- Poszytek, K.; Ciężkowska, M.; Skłodowska, A.; Drewniak, Ł. Microbial Consortium with High Cellulolytic Activity (MCHCA) for Enhanced Biogas Production. Front. Microbiol. 2016, 7, 324. [Google Scholar] [CrossRef]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities—A review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Blades, L.; Morgan, K.; Douglas, R.; Glover, S.; De Rosa, M.; Cromie, T.; Smyth, B. Circular Biogas-Based Economy in a Rural Agricultural Setting. Energy Procedia 2017, 123, 89–96. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; García-Cascallana, J.; Gómez, X. Biogas Production from Organic Wastes: Integrating Concepts of Circular Economy. Fuels 2021, 2, 144–167. [Google Scholar] [CrossRef]
- Lubańska, A.; Kazak, J.K. The Role of Biogas Production in Circular Economy Approach from the Perspective of Locality. Energies 2023, 16, 3801. [Google Scholar] [CrossRef]
- MIDAGRI. Boletín Estadístico Mensual “EL AGRO EN CIFRAS”—2023. [Internet]. 2024. Available online: https://cdn.www.gob.pe/uploads/document/file/5941243/4024332-boletin-mensual-el-agro-en-cifras-diciembre-2023.pdf?v=1710003696 (accessed on 5 August 2024).
- MIDAGRI. Producción y Comercio de la Tara en Perú [Internet]. Perú. 2019. Available online: https://cdn.www.gob.pe/uploads/document/file/419835/produccion-comercio-de-la-tara-peru.pdf (accessed on 5 August 2024).
- Condori Tintaya, F.; Vildoso González, V. Evaluación de la oferta exportable de tara (Caesalpinia spinosa) y su rentabilidad en la región tacna. Cienc. Desarro. 2019, 31–35. [Google Scholar] [CrossRef]
- Barrena, M.; Gamarra, O.; Milla, M.; Fellenberg, T.; Ordinola, C. Optimización de la producción de biogas a escala de laboratorio a partir de estiércol de bovino, lactosuero y totora (Scirpus californicus). INDES Rev. Investig. Desarro. Sustentable 2017, 3, 60–66. [Google Scholar] [CrossRef]
- Garcia Saldaña, W.E. Influencia de Inóculos de Melaza y Harina de Tara (Caesalpinia spinosa) en la Producción de Biogás a Partir de Estiércol de Ganado Vacuno; Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas: Chachapoyas, Peru, 2022. [Google Scholar]
- Kabaivanova, L.; Petrova, P.; Hubenov, V.; Simeonov, I. Biogas Production Potential of Thermophilic Anaerobic Biodegradation of Organic Waste by a Microbial Consortium Identified with Metagenomics. Life 2022, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Bolzonella, D.; Pavan, P.; Fatone, F.; Cecchi, F. Mesophilic and thermophilic anaerobic co-digestion of waste activated sludge and source sorted biowaste in pilot- and full-scale reactors. Renew. Energy 2013, 55, 260–265. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Postigo, V.; Sánchez, A.; Cabellos, J.M.; Arroyo, T. New Approaches for the Fermentation of Beer: Non-Saccharomyces Yeasts from Wine. Fermentation 2022, 8, 280. [Google Scholar] [CrossRef]
- Mamani, J.; Llumipanta, F.; Ramos, S.; Rea, J.; Alucho, J.; Llanos, F.; Pilco, J.C. Sistemas de producción de biogás: Fundamento, técnicas de mejora, ventajas y desventajas. Agroindustrial Sci. 2021, 11, 239–247. [Google Scholar] [CrossRef]
- Onen Cinar, S.; Nsair, A.; Wieczorek, N.; Kuchta, K. Long-Term Assessment of Temperature Management in an Industrial Scale Biogas Plant. Sustainability 2022, 14, 612. [Google Scholar] [CrossRef]
- Dhungana, B.; Lohani, S.P.; Marsolek, M. Anaerobic Co-Digestion of Food Waste with Livestock Manure at Ambient Temperature: A Biogas Based Circular Economy and Sustainable Development Goals. Sustainability 2022, 14, 3307. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- León Torres, A.; Nomberto Rodríguez, C.; Mendoza Arevalo, G.A. Diseño e implementación de una planta piloto de producción de Biogás, Biol. Arnaldoa 2019, 26, 1017–1032. [Google Scholar]
- Atelge, M.R.; Senol, H.; Djaafri, M.; Hansu, T.A.; Krisa, D.; Atabani, A.; Eskicioglu, C.; Muratçobanoğlu, H.; Unalan, S.; Kalloum, S.; et al. A Critical Overview of the State-of-the-Art Methods for Biogas Purification and Utilization Processes. Sustainability 2021, 13, 11515. [Google Scholar] [CrossRef]
- Murillo-Roos, M.; Uribe-Lorío, L.; Fuentes-Schweizer, P.; Vidaurre-Barahona, D.; Brenes-Guillén, L.; Jiménez, I.; Arguedas, T.; Liao, W.; Uribe, L. Biogas Production and Microbial Communities of Mesophilic and Thermophilic Anaerobic Co-Digestion of Animal Manures and Food Wastes in Costa Rica. Energies 2022, 15, 3252. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Mukherjee, M.; Patel, A. Assessment of Sustainable Biogas Production from Co-Digestion of Jatropha De-Oiled Cake and Cattle Dung Using Floating Drum Type Digester under Psychrophilic and Mesophilic Conditions. Clean Technol. 2022, 4, 529–541. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, W.-K.; Wu, Q.-C.; Zhang, F.; Li, W.-J.; Yang, Z.-M.; Bo, Y.-K.; Yang, H.-J. The Effect of Different Lactic Acid Bacteria Inoculants on Silage Quality, Phenolic Acid Profiles, Bacterial Community and In Vitro Rumen Fermentation Characteristic of Whole Corn Silage. Fermentation 2022, 8, 285. [Google Scholar] [CrossRef]
- Hupp, B.; Pap, B.; Farkas, A.; Maróti, G. Development of a Microalgae-Based Continuous Starch-to-Hydrogen Conversion Approach. Fermentation 2022, 8, 294. [Google Scholar] [CrossRef]
- Kapoor, R.; Ghosh, P.; Kumar, M.; Sengupta, S.; Gupta, A.; Kumar, S.S.; Vijay, V.; Kumar, V.; Kumar Vijay, V.; Pant, D. Valorization of agricultural waste for biogas based circular economy in India: A research outlook. Bioresour. Technol. 2020, 304, 123036. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz, J. Energy and Economic Balance between Manure Stored and Used as a Substrate for Biogas Production. Energies 2022, 15, 413. [Google Scholar] [CrossRef]
- Gao, M.; Wang, D.; Wang, Y.; Wang, X.; Feng, Y. Opportunities and Challenges for Biogas Development: A Review in 2013–2018. Curr. Pollut. Rep. 2019, 5, 25–35. [Google Scholar] [CrossRef]






| Systems | Treatments | |||||
|---|---|---|---|---|---|---|
| A | T1 | T2 | T3 | T4 | T5 | T6 |
| (1:5) | (1:3) | (1:2) | (1:5) | (1:3) | (1:2) | |
| M + HT | M + HT | M + HT | ||||
| B | (1:5) | (1:3) | (1:2) | (1:5) | (1:3) | (1:2) |
| M + HT | M + HT | M + HT | ||||
| Treatments | Variables | Useful Capacity of the Biodigester mL | |||
|---|---|---|---|---|---|
| Manure | Molasses | Caesalpinia spinosa Meal | Water | ||
| g | mL | g | mL | ||
| T1 | 400 | 300 | 30 | 2000 | 2800 |
| T2 | 400 | 120 | 18 | 1200 | 2800 |
| T3 | 400 | 40 | 12 | 800 | 2800 |
| T4 | 400 | - | - | 2000 | 2800 |
| T5 | 400 | - | - | 1200 | 2800 |
| T6 | 400 | - | - | 800 | 2800 |
| Samples and Treatments | Initial pH |
|---|---|
| Water | 7.27 |
| Manure | 7.60 |
| Molasses | 4.98 |
| Caesalpinia spinosa meal | 5.90 |
| T1 | 6.5 |
| T2 | 7.00 |
| T3 | 6.8 |
| T4 | 6.6 |
| T5 | 6.5 |
| T6 | 6.6 |
| Treatments | ||||||
|---|---|---|---|---|---|---|
| Total solids (%) | T1 | T2 | T3 | T4 | T5 | T6 |
| 13.29 | 22.5 | 39.2 | 11.37 | 20.3 | 36.3 | |
| Systems | Minimum | Maximum | Median | Media | Standard Deviation |
|---|---|---|---|---|---|
| Controlled heating | 0.0 | 2135.0 | 595.0 | 619.6 | 419.1 |
| Solar heating | 0.0 | 2350.0 | 560.0 | 610.9 | 426.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gosgot Angeles, W.; Garcia Saldaña, W.; Oliva-Cruz, M.; Barrena Gurbillon, M.Á.; Ordinola Ramirez, C.M.; Gamarra-Torres, O.A.; Mori Servan, D.C. Influence of Molasses and Caesalpinia spinosa Meal Inoculums on Biogas Production from Cattle Manure. Fermentation 2024, 10, 452. https://doi.org/10.3390/fermentation10090452
Gosgot Angeles W, Garcia Saldaña W, Oliva-Cruz M, Barrena Gurbillon MÁ, Ordinola Ramirez CM, Gamarra-Torres OA, Mori Servan DC. Influence of Molasses and Caesalpinia spinosa Meal Inoculums on Biogas Production from Cattle Manure. Fermentation. 2024; 10(9):452. https://doi.org/10.3390/fermentation10090452
Chicago/Turabian StyleGosgot Angeles, Wildor, Willan Garcia Saldaña, Manuel Oliva-Cruz, Miguel Ángel Barrena Gurbillon, Carla M. Ordinola Ramirez, Oscar Andrés Gamarra-Torres, and Diana Carina Mori Servan. 2024. "Influence of Molasses and Caesalpinia spinosa Meal Inoculums on Biogas Production from Cattle Manure" Fermentation 10, no. 9: 452. https://doi.org/10.3390/fermentation10090452







