Abstract
Odor emissions from animal manure present a significant environmental challenge in livestock farming, impacting air quality and farm sustainability. Traditional methods, such as chemical additives and manure treatment, can be costly, labor-intensive, and less eco-friendly. Therefore, this study investigated the effectiveness of microbial feed additives in reducing these odors. Conducted over three months in 2022 on a Korean beef cattle farm with 20 cattle, the experiment involved feeding a mixture of four microbial strains—Bacillus subtilis KNU-11, Lactobacillus acidophilus KNU-02, Lactobacillus casei KNU-12, and Saccharomyces cerevisiae KNU-06. Manure samples were collected from an experimental group (n = 9) and a control group (n = 11), with microbial community changes assessed through 16S ribosomal RNA gene amplicon sequencing. The results demonstrated significant reductions in specific odorous compounds in the experimental group compared to the control group: ammonia decreased by 64.1%, dimethyl sulfide by 81.3%, butyric acid by 84.6%, and isovaleric acid by 49.8%. Additionally, there was a notable shift in the microbiome, with an increase in the relative abundance of Ruminococcaceae and Prevotellaceae microbes associated with fiver degradation and fermentation, while the control group had higher levels of Bacteroidota and Spirochaetota, which are linked to pathogenicity. This study demonstrates that probiotics effectively alter intestinal microbiota to enhance microorganisms associated with odor mitigation, offering a promising and more sustainable approach to reducing odor emissions in livestock farming.
1. Introduction
The livestock industry is essential not only for providing high-quality animal protein but also for its diverse roles, including educational and emotional support, regional community development, and tourism [1,2,3,4]. It plays a significant part in agriculture, accounting for approximately 40% of the sector in Korea [5]. However, livestock farming also presents complex environmental, social, and economic challenges [6]. Among these issues, odor emissions from livestock manure are particularly pressing, causing considerable distress to nearby residents. In cattle farming, odors are especially persistent and difficult to manage. Furthermore, traditional methods for mitigating these odors, such as chemical additives and manure treatment, can be costly, labor-intensive, and less environmentally friendly [7]. Therefore, finding sustainable solutions for managing livestock odors is essential. Previous studies have reported that odorous compounds are generated through the fermentation of undigested nutrients in animal feed by microbes under anaerobic conditions [8,9]. Research has also explored reducing these odors by altering the gut microbiota of livestock [10,11,12]. This approach offers a practical advantage, as it can be easily implemented by adding treatments to feed or drinking water, without requiring additional equipment or facility modifications. Such methods have great potential for widespread adoption if supported adequately [13,14].
While there is evidence that probiotic intake can alter the gut microbiota of livestock [13,15,16], the use of probiotics in livestock farming is generally focused on weight gain, disease prevention, and increased milk production [17,18]. Moreover, previous studies on the odor reduction effects of probiotics have often been confined to in vitro experiments [8] or have had limited scope across different livestock species [12,19,20].
The novelty in this study lies in its focus on the in vivo application of probiotics as a sustainable solution for odor management in cattle farming. While the use of microbial feed additives as a solution to this problem has been highlighted, its effectiveness remains debated [18]. Unlike previous studies, this research aims to advance the application of probiotics specifically within the context of the Korean livestock industry. Conducted in collaboration with the Gunwi Agricultural Technology Center and a Korean beef cattle farm in Gunwi-gun, this study seeks to address the gaps in existing research by investigating the feasibility of using probiotics as part of a comprehensive odor reduction strategy. By analyzing changes in gut microbial communities, this study aims to identify the microorganisms associated with odor and propose a biological solution to the environmental challenges facing the Korean livestock sector [3,21,22].
2. Materials and Methods
2.1. Experimental Design
The experiment was conducted over three months, from September to November 2023. Twenty healthy Korean male beef cattle (11–12 months old), sourced from a Korean beef farm in Gunwi, Republic of Korea (29-2, Hapyeong-gil, Hyoryeong-myeon, Gunwi-gun, Daegu), were divided into two groups: a control group (n = 9) and an experimental group (n = 9). Both groups were raised under identical conditions typical of livestock farms, except that the experimental group received probiotics in the drinking water [6,21]. The cattle were housed in separate open barns for each group, with each barn measuring 5 × 10 m. The barns of the control and experimental groups were located at least 10 m apart. Both groups were fed “Clean Young Calf” feed (Gunwi Livestock Cooperative, Gunwi, Republic of Korea), comprising 19.0% crude protein, 2.0% crude fat, 20.0% crude fiber, 10.0% crude ash, 0.50% calcium, 0.80% phosphorus, and 74.0% total digestible nutrients. During the experiment, the average temperature, rainfall, and relative humidity were 14.9 °C, 76.14 mm, and 68%, respectively.
2.2. Bacterial Culture and Administration of Strains
The experiment utilized four microbial strains: Bacillus subtilis KNU-11, Lactobacillus casei KNU-12, Lactobacillus acidophilus KNU-02, and Saccharomyces cerevisiae KNU-06. The culture media used for each strain were as follows: LB broth (BD Difco, Franklin Lakes, NJ, USA) for B. subtilis KNU-11, MRS broth (BD Difco, Franklin Lakes, NJ, USA) for L. casei and L. acidophilus, and YPD broth (BD Difco, Franklin Lakes, NJ, USA) for S. cerevisiae [8,15,23,24]. These strains were selected based on previous studies demonstrating their efficacy in reducing odors in various livestock environments. The strains were individually cultured in 300 L fermenters under the following conditions: 100 rpm and 30 °C for 1 d for B. subtilis KNU-11 and S. cerevisiae KNU-06, and 30 rpm and 37 °C for 3 d for L. acidophilus KNU-02 and L. casei KNU-12. Subsequently, the cultured broths of these four strains were mixed in equal ratios to prepare a composite probiotic with a concentration of 108 cells/mL. This probiotic solution was diluted 250-fold in the cattle’s drinking water and administered ad libitum throughout the day.
2.3. Odor Measurement
Odor measurement was conducted at the farm boundary following the procedures outlined in the Notice on Odor Status Survey Procedures and Methods. Selected ion flow tube mass spectrometry (Thomson Environmental Systems, Kirrawee NSW 2232, Australia) was employed for this purpose [7]. Measurements were taken at the beginning and the end of the experiment. The specific odorants measured included ammonia, hydrogen sulfide, dimethyl sulfide, methyl mercaptan, isovaleric acid, butyraldehyde, n-butyric acid, propionaldehyde, and valeraldehyde, all of which are substances designated under the Odor Prevention Law [6]. The concentrations of these odorants were expressed in parts per billion (ppb), and the changes in their levels before and after the experiment were compared.
For odor sampling, a Gas Collector 10 (SANT, Serial Number: S23030) was used, which is specifically designed for air sample collection. This equipment was operated under a pressure of 1.2 bar and a flow rate of 2.5 L/min, ensuring precise and consistent sample collection. The samples were collected using Tedlar bags (Sigma-Aldrich, St. Louis, MO, USA) attached to the Gas Collector 10, and each sampling session lasted for approximately 5 min to ensure adequate sample volume for analysis. The samples were taken from the farm boundary, ensuring they accurately represented the ambient air at the time of collection. After collection, the samples were immediately sealed and transported to the laboratory within 24 h to maintain sample integrity. This detailed sampling method, in conjunction with the specifications of the Gas Collector 10, was crucial in ensuring the accuracy and reproducibility of the odor measurements, allowing for a reliable comparison of odorant concentrations before and after the experiment [7].
2.4. Sample Collection and DNA Extraction
To assess the impact of probiotics on gut microbiota, fecal samples were collected from the cattle. Sampling began before probiotic administration and continued weekly for approximately three months, starting three weeks after the initiation of probiotic treatment. Samples were collected from both the control and experimental groups. To prevent contamination, disposable overshoes were worn before entering the livestock area, and fecal samples were transferred to sterile 50 mL conical tubes. Each sampling was performed in triplicate at each time point. The collected samples were stored at −70 °C until DNA extraction, which was performed using the QIAamp PowerFecal Pro DNA Kit (QIAGEN, Hilden, Germany), following the manufacturer’s instructions [25].
2.5. Microbial Community Analysis
For microbial community analysis, DNA from the samples was amplified using primers targeting the V4 region of the 16S rRNA gene (Forward: 5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCTGTGNCAGCMGCCGCGGTRA-3′, Reverse: 5′-ACACTCTTTCCCTACACGACGCTCTTCCGATCTGTGNCAGCMGCCGCGGTRA-3′). The amplified DNA products were purified and sequenced using the MiSeq platform (Illumina, Inc., San Diego, CA, USA) at the KNU NGS Core Facility (Daegu, Republic of Korea). The resulting sequencing data were processed to generate Amplicon Sequence Variants (ASVs) using Quantitative Insights Into Microbial Ecology 2 (QIIME2, version 2023.2.0) and Divisive Amplicon Denoising Algorithm 2 (DADA2) pipelines. Briefly, reads with a Phred quality score below 4 were removed, and sequences were trimmed to a length of 300 at positions where the median Phred quality score dropped below 30. The resulting ASVs were taxonomically classified using the SILVA 138 database, and sequences corresponding to mitochondria, chloroplasts, or unclassified reads were excluded. The feature counts per sample ranged from 21,125 to 77,230, and the sample counts were normalized to 21,125 by rarefying. Ultimately, 17,626 ASVs were identified.
For microbial community analysis, data were analyzed using R (version 4.3.0). For relative abundance analysis, only ASVs present in more than 10% of samples with an abundance greater than 1% were retained to minimize the impact of low mean and high coefficients of variation. Alpha diversity, representing microbial species diversity within samples, was calculated using Chao1, Shannon, and Inverse Simpson indices, with statistical significance assessed via the Mann–Whitney test to determine p-values [11,26]. Beta diversity, indicating diversity between samples, was evaluated using Bray–Curtis distance analysis and visualized through principal coordinate analysis (PCoA). The statistical significance of dispersion in beta diversity among samples was determined using permutational multivariate analysis of variance (PERMANOVA) and analysis of similarities (ANOSIM) [22,27].
Additionally, linear discriminant analysis (LDA) effect size (LEfSe) algorithm was employed to identify differentially abundant taxa between the control and experimental groups. LEfSe combines standard tests for statistical significance with additional tests for biological consistency and effect size estimation, allowing for the identification of biomarkers that differ between groups [18]. The LDA score was used to estimate the effect size of each differentially abundant feature, with a threshold of 2.0 used to determine the significance of features. The analysis was conducted using the LEfSe software package 1.14.0, and results were visualized to highlight the taxa most significantly associated with each group [19,26].
3. Results
3.1. Comparison of Odorant Reduction Rates
The percentage reduction in odorant levels, including key compounds such as ammonia, dimethyl sulfide, isovaleric acid, butyric aldehyde, butyric acid, and valeraldehyde, was meticulously analyzed between the control and experimental groups. Reduction rates were determined using the formula (S − S0)/S0, where S represents the odorant concentration at the conclusion of the experiment, and S0 denotes the initial concentration before the experiment commenced. The results revealed that the experimental group, which received probiotic feed additives, exhibited significantly higher reduction rates for these odorous compounds compared to the control group (as detailed in Table 1).

Table 1.
Measured values of designated malodor-producing substances. Data are expressed as mean values from three repeated measurements.
3.2. Fecal Microbiota Composition
The relative abundance of taxa at the phylum level was analyzed by averaging the pre- and post-experiment results within each group. The experimental group also showed a reduction in the relative abundance of Desulfobacterota, a phylum associated with sulfur compound metabolism, indicating a potential decrease in sulfur-related odors. The reduction in Desulfobacterota from 0.57% to 0.17% in the control group and from 0.76% to 0.16% in the experimental group highlights the role of probiotics in mitigating sulfur-related odors. In both the control and experimental groups, Bacteroidota and Bacillota were the predominant phyla, followed by Pseudomonadota, Spirochaetota, and Verrucomicrobiota (Table 2). These phyla are commonly reported as dominant in bovine fecal microbiota. Notably, while the control group showed no significant changes in relative abundance throughout the experimental period, the experimental group exhibited a marked shift: Bacteroidota decreased from 43.36% to 39.30%, and Bacillota increased from 47.83% to 56.42%, indicating an approximate 4.1–8.6% change in both directions. These phylum-level changes, observed exclusively in the experimental group, may be attributed to the influence of probiotics.

Table 2.
The relative abundance of various phyla before and after the experiment.
3.3. Microbial Diversity of Cattle Gut
Alpha diversity was assessed using the Chao1, Shannon, and Inverse Simpson indices to compare microbial community diversity within samples from the control and experimental groups on a monthly basis (Table 3). Over time, the experimental group exhibited an increasing trend in microbial diversity indices, whereas the control group showed no significant changes. Additionally, the p-value for the difference in mean diversity indices between the two groups decreased over time, indicating a growing divergence in gut microbial diversity between the experimental and control groups.

Table 3.
Values and statistical significance of alpha diversity indices.
Beta diversity analysis further supported these findings, revealing significant differences in microbial community diversity between the control and experimental groups throughout the study period (Figure 1). The results illustrate the changes in microbial community diversity between the experimental and control groups over each month of the study. Significant differences between the groups were observed in each monthly analysis, with a trend of increasing divergence as the experiment progressed. The p-values derived from PERMANOVA and ANOSIM tests indicate that these differences were statistically significant throughout the study period. These findings suggest that continuous probiotic intake had a pronounced impact on the gut microbial communities. Additionally, LEfSe analysis, with a p-value cutoff of 0.05, identified representative microorganisms in each group (Figure 2). Bacteroidaceae and Spirochaetaceae were prevalent in the control group, while Ruminococcaceae and Prevotellaceae were more abundant in the experimental group. The analysis highlighted significantly different microorganisms between the experimental and control groups. Bacteroidaceae and Spirochaetaceae were found to be prevalent in the control group, while Ruminococcaceae and Prevotellaceae were more abundant in the experimental group. The increased presence of beneficial microbes, such as Ruminococcaceae and Prevotella, in the experimental group facilitated ruminal fermentation and positively influenced the experimental outcomes.
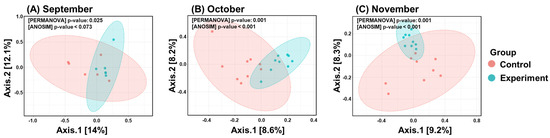
Figure 1.
Comparison of beta diversity between experimental and control groups. The plots illustrate the microbial community diversity for each group across the experimental period: (A) September, (B) October, and (C) November. p-values, calculated using PERMANOVA and ANOSIM, are displayed in the upper right corner of each plot.
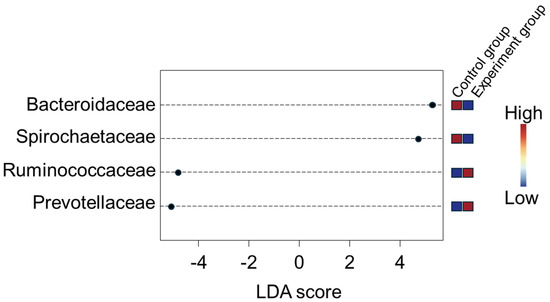
Figure 2.
Biomarkers identified using LEfSe. Red boxes indicate a high abundance of the microorganisms in the corresponding group, while blue boxes represent a lower abundance or relative absence in that group.
4. Discussion
The findings from this study suggest that probiotic microbial feed additives can significantly reduce odor emissions in cattle farming. Specifically, the experimental group exhibited notable reductions in various odorous compounds compared to the control group, including compounds such as ammonia, dimethyl sulfide, and butyric acid. These observations support the hypothesis that probiotics can effectively mitigate odor emissions from livestock. The observed reduction in odorous compounds is likely attributable to changes in the gut microbiota induced by the probiotic feed additives. The probiotic strains used in this study, Bacillus subtilis KNU-11, Lactobacillus acidophilus KNU-02, Lactobacillus casei KNU-12, and Saccharomyces cerevisiae KNU-06, are known for their ability to enhance gut health and reduce harmful emissions. Previous studies have shown that these strains can alter microbial metabolism, resulting in reduced production of volatile fatty acids and sulfur compounds, which are major contributors to malodor in livestock farms [13,19,28].
This enhanced reduction can be attributed to the active modification of the gut microbiota by the probiotics, leading to decreased production of volatile compounds associated with unpleasant odors. These findings are consistent with earlier studies that reported similar reductions in volatile fatty acids within livestock feces and diminished sulfur compound emissions, further reinforcing the effectiveness of probiotics in odor mitigation. The observed reductions in odorants such as ammonia and sulfur compounds suggest that probiotics play a crucial role in altering microbial metabolism, thereby reducing the overall malodor in livestock environments. Additionally, this study provides further evidence that integrating probiotics into livestock management practices could offer a sustainable and eco-friendly solution to persistent odor issues in agricultural settings.
The use of probiotics in cattle has been explored in various contexts, primarily for improving feed efficiency, enhancing growth rates, and preventing disease [14,15,16]. The current applications of probiotics in cattle typically focus on promoting a balanced gut microbiome, which can lead to better nutrient absorption and overall animal health [9,12,25]. Additionally, probiotics are used to reduce the incidence of digestive disorders such as asidosis and bloat [24]. The bacterial strains used in farm applications often include species of Lactobacillus, Bifidobacterium, and Bacillus, which are selected for their resilience in the gastrointestinal tract and their ability to outcompete pathogenic bacteria [18,20]. In this study, the selected strains were chosen based on their previous demonstrated efficacy in reducing odors in livestock environments, specifically targeting the reduction of compounds that contribute to malodor [15,23,24].
However, it is important to address potential limitations in the study, particularly regarding the differences in sample sizes between the experimental (n = 9) and control (n = 11) groups. While robust statistical methods such as PERMANOVA and ANOSIM were employed, which are designed to handle variations in group sizes to some extent, no specific adjustments were made to directly account for the sample size disparity. This could potentially introduce bias or affect the reliability of the results. We acknowledge this s a limitation and recognize the importance of addressing this issue in future studies. Applying more sophisticated statistical techniques, such as weighted analyses or bootstrapping methods, could provide a more accurate assessment of the impact of probiotic supplementation of odor reduction [22,27].
Moreover, while the mechanisms by which probiotics exert their effects on the host are not fully elucidated, several potential mechanisms have been proposed, including the normalization of gut microbiota, competitive exclusion of pathogens, bacteriocin production, enhanced enzyme activity and volatile fatty acid production, cell adhesion, antagonism and mucin production, immune system modulation, and gut–brain axis interactions [21,26]. Collectively, these mechanisms suggest that probiotics influence gut microbial communities. Therefore, the observed increase in gut microbial diversity in the experimental group is likely attributed to continuous probiotic intake.
The study’s findings indicate that continuous probiotic intake influenced the gut microbiota of the cattle, as evidenced by the significant changes in microbial diversity and community composition. Alpha and beta diversity indices showed an increase in microbial diversity in the experimental group, which is consistent with improved gut health. Probiotics are known to enhance microbial diversity by promoting beneficial bacteria and inhibiting pathogenic species [6,21,26]. The observed increase in microbial diversity and the presence of beneficial microbes in the experimental group can be attributed to the continuous intake of probiotics, which facilitate competitive exclusion of pathogens, production of antimicrobial substances, and modulation of the host immune system [6,25,29].
This suggests that probiotics increased the relative abundance of microbes associated with fiber degradation and fermentation, while the control group had a higher prevalence of microorganisms linked to pathogenicity. These shifts in microbial communities help explain the mechanisms by which probiotic intake contributes to odor reduction.
However, it is notable that the concentration of hydrogen sulfide actually increased after probiotic treatment. This counterintuitive result may be due to several factors, including the potential for certain probiotic strains to produce hydrogen sulfide as a byproduct of sulfur-containing amino acid metabolism [11,19]. Alternatively, the probiotics may have altered the microbial community in a way that increased the abundance of hydrogen sulfide producing bacteria, or there may have been an increase in the availability of sulfur-containing substrates in the gut. This unexpected outcome highlights the complexity of microbial interactions in the gut and suggests that while probiotics can reduce certain odorous compounds, they may also inadvertently increase others, depending on the specific strains and environmental conditions involved [7,10]. Further investigation into the metabolic pathways affected by these probiotics is necessary to fully understand this phenomenon.
Bacteroidaceae, a family within the Bacteroidota phylum, is known to be abundant but not the dominant contributor to fiber degradation [27]. Spirochaetaceae, belonging to the Spirochaetota phylum, is adapted to oxidative stress and associated with skin or mucosal diseases in various mammals, including several pathogenic species recognized as major gut pathogens [29]. Conversely, previous metatranscriptomic studies have shown that enzymes necessary for fiber degradation, particularly those from the glycoside hydrolase family, are actively expressed by Ruminococcus within Ruminococcaceae and Prevotella within Prevotellaceae [22]. This suggests that the probiotics influenced gut microbiota, resulting in distinct microbial profiles between the experimental and control groups.
Overall, combining data on odor reduction, microbial community composition, and microbial diversity changes indicates that continuous probiotic intake influenced the gut microbiota of the cattle. However, the effects were not universally pronounced, as some odorous compounds did not exhibit a substantial reduction, indicating that probiotics alone are not a complete solution for odor mitigation. The complex mechanisms underlying the impact of probiotics on odor reduction remain unclear, underscoring the need for further research in this area. Nonetheless, the simplicity of applying microbial agents and their potential for synergistic effects with other odor reduction methods emphasize the importance of continued research into eco-friendly odor management in sustainable livestock farming. Future studies should focus on identifying more effective strains, analyzing metabolic by-products, assessing additional effects on weight gain, evaluating long-term consumption impacts, and exploring effects across different livestock species.
The microbial community analysis revealed significant changes in the relative abundance of certain bacterial phyla in the experimental group. The decrease in Bacteroidota and the increase in Bacillota suggest that probiotics altered the composition of gut microbiota, promoting the growth of bacteria associated with odor reduction. The increase in beneficial bacteria such as Ruminococcaceae and Prevotella, which are involved in fiber degradation and fermentation, further supports the positive impact of probiotics on gut health and odor mitigation [18,22].
While our study focused on ASVs with relative abundances greater than 1%, we acknowledge the significance of low-abundance taxa and suggest that future research could utilize deeper sequencing or alternative statistical approaches to explore the roles of these rare microbial taxa. This would provide a more comprehensive understanding of the microbial dynamics involved [8,30].
These results have significant implications for sustainable livestock management. Reducing odor emissions not only improves the living conditions for livestock and farm workers but also minimizes the environmental impact on surrounding communities. The use of probiotics as feed additives offers a practical and eco-friendly solution to the persistent problem of livestock odor [6,14,28]. Moreover, the ease of administering probiotics through drinking water makes this method suitable for widespread adoption in livestock farming.
While the study confirms the effectiveness of probiotics in reducing odor emissions, it also highlights the need for further research. The reduction rates of certain odorous compounds were not universally significant, suggesting that probiotics alone may not be a complete solution for odor mitigation [13,16,24]. Future studies should focus on identifying more effective probiotic strains, understanding the metabolic pathways involved in odor reduction, and assessing the long-term effects of probiotic intake on livestock health and productivity. Additionally, evaluating the impact of probiotics on different livestock species and under varying environmental conditions would provide a comprehensive understanding of their potential benefits [18,22].
5. Conclusions
This study conclusively supports the hypothesis that probiotic microbial feed additives significantly reduce odor emissions on cattle farms by altering gut microbiota and decreasing the concentration of odorous compounds. These results affirm the potential of probiotics to enhance environmental sustainability in livestock operations. While promising, the findings also underscore the importance of continued research to address variability in effectiveness and explore the long-term impacts of probiotic use in diverse farming conditions. This research paves the way for developing more effective probiotic solutions, advancing sustainable livestock management practices that balance productivity with environmental stewardship.
Author Contributions
Conceptualization, J.-H.S. and T.-K.H.; methodology, M.-K.P., W.K., Y.-J.P. and Y.J.; software, W.K.; validation, M.-C.K., Y.-J.P. and H.S.; formal analysis, M.-C.K.; investigation, M.-K.P.; resources, T.-K.H.; data curation, D.S.; writing—original draft preparation, W.K. and M.-K.P.; writing—review and editing, M.-K.P.; visualization, M.-K.P.; supervision, J.-H.S.; project administration, J.-H.S.; funding acquisition, J.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Korea Basic Science Institute (National Research Facilities and Equipment Center, 2021R1A6C101A416) funded by the Ministry of Education, the project to train professional personnel in biological materials by the Ministry of Environment, Republic of Korea.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Kyungpook National University (protocol code 2024-0284 and date of approval).
Data Availability Statement
The data utilized in this study were solely for analysis purposes, and the rights to the data belong to the respective farms. As such, the data are not available for public use or distribution.
Acknowledgments
We express our appreciation and especially thank the support and cooperation of the KNU NGS Core Facility for providing a MiSeq sequencer.
Conflicts of Interest
Authors Min-Kyu Park, Yeong-Jun Park, Min-Chul Kim, HyunWoo Son, DaeWeon Seo and Jae-Ho Shin were employed by the company Microbalance Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Jęczmyk, A.; Uglis, J.; Steppa, R. Can animals be the key to the development of tourism: A case study of livestock in agritourism. Animals 2021, 11, 2357. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Havlik, P.; Valin, H.; Notenbaert, A.; Rufino, M.C.; Thornton, P.K.; Blümmel, M.; Weiss, F.; Grace, D.; Obersteiner, M. Biomass use, production, feed efficiencies, and greenhouse gas emissions from global livestock systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20888–20893. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.W.; Overballe-Petersen, S.; Ermini, L.; Der Sarkissian, C.; Haile, J.; Hellstrom, M.; Spens, J.; Thomsen, P.F.; Bohmann, K.; Cappellini, E.; et al. Ancient and modern environmental DNA. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130383. [Google Scholar] [CrossRef]
- Chung, K.Y.; Lee, S.H.; Cho, S.H.; Kwon, E.G.; Lee, J.H. Current situation and future prospects for beef production in South Korea—A review. Asian Australas. J. Anim. Sci. 2018, 31, 951. [Google Scholar] [CrossRef]
- Kesavan, P.; Swaminathan, M. Ethical, social, environmental and economic issues in animal agriculture. In Applications of Gene-Based Technologies for Improving Animal Production and Health in Developing Countries; Springer: Dordrecht, The Netherlands, 2005; pp. 447–462. [Google Scholar]
- Varel, V.H.; Wells, J.E.; Berry, E.D.; Miller, D.N. Manure odor potential and Escherichia coli concentrations in manure slurries of feedlot steers fed 40% corn wet distillers grains. J. Environ. Qual. 2010, 39, 1498–1506. [Google Scholar] [CrossRef]
- Nowocień, K.; Sokołowska, B. Bacillus spp. as a new direction in biocontrol and deodorization of organic fertilizers. AIMS Environ. Sci. 2022, 9, 95–105. [Google Scholar] [CrossRef]
- Varada, V.V.; Kumar, S.; Chhotaray, S.; Tyagi, A.K. Host-specific probiotics feeding influence growth, gut microbiota, and fecal biomarkers in buffalo calves. AMB Express 2022, 12, 118. [Google Scholar] [CrossRef]
- Alam, J.; Jeong, C.D.; Mamuad, L.L.; Sung, H.G.; Kim, D.W.; Cho, S.B.; Lee, K.; Jeon, C.O.; Lee, S.S. Bacterial community dynamics during swine in vitro fermentation using starch as a substrate with different feed additives for odor reduction. Asian Australas. J. Anim. Sci. 2012, 25, 690–700. [Google Scholar] [CrossRef]
- Alipour, M.J.; Jalanka, J.; Pessa-Morikawa, T.; Kokkonen, T.; Satokari, R.; Hynönen, U.; Iivanainen, A.; Niku, M. The composition of the perinatal intestinal microbiota in cattle. Sci. Rep. 2018, 8, 10437. [Google Scholar] [CrossRef]
- Ribeiro, G.O.; Oss, D.B.; He, Z.; Gruninger, R.J.; Elekwachi, C.; Forster, R.J.; Yang, W.; Beauchemin, K.A.; McAllister, T.A. Repeated inoculation of cattle rumen with bison rumen contents alters the rumen microbiome and improves nitrogen digestibility in cattle. Sci. Rep. 2017, 7, 1276. [Google Scholar] [CrossRef] [PubMed]
- Nalla, K.; Manda, N.K.; Dhillon, H.S.; Kanade, S.R.; Rokana, N.; Hess, M.; Puniya, A.K. Impact of probiotics on dairy production efficiency. Front. Microbiol. 2022, 13, 805963. [Google Scholar] [CrossRef] [PubMed]
- Uyeno, Y.; Shigemori, S.; Shimosato, T. Effect of probiotics/prebiotics on cattle health and productivity. Microbes Environ. 2015, 30, 126–132. [Google Scholar] [CrossRef]
- Lan, R.; Koo, J.; Kim, I. Effects of Lactobacillus acidophilus supplementation on growth performance, nutrient digestibility, fecal microbial and noxious gas emission in weaning pigs. J. Sci. Food Agric. 2017, 97, 1310–1315. [Google Scholar] [CrossRef]
- Lambo, M.T.; Chang, X.; Liu, D. The recent trend in the use of multistrain probiotics in livestock production: An overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed. Sci. Technol. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Várhidi, Z.; Máté, M.; Ózsvári, L. The use of probiotics in nutrition and herd health management in large Hungarian dairy cattle farms. Front. Vet. Sci. 2022, 9, 957935. [Google Scholar] [CrossRef]
- Hu, J.; Kim, I.H. Effect of Bacillus subtilis C-3102 spores as a probiotic feed supplement on growth performance, nutrient digestibility, diarrhea score, intestinal microbiota, and excreta odor contents in weanling piglets. Animals 2022, 12, 316. [Google Scholar] [CrossRef]
- Welch, C.B.; Ryman, V.E.; Pringle, T.D.; Lourenco, J.M. Utilizing the gastrointestinal microbiota to modulate cattle health through the microbiome-gut-organ axes. Microorganisms 2022, 10, 1391. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Terry, S.A.; Badhan, A.; Wang, Y.; Chaves, A.V.; McAllister, T.A. Fibre digestion by rumen microbiota—A review of recent metagenomic and metatranscriptomic studies. Can. J. Anim. Sci. 2019, 99, 678–692. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, Y.H.; Yoo, J.H.; Park, J.Y.; Shim, M.Y. The Malodor Decreasing Effect of Saccharomyces cerevisiae on Decomposing Waste Egg. Korean J. Environ. Biol. 2016, 34, 177–182. [Google Scholar] [CrossRef]
- Naidu, A.S.; Xie, X.; Leumer, D.A.; Harrison, S.; Burrill, M.J.; Fonda, E.A. Reduction of sulfide, ammonia compounds, and adhesion properties of Lactobacillus casei strain KE99 in vitro. Curr. Microbiol. 2002, 44, 196–205. [Google Scholar] [CrossRef]
- Pinloche, E.; McEwan, N.; Marden, J.P.; Bayourthe, C.; Auclair, E.; Newbold, C.J. The effects of a probiotic yeast on the bacterial diversity and population structure in the rumen of cattle. PLoS ONE 2013, 8, e67824. [Google Scholar] [CrossRef]
- Halloran, K.; Underwood, M.A. Probiotic mechanisms of action. Early Hum. Dev. 2019, 135, 58–65. [Google Scholar] [CrossRef]
- Dai, X.; Tian, Y.; Li, J.; Su, X.; Wang, X.; Zhao, S.; Liu, L.; Luo, Y.; Liu, D.; Zheng, H.; et al. Metatranscriptomic analyses of plant cell wall polysaccharide degradation by microorganisms in the cow rumen. Appl. Environ. Microbiol. 2015, 81, 1375–1386. [Google Scholar] [CrossRef]
- Alam, M.J.; Islam, M.; Jeon, C.-O.; Lee, K.; Kim, S.-H.; Yang, C.-J.; Kabir, M.E.; Lee, S.-S. In vitro assessment of probiotic potential of selected bacteria isolated from pig faeces with potential application of odour reduction. Int. J. Vet. Sci. Med. 2021, 9, 22–30. [Google Scholar] [CrossRef]
- Hampson, D.J.; Ahmed, N. Spirochaetes as intestinal pathogens: Lessons from a Brachyspira genome. Gut Pathog. 2009, 1, 10. [Google Scholar] [CrossRef]
- Lein-Jöbstl, D.; Schornsteiner, E.; Mann, E.; Wagner, M.; Drillich, M.; Schmitz-Esser, S. Pyrosequencing reveals diverse fecal microbiota in Simmental calves during early development. Front. Microbiol. 2014, 5, 622. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).