Abstract
Glucosidases are important enzyme largely used in food industry; for this reason, different research studies have been aimed at investigating new producing microorganisms and cheap growth medium that can help to minimize their production costs and time. Food by-products and wastes are considered low-cost substrates that can play an important role from the perspective of a circular economy concept. Brewer’s spent grain (BSG) is the most abundant by-product of beer production that, thanks to its chemical and nutritional composition, has recently been re-evaluated for its application in various sectors. The aim of the present study was to induce the production of β-glucosidase in Wickerhamomyces anomalus BS91 using BSG as the main component. The results obtained during our research show that BSG is an attractive by-product of beer industry that can be used for the production of glucosidase. Enzyme activity obtained using this microorganism was equal to 369.7 ± 8.0 U/mL, six time greater than that observed in conventional nutritional medium (59.0 ± 5.7 U/mL). Also, we conducted additional research concerning β-glucosidase localization, and the obtained results show that the enzyme is tightly bound to the yeast cell wall, and this can probably greatly affect its stability since it is being protected by the cell wall itself.
1. Introduction
β-glucosidases are important enzymes, naturally present in vegetable tissues or produced by some microorganisms, involved in the hydrolysis of glycosidic bonds connected to the terminal non-reducing glucosides and oligosaccharides [1]. Although β-glucosidases have several industrial applications, one of the most popular is in the food sector [2] and, in particular, in wine and beer production, where their activity on glycosidic substrates has the potential for aroma release [3].
Yeasts belonging to either Saccharomyces or non-Saccharomyces yeast are part of the alcoholic beverage fermentation process. Non-Saccharomyces yeasts are usually present during spontaneous fermentation, and their role in improving beverage quality, especially in terms of aroma and flavor has been extensively studied, since they are the major producers of β-glucosidase. In addition, enzymes from yeasts isolated from grapes and musts are the most suitable enzymes for flavor and aroma improvement in wine, since they are not inhibited by higher ethanol and sugar concentrations, and their optimal pH and temperature value is close to that of winemaking [4]. β-glucosidases are also useful in the wine industry for producing less intensely colored wines, such as rose wine and white wine [5], due to the ability of some β-glucosidases to hydrolyze anthocyanins.
Aroma and flavor are also crucial for beer production. Hop and yeast activity are key factors for the complex combination of aroma and flavor, even though these ingredients are used in smaller quantities compared to water and malted cereals. This can be explained, in part, by the fact that hops contain odorless terpene glycosides, which are hydrolyzed by microbial enzymes, including β-glucosidases, for releasing the flavors and aromas of the finished beer [3]. Similar to winemaking, even in beer production [3], the activity of exogenous β-glucosidases, using specific yeast strains, can optimize the release of aromatic molecules that are linked to glycosides, thus improving the aromatic quality of beer [6].
Among the non-Saccharomyces yeasts responsible for wine and beer aroma complexity, the yeast Wickerhamomyces anomalus, formerly known as Pichia anomala, plays an important role [7,8]. W. anomalus can also be used in wine and beer aroma improvement [9,10]. This yeast can be isolated from several natural habitats, including grapes, wines, and fermented vegetables, due to its ability to tolerate extreme environmental conditions like oxidative and osmotic stress, high salt concentrations, and ability to grow in a wide range of temperatures (3–37 °C) and pH ranges (2.0–12.4) [11]. The interest in this yeast species also relies on its Qualified Presumption of Safety (QPS) status for use in the food industry [12], verified by the European Food Safety Authority (EFSA), which facilitates its applicability.
Furthermore, thanks to W. anomalus’s ability to grow on a wide range of carbon sources, different studies have shown the potential of usage of this yeast strain in combination with low-cost substrates in the production of biosurfactant, biodiesel, and ethanol [13]. Among low-cost carbon sources, brewer’s spent grain (BSG) has recently attracted a lot of interest for its potential application in different industrial sectors [14]. Since beer is being produced at large scale, huge amounts of by-products, including high quantities of BSG are also being produced [15]. It is estimated that for every 100 liters of beer produced, 14 to 20 kg of brewer’s spent grain is generated [16].
Such by-products have high water and organic matter content, and are rich in carbohydrates, proteins, amino acids, minerals, vitamins, and phenolic compounds. Therefore, their use in industrial bioprocesses, rather than disposal in landfills, has to be encouraged. Some studies have also been conducted in order to determine whether BSG is a sustainable substrate for the microbial production of fatty acids, carotenoids, ethanol, biopolymers, proteins, and fermentable sugars [14]. Also, in some cases, BSG is used for the production of different enzymes using filamentous fungi [17,18,19,20].
Although different authors [21,22,23] have studied the characteristics and composition of by-products in the beer production industry and their potential application in biotechnology, to the best of our knowledge, very little research has been conducted in the context of using BSG for the induction of β-glucosidase production in W. anomalus.
Therefore, the aim of the present study was to verify the possibility of using brewer’s spent grain (BSG) as the main substrate component in culture medium to induce the production of β-glucosidase in Wickerhamomyces anomalus BS91. Additionally, during our research, we tried to determine the exact enzyme location in W. anomalus BS91 cells.
2. Materials and Methods
2.1. Brewers Spent Grain
Brewers’ spent grain (BSG) was provided by the “24 Baroni” brewery, a craft brewery located in Nicosia (EN), Italy. The sample provided by the company consisted of 60% barley malt (Pilsner) and 40% wheat and was used to produce top-fermented Blond Ale. The initial moisture content was equal to 75.1 ± 0.05%. The sample was immediately transported to the Di3A (Dipartimento di Agricoltura, Alimentazione e Ambiente, University of Catania, Italy) and left for drying in a laboratory oven(Thermo Fisher Scientific, Langenselbold, Germany) at 55 ± 1 °C for 48 h.
2.2. Physico-Chemical Characterization of Brewers’ Spent Grain (BSG)
The BSG was evaluated for its moisture content using an electronic moisture balance (Eurotherm, Gibertini®, Novate Milanese, Italy), and for its water activity (aw) using Aqualab Vapor Sorption (Decago Device, Pullman, WA, USA).
Total protein, ash, lipids, and fiber were evaluated following the methodologies reported by the Association of Official Analytical Chemist (AOAC), briefly described below. Protein content was evaluated using the Kjeldahl method using a conversion factor of 6.25, while ash was determined following the incineration of the samples in a Muffle (Zetalab s.r.l. Padova, Italy) at 600 °C for 2 h [24]. Fat content was determined using the Soxhelt apparatus [25]. Total dietary fiber was determined in accordance with the AOAC 991.43 method [26] using the enzymatic assay kit Total Dietary Fiber (Megazyme International Ireland Ltd., Wiclkow, Ireland), following the manufacturer’s instructions. Reducing sugars were determined using the Fehling method. This method included using two Fehling solutions, namely Fehling A and Fehling B solutions, mixed in equal volumes [27]. The total carbohydrate content was calculated using the following formula: Total carbohydrates% = 100 − (proteins% + lipids% + moisture% + ash%).
2.3. Microorganism and Culture Conditions
The yeast strain used in this study belonged to the Di3A collection (Department of Agriculture, Food and Environment, University of Catania, Sicily, Italy). The W. anomalus BS91 strain was previously identified by sequencing the D1/D2 region of the 26S rRNA gene and was extensively characterized for its biocontrol capacity [28,29,30,31]. W. anomalus BS91 was stored at 4 °C on Yeast Extract Peptone Dextrose Agar (YPDA) formulated as follows: yeast extract, 10 g; peptone, 10 g; dextrose, 20 g; and agar, 20 g (Oxoid, Basingstoke, UK) per liter of distilled water.
Broth was sterilized at 121 °C for 15 min and let to cool at room temperature. Sterile sodium chloride (NaCl) solution at 0.9% (m/V) was used to suspend W. anomalus BS91 yeast cells grown on YPDA for 72 h at 25 °C, and the yeast cell number was determined using a Burker chamber. After that, the suspension was used for broth inoculation, where the initial cell concentration was 105 Unit Forming Colony (UFC)/ mL. The inoculated media were divided into 12 conical tubes, each containing 50 ml of suspension (three for each withdrawal time), and the tubes were incubated at 25 ± 2 °C on an orbital shaker (Fisher Scientific Italia, Segrate, Milano, Italy) (170 rpm) for 96 h. Conventional YPD broth was inoculated and incubated in the same conditions, in order to compare the growth of W. anomalus BS91 and its enzyme production in two different media.
2.4. Preparation of Fermentation Medium for β-Glucosidase Induction
The BSG, characterized and dried as reported in Section 2.2, was reduced to flour using an electric blender (Silver Crest, Germany), which was additionally sieved using 38 mesh sieves (0.5 mm) in order to standardize the particle size. The obtained brewers’ spent grain flour (BSG-F) was suspended at a concentration equal to 5% (m/V) in water with a 1% (m/V) Yeast Nitrogen base (YNB, Biolife, Milan, Italy).
2.5. Evaluation of Yeast Growth and β-Glucosidase Production in Experimental Medium Containing BSG-F
2.5.1. Microbial Count
The growth rate of W. anomalus BS91 in the experimental media formulated with 5% (m/V) BSG-F, prepared as previously described, was evaluated by viable plate counting after 18, 24, 48, 72, and 96 h of incubation (25 ± 2 °C in an orbital shaker) and compared with the growth of the yeast in YPD media.
Immediately after inoculation and at each above-mentioned time point, 1 mL of broth was taken from each conical tube, serially diluted, and subsequently spread-plated (100 μL) on Sabouraud Dextrose Agar (SDA, Oxoid, Basingstoke, UK) medium. Growth was assessed using the plate counts of colonies grown on SDA after 48 and 72 h of incubation at 25 ± 2 °C and expressed as log CFU/mL.
2.5.2. β-Glucosidase Activity
The production of β-glucosidase in 5% BSG-F and in YPD was evaluated at the same withdrawal times considered for the microbial count (0, 18, 24, 48, 72, and 96 h of incubation at 25 ± 2 °C on an orbital shaker).
An assay was conducted following the procedure described by Restuccia et al. [32], with a small modification reported in Parafati et al. [33].
The β-glucosidase activity was evaluated trough a quantitative assay that involves the use of p-nitrophenyl-β-D-glucoside (Sigma-Aldrich, Merck Life Science S. r.l., Milano, Italy) as the substrate. In brief, yeast suspension (250 μL), grown in the experimental BSG-F media or in YPD media up to 96 h, was mixed with 250 μL of a solution of p-nitrophenyl-β-D-glucoside dissolved in 100 mM citrate-phosphate buffer (pH 5.0) in quantities suitable to achieve a final concentration equal to 5 mM. After 30 min at 30 °C, 625 μL of 1 M sodium carbonate was added to stop the reaction.
The β-glucosidase activity was evaluated by measuring the amount of p-nitrophenol liberated from the chromogenic substrates estimated by the absorbance obtained at 400 nm with a Perkin Elmer lambda 25 Ultraviolet–Visible spectrometer (PerkinElmer Inc., Waltham, WA, USA). A suitable blank was used for each assay. The results were expressed as enzyme units (U) liberating 1 nmol of p-nitrophenol per milliliter (nmol/mL) under the assay conditions and expressed as U/mL.
The analyses were carried out in three independent replicates, and the final result was expressed as the mean value ± standard deviation of the mean.
2.6. Evaluation of Enzyme Localization
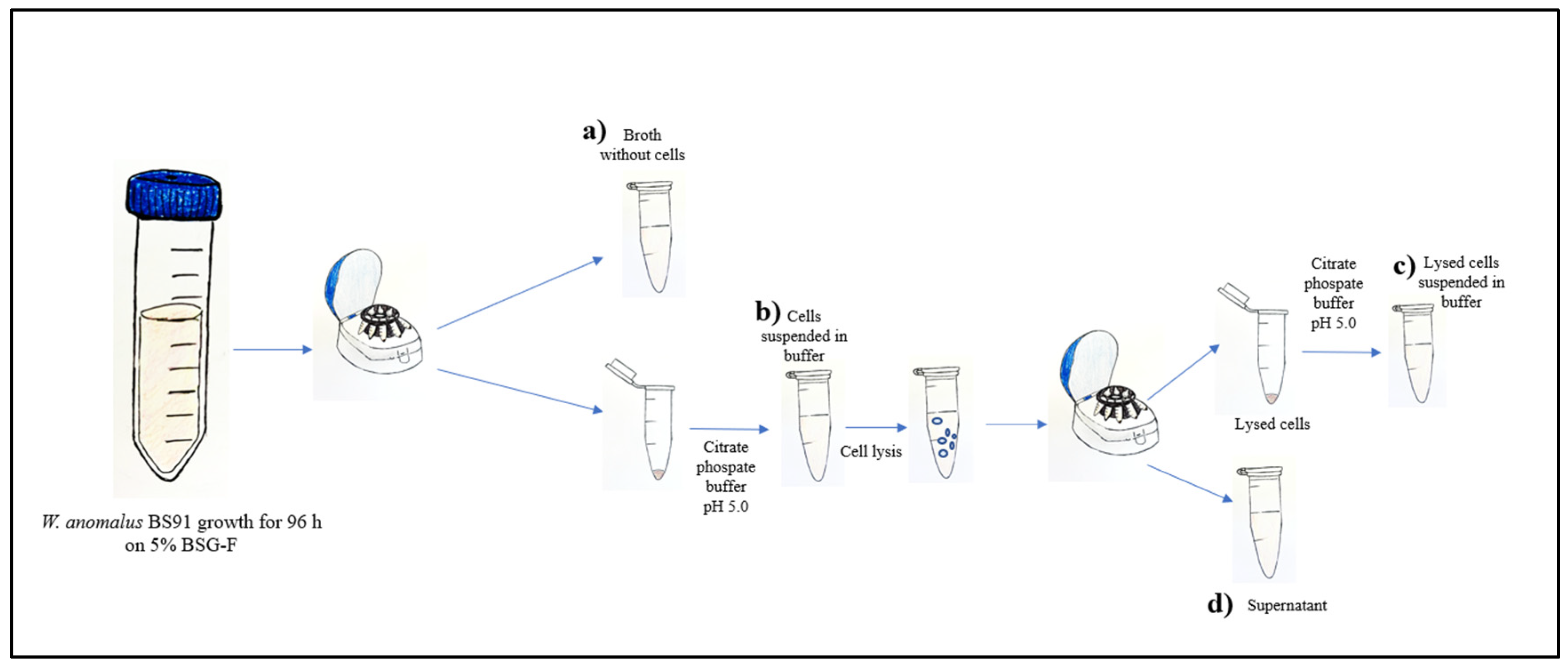
Enzyme localization was carried out by following the protocol reported by Pitson and co-workers [34] with minor modifications. In brief, 1 mL of 5% BSG-F inoculated with W. anomalus BS91 was taken after 96 h of incubation at 25 ± 2 °C on a rotary shaker and centrifuged with a benchtop mini-microcentrifuge (MULTISPIN 12, Giorgio Bormac S.r.l.
Carpi, (Modena, Italy) at 10,000 rpm for 60 min. After centrifugation, the supernatant was filtered using a 0.22 μm membrane filter and stored for subsequent analysis, while the precipitate was washed twice and resuspended in the same amount of citrate-phosphate buffer (pH 5.0). Additionally, the precipitate was subjected to mechanical cell lysis using glass beads, with alternate phases of vortexing and ice incubation. Complete cell fragmentation was confirmed under an optical microscope (Olympus Optical Co., Postfach, Hamburg, Germany). The supernatant, containing the intracellular cell content, was separated by centrifugation (10,000 rpm for 60 min) from lysed cells and filtered using a 0.22 μm membrane filter, while the precipitate was resuspended in citrate-phosphate buffer (pH 5.0). The β-glucosidase activity was assessed (as reported in Section 2.4), in all obtained fractions, as shown in Figure 1.
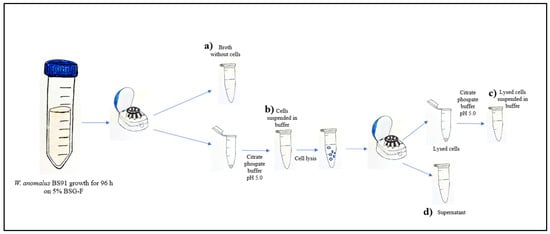
Figure 1.
Enzyme localization procedure. (a) broth medium separated from the cells, (b) whole cells resuspended in citrate-phosphate buffer (pH 5.0) (c) lysed cells resuspended in citrate-phosphate buffer (pH 5.0), and (d) supernatant containing the intracellular cell content. This analysis allowed us to determine the level of β-glucosidase activity in the extracellular fraction, intracellular fraction, and cell wall fraction.
2.7. Statistical Analysis
Data obtained from the above-described experiments were analyzed using Microsoft Excel (2013), and each final value, resulting from at least three independent repetitions, was expressed as the mean value ± standard deviation. The significant differences between enzymatic activity detected in W. anomalus BS91 cultivated in different conditions and at different incubation times was determined with one-way ANOVA (p < 0.05) with Fisher’s least significant difference (LSD) test, using the statistical package software Minitab™, version 20.0.
3. Results
3.1. Physico-Chemical Evaluation and Nutritional Composition of BSG
Table 1 shows the nutritional composition of the BSG. The Drying treatment conducted at 55 °C for 48 h was sufficient to strongly reduce the humidity (75.1 ± 0.05%) of the BSG, which, at the end of the process, resulted in the following values of moisture (M%) and water activity (aw): 3.73 ± 0.04% and 0.35 ± 0.02%, respectively. The protein content of the sample was 20.13 ± 0.37%, in line with those values reported by previous research studies [35,36,37], which showed values ranging between 16.40% and 23.40%.

Table 1.
Results of physico-chemical characterization of BSG.
The total carbohydrates value (Table 1) is equal to 70.72 ± 0.04%, which includes complex and simple sugars and fiber, defined as types of carbohydrates that the human body cannot digest [38]. The latter is mostly found in the walls, as BSG mostly constitutes of the walls of the husk–pericarp–seed coat, and it is mainly composed of hemicellulose, cellulose, and lignin [36,39]. In our sample, fiber content was around 65.00 ± 0.06%, which is similar to the content determined by Mussato et al. [36].
Additionally, the reduced sugar content in the BSG samples was around 2.50 ± 0.03%, which is in accordance with the values reported by Bravi et al. [40].
3.2. Comparison of Growth of W. anomalus BS91 in BSG-F and YPD Media
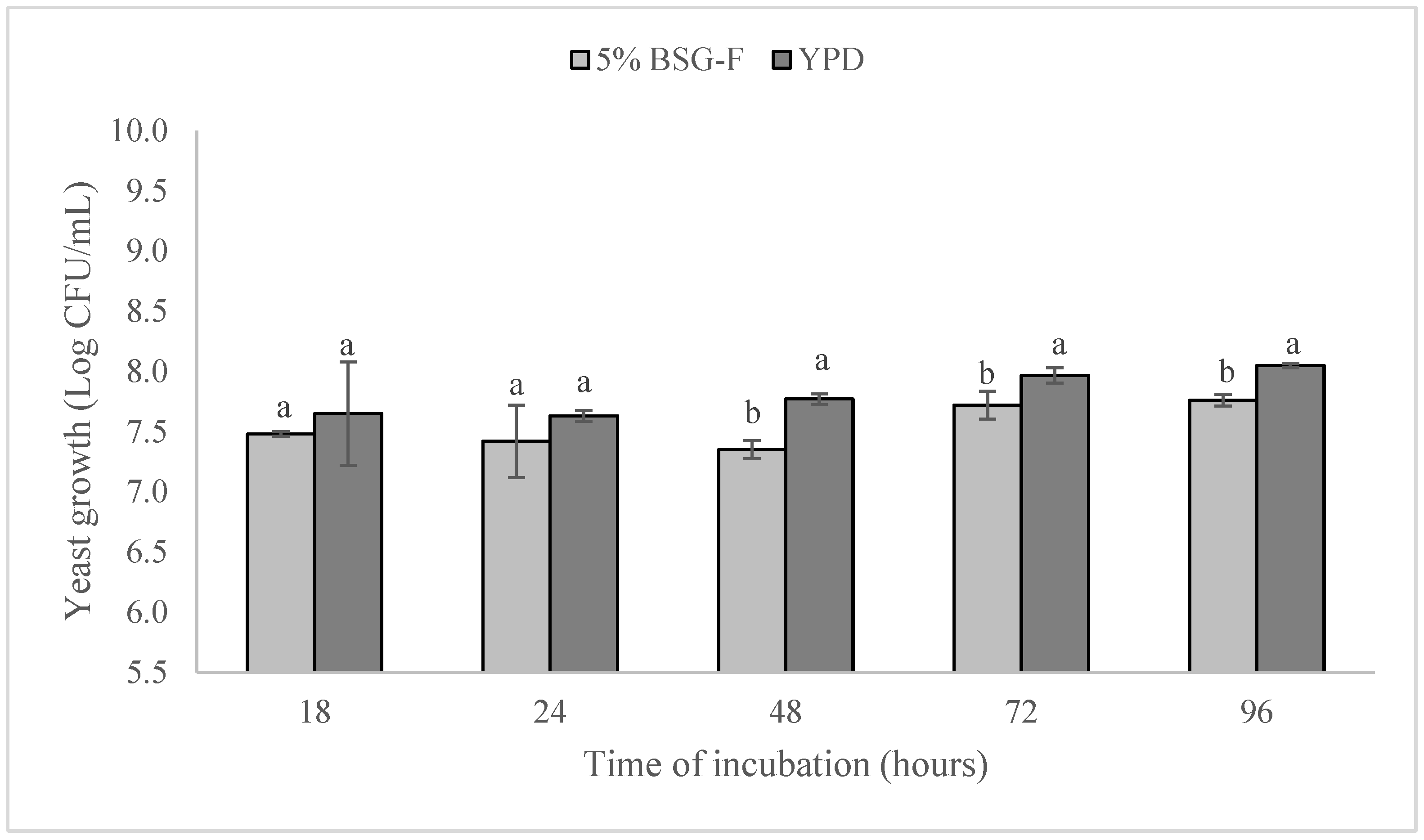
Figure 2 shows the growth of W. anomalus BS91 both in experimental 5% BSG-F and YPD media. Starting from an initial concentration, in all media, of 5.5 ± 0.1 log CFU/mL, and after 18 h of incubation, W. amomalus BS91 growth started to increase, but no significant differences (p > 0.05) were observed between the count of the yeast in the different cultivation media. At this point in time, in fact, the values of 7.48 ± 0.43 and 7.65 ± 0.02, represented in log CFU/mL, were detected in 5% BSG-F and YPD broth, respectively.
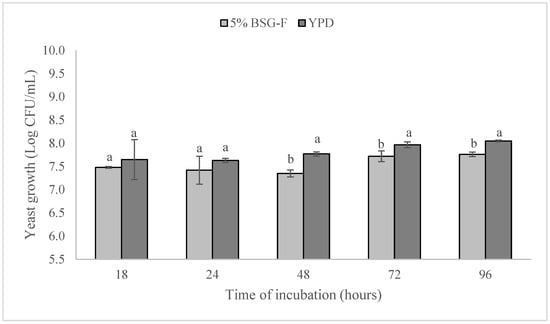
Figure 2.
Growth comparison of yeast W. anomalus BS91 in 5% BSG-F and YPD media during 96 h of cultivation (25 ± 2 °C). Within each time point (18, 24, 48, 72, or 96 h), columns with different letters show significant differences according to Fisher’s least significant difference test (p < 0.05). Vertical bars indicate the standard deviation of the mean.
After 48 h and up to 96 h of cultivation, a significant (p < 0.05) difference in W. anomalus BS91 growth was registered between the experimental media containing 5% BSG-F and YPD. In particular, at 48, 72, and 96 h, it is possible to notice a lower growth of W. anomalus BS91 in the medium containing 5% BSG-F in comparison to YPD, registering at the end of the incubation period (96 h) with values of 7.76 ± 0.02 and 8.05 ± 0.05, expressed as log CFU/mL, respectively.
Despite the differences observed for W. anomalus BS91 growth in the two-culture media (5% BSG-F and YPD) at different times points (18, 24, 48, 72, and 96 h), it can be seen that BSG-F is a good substrate that can support the growth W. anomalus BS91, probably thanks to the presences of high content of reducing sugar and proteins (Table 1).
3.3. Estimation of β-Glucosidase Activity in Experimental Medium Containing BSG-F
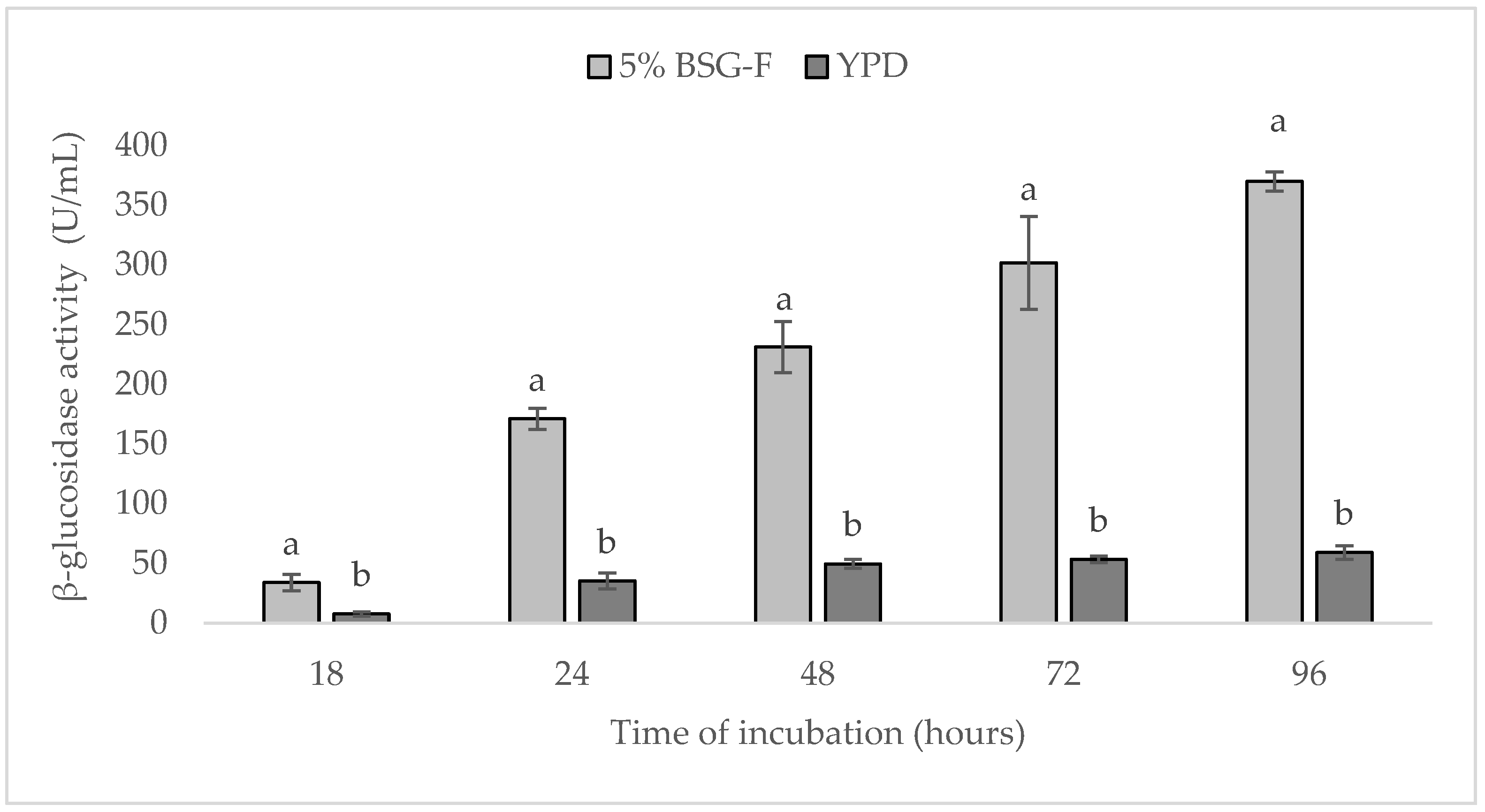
When compared for its β-glucosidase activity, in BSG-F and YPD media, a great difference was observed in the activity of an enzyme, which can be seen in Figure 3. This difference was noticeable already after 18 h of cultivation, where values of 33.9 ± 6.8 and 7.7 ± 1.9, represented as U/mL, were, respectively, determined.
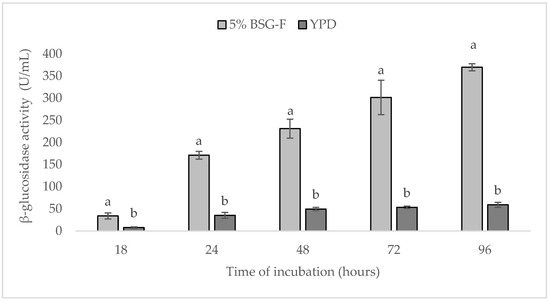
Figure 3.
Comparison of β-glucosidase activity (U/mL) of yeast W. anomalus BS91 in 5% BSG-F and YPD media during 96 h of cultivation (25 ± 2 °C). Within each time point (18, 24, 48, 72 or 96 h), columns with different letters shows significant differences according to Fisher’s least significant difference test (p < 0.05). Vertical bars indicate the standard deviation of the mean.
In YPD media, the glucosidase activity increased slightly during incubation, showing the highest values after 96 h (Figure 3); nevertheless, between 72 h and 96 h of incubation, no significant differences (p > 0.05) in enzymatic activity were detected, registering the values of 53.4 ± 2.8 and 59.0 ± 5.7 U/mL. In contrast, β-glucosidase activity strongly increased in BSG-F during incubation. After 24 h, in fact, the value of 171.0 ± 9.0 U/mL was recorded, which more than doubled at the end of the incubation period (96 h), registering the significantly (p < 0.05) higher value of 369.7 ± 8.0 U/mL.
3.4. Enzyme Localization
In order to evaluate the enzyme localization, yeast cultivated in 5% BSG-F media was subjected to the procedure described in Section 2.6 and represented in Figure 1.
From Table 2, which shows β-glucosidase activity in the different cell fractions, it can be seen that W. anomalus BS91 did not produce extracellular β-glucosidase; instead, based on our results, it seems that the enzyme is bounded to the cell wall, which was concluded based on a value of 366.1 ± 1.9 (U/mL), determined using cell lysate resuspended in buffer. Negligible enzymatic activity was detected in extracellular or intracellular fractions in comparison to that evidenced in wall-bound fractions (Table 2).

Table 2.
β-glucosidase activity in different W. anomalus BS91 fractions.
4. Discussion
The by-products that can be obtained during beer processing are characterized by great variability in chemical composition [41]; nevertheless, the BSG used in the present study showed a nutritional content similar to that already found in other studies by various authors [40,42,43].
Our research showed that, based on its nutritional composition, BSG represents suitable substrate that is able to support the growth of W. anomalus BS91. This observation is based on the fact that when compared to a control medium, a similar cell number was detected, meaning that the BSG-F medium contained all components necessary for yeast growth (Table 1).
Furthermore, regarding the enzyme activity, the W. anomalus BS91 growth up to 96 h in media containing 5% BSG-F showed high β-glucosidase activity that was almost six times greater than that of YPD (Figure 3).
Due to its carbohydrate content, BSG has already been used in different research studies as substrate for fungi like Aspergillus niger, Trichoderma reesei, Thermoascus aurantiacus [44], Fusarium oxysporum, Neurospora crassa [45], and Penicillium sp. HC1 [46] in order to enzymatically convert polysaccharides to monosaccharides. The above-mentioned filamentous fungi can, in fact, produce a wide variety of enzymes such as endoglucanase, cellobiohydrolase, β-glucosidase, and xylanase, with concentrations that reach their highest value around 8–12 days of fermentation [46,47]. Nishida eta al [47] reported that, when using wheat bran as the growing substrate for Aspergillus awamori in solid-state fermentation, it is possible to obtain great β-glucosidase activity equal to 652 ± 50 U/g of substrate. Filamentous fungi generally produce a great amount of extracellular cell-wall-degrading enzymes and for this reason are the most used microorganisms for their commercial production [48]. The use of extracellular enzymes, whose isolation is simpler compared to extraction from the cell, is often associated with immobilization techniques that allow them to increase their stability [49].
To the best of our knowledge, no research study has been conducted employing BSG as substrate for the increase in β-glucosidase production in W. anomalus.
In the present study, the β-glucosidases were probably induced, in W. anomalus BS91, by the presence of high fiber content (Table 1). BSG dietary fiber can be divided by solubility in soluble dietary fiber that includes β-glucans, pectic polysaccharides, arabinogalactans, high-branched arabinoxylan, and xyloglucans, and insoluble dietary fiber is mainly represented by lignin, cellulose, low-branched arabinoxylan, xyloglucans, and galactomannans [50]. In particular, cellulose, which in BSG constitutes approximately 17% of the total dietary fiber [36], represents the substrate for cellulolytic enzymes, and for its complete hydrolysis, the combined action of endoglucanase, exoglucanase, and beta-glucosidase is required [51].
Also, recent studies have shown that the total dietary fiber content ranging from 46.4% to up to more than 50% can significantly increase the β-glucosidase secretion in Kluyveromyces marxianus [52,53].
Therefore, great β-glucosidase activity of W. anolmalus BS91 was probably induced by the nutritional composition of BSG (Table 1), which contains a good amount of fiber (65.00 ± 0.06%) that reached a concentration of approximately 3.2%, in the formulation of the experimental media containing 5% BSG-F.
Based on our results, it can be concluded that W. anomalus BS91 is a good enzyme producer that is able to grow faster in comparison to filamentous fungi and is thus able to reduce fermentation times.
Moreover, depending on the final application, the use of enzymes bound to the cell wall could represent a great resource, given by the simplicity and cost-effectiveness that comes from the cell recovery.
Since beta-glucosidases represent a major segment of the global enzyme market (about 11% in 2021) [54], reducing problems related to BSG management could lead to an industrial production of this enzyme, exploiting this by-product’s current limited used [14]. For this reason, future research studies should be conducted to evaluate the induction of W. anomalus BS91 β-glucosidase in solid-state fermentation in order to take advantage of the high humidity of BSG and to bypass the economic loss deriving from drying operations. Furthermore, extremely expensive sterilization processes could be reduced or eliminated depending on the amount of inoculum used and its ability to overpower the existing microbial population.
5. Conclusions
The present study demonstrated that BSG is an attractive by-product of the beer industry that can be used to increase β-glucosidase production in yeast W. anomalus BS91. The results show that BSG-F, used as an ingredient in the experimental media, increased yeast enzymatic activity by six times compared to YPD medium. The experiments, whose purpose was to determine enzyme location, evidenced that the yeast W. anomalus BS91 produces a wall-bound β-glucosidase enzyme that could potentially be used in bioconversion processes. The cell wall can, in fact, represent a natural support that improves enzyme stability, which can potentially exclude the necessity for using additional immobilization techniques. Further investigations will be carried out in order to characterize the W. anomalus BS91 β-glucosidase and to evaluate its application in a food matrix for improving its sensory characteristics.
Author Contributions
Conceptualization, L.P. and C.R.; methodology, R.P. and L.P.; software, I.P. and F.P.; formal analysis, L.P., F.P. and I.P.; investigation, L.P. and R.P.; data curation, L.P., R.P. and C.R.; writing—original draft, L.P.; writing—review and editing, L.P. and C.R.; supervision, C.R. and B.F. All authors have read and agreed to the published version of the manuscript.
Funding
L.P. was supported by the funding “PON Ricerca e Innovazione, D.M.1062/21”—Contratti di ricerca from the Italian Ministry of University (MUR)–CUP E61B21004310007. I.P. was supported by the On FOODS project—“Research and innovation network on food and nutrition Sustainability, Safety and Security—Working ON Foods”—CUP E63C22002060006.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed in this study are available within the manuscript and are available from the corresponding authors upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liang, Z.; Fang, Z.; Pai, A.; Luo, J.; Gan, R.; Gao, Y.; Lu, J.; Zhang, P. Glycosidically Bound Aroma Precursors in Fruits: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.R.K.; Esen, A. β-Glucosidases. Cell. Mol. Life Sci. 2010, 67, 3389. [Google Scholar] [CrossRef] [PubMed]
- de Morais Souto, B.; Florentino Barbosa, M.; Marinsek Sales, R.M.; Conessa Moura, S.; de Rezende Bastos Araújo, A.; Ferraz Quirino, B. The Potential of β-Glucosidases for Aroma and Flavor Improvement in the Food Industry. Microbe 2023, 1, 100004. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Spagna, G.; Palmeri, R.; Torriani, S. Assessment of β-Glucosidase Activity in Selected Wild Strains of Oenococcus oeni for Malolactic Fermentation. Enzym. Microb. Technol. 2004, 34, 292–296. [Google Scholar] [CrossRef]
- Monteiro, L.M.O.; Pereira, M.G.; Vici, A.C.; Heinen, P.R.; Buckeridge, M.S.; de Lourdes Teixeira de Moraes Polizeli, M. Efficient Hydrolysis of Wine and Grape Juice Anthocyanins by Malbranchea pulchella β-Glucosidase Immobilized on MANAE-Agarose and ConA-Sepharose Supports. Int. J. Biol. Macromol. 2019, 136, 1133–1141. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could Non-Saccharomyces Yeasts Contribute on Innovative Brewing Fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces anomalus in Winemaking. Fermentation 2018, 4, 68. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, X.; Zhang, Z.; Jiang, Q.; Yang, F.; Yu, P.; Xia, W.; Liu, S. Application of Wickerhamomyces anomalus and Pichia fermentans to Improve the Aroma of Fermented Sour Fish. LWT 2024, 192, 115725. [Google Scholar] [CrossRef]
- Li, Q.; Du, B.; Chen, X.; Zhao, Y.; Zhu, L.; Ma, H.; Sun, B.; Hao, J.; Li, X. Microbial Community Dynamics and Spatial Distribution of Flavor Compound Metabolism during Solid-State Fermentation of Baijiu Enhanced by Wickerhamomyces anomalus. Food Biosci. 2024, 59, 103909. [Google Scholar] [CrossRef]
- Fredlund, E. Central Carbon Metabolism of the Biocontrol Yeast Pichia anomala: Influence of Oxygen Limitation. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2004. [Google Scholar]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 15: Suitability of Taxonomic Units Notified to EFSA until September 2021. EFSA J. 2022, 20, e07045. [Google Scholar] [CrossRef] [PubMed]
- Ben Atitallah, I.; Ntaikou, I.; Antonopoulou, G.; Alexandropoulou, M.; Brysch-Herzberg, M.; Nasri, M.; Lyberatos, G.; Mechichi, T. Evaluation of the Non-Conventional Yeast Strain Wickerhamomyces anomalus (Pichia anomala) X19 for Enhanced Bioethanol Production Using Date Palm Sap as Renewable Feedstock. Renew. Energy 2020, 154, 71–81. [Google Scholar] [CrossRef]
- Bachmann, S.A.L.; Calvete, T.; Féris, L.A. Potential Applications of Brewery Spent Grain: Critical an Overview. J. Environ. Chem. Eng. 2022, 10, 106951. [Google Scholar] [CrossRef]
- Rocha dos Santos Mathias, T.; Moretzsohn de Mello, P.P.; Servulo, E. Solid Wastes in Brewing Process: A Review. J. Brew. Distill. 2014, 5, 43. [Google Scholar] [CrossRef]
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, Wastewater and Waste Management in Brewing Industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Faria, N.T.; Marques, S.; Ferreira, F.C.; Fonseca, C. Production of Xylanolytic Enzymes by Moesziomyces spp. Using Xylose, Xylan and Brewery’s Spent Grain as Substrates. New Biotechnol. 2019, 49, 137–143. [Google Scholar] [CrossRef]
- Adeniran, H.A.; Abiose, S.H.; Ogunsua, A.O. Production of Fungal β-Amylase and Amyloglucosidase on Some Nigerian Agricultural Residues. Food Bioprocess Technol. 2010, 3, 693–698. [Google Scholar] [CrossRef]
- Da Silva Menezes, B.; Rossi, D.M.; Ayub, M.A.Z. Screening of Filamentous Fungi to Produce Xylanase and Xylooligosaccharides in Submerged and Solid-State Cultivations on Rice Husk, Soybean Hull, and Spent Malt as Substrates. World J. Microbiol. Biotechnol. 2017, 33, 58. [Google Scholar] [CrossRef]
- Aita, B.C.; Spannemberg, S.S.; Schmaltz, S.; Zabot, G.L.; Tres, M.V.; Kuhn, R.C.; Mazutti, M.A. Production of Cell-Wall Degrading Enzymes by Solid-State Fermentation Using Agroindustrial Residues as Substrates. J. Environ. Chem. Eng. 2019, 7, 103193. [Google Scholar] [CrossRef]
- Dos Santos Mathias, T.R.; Alexandre, V.M.F.; Cammarota, M.C.; de Mello, P.P.M.; Sérvulo, E.F.C. Characterization and Determination of Brewer’s Solid Wastes Composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- Kurnik, K.; Krzyżyński, M.; Treder, K.; Tretyn, A.; Tyburski, J. Study on Utilizing Solid Food Industry Waste with Brewers’ Spent Grain and Potato Pulp as Possible Peroxidase Sources. J. Food Biochem. 2018, 42, 12446. [Google Scholar] [CrossRef]
- Emmanuel, J.K.; Nganyira, P.D.; Shao, G.N. Evaluating the Potential Applications of Brewers’ Spent Grain in Biogas Generation, Food and Biotechnology Industry: A Review. Heliyon 2022, 8, e11140. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, [CD-ROM]; Association of Official Analysis Chemists International: Rockville, MA, USA, 2007; Volume 1. [Google Scholar]
- Association of Official Analytical Chemists. Official Method 922.06 Fat in Flour; Acid Hydrolysis Method. In Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Rockville, MA, USA, 2012. [Google Scholar]
- Lee, S.C.; Prosky, L.; Vries, J.W. De Determination of Total, Soluble, and Insoluble Dietary Fiber in Foods—Enzymatic-Gravimetric Method, MES-TRIS Buffer: Collaborative Study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Jones, J.K.L.; Stoodley, R.J. Fractionation Using Copper Complexes. Methods Carbohydr. Chem. 1965, 5, 36–38. [Google Scholar]
- Platania, C.; Restuccia, C.; Muccilli, S.; Cirvilleri, G. Efficacy of Killer Yeasts in the Biological Control of Penicillium digitatum on Tarocco Orange Fruits (Citrus sinensis). Food Microbiol. 2012, 30, 219–225. [Google Scholar] [CrossRef]
- Muccilli, S.; Wemhoff, S.; Restuccia, C.; Meinhardt, F. Exoglucanase-Encoding Genes from Three Wickerhamomyces anomalus Killer Strains Isolated from Olive Brine. Yeast 2013, 30, 33–43. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol Ability and Action Mechanism of Food-Isolated Yeast Strains against Botrytis cinerea Causing Post-Harvest Bunch Rot of Table Grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. The Effect of Locust Bean Gum (LBG)-Based Edible Coatings Carrying Biocontrol Yeasts against Penicillium digitatum and Penicillium italicum Causal Agents of Postharvest Decay of Mandarin Fruit. Food Microbiol. 2016, 58, 87–94. [Google Scholar] [CrossRef]
- Restuccia, C.; Muccilli, S.; Palmeri, R.; Randazzo, C.L.; Caggia, C.; Spagna, G. An Alkaline β-Glucosidase Isolated from an Olive Brine Strain of Wickerhamomyces anomalus. FEMS Yeast Res. 2011, 11, 487–493. [Google Scholar] [CrossRef]
- Parafati, L.; Palmeri, R.; Pitino, I.; Restuccia, C. Killer Yeasts Isolated from Olive Brines: Technological and Probiotic Aptitudes. Food Microbiol. 2022, 103, 103950. [Google Scholar] [CrossRef]
- Pitson, S.M.; Seviour, R.J.; McDougall, B.M. Induction and Carbon Source Control of Extracellular β-Glucosidase Production in Acremonium persicinum. Mycol. Res. 1999, 103, 161–167. [Google Scholar] [CrossRef]
- Kemppainen, K.; Rommi, K.; Holopainen, U.; Kruus, K. Steam Explosion of Brewer’s Spent Grain Improves Enzymatic Digestibility of Carbohydrates and Affects Solubility and Stability of Proteins. Appl. Biochem. Biotechnol. 2016, 180, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ Spent Grain: Generation, Characteristics and Potential Applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Yu, D.; Sun, Y.; Wang, W.; O’Keefe, S.F.; Neilson, A.P.; Feng, H.; Wang, Z.; Huang, H. Recovery of Protein Hydrolysates from Brewer’s Spent Grain Using Enzyme and Ultrasonication. Int. J. Food Sci. Technol. 2020, 55, 357–368. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Ma, S.; Wang, X.X.; Zheng, X.L. Modification and Application of Dietary Fiber in Foods. J. Chem. 2017, 2017, 9340427. [Google Scholar] [CrossRef]
- Allegretti, C.; Bellinetto, E.; D’arrigo, P.; Griffini, G.; Marzorati, S.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A.; et al. Towards a Complete Exploitation of Brewers’ Spent Grain from a Circular Economy Perspective. Fermentation 2022, 8, 151. [Google Scholar] [CrossRef]
- Bravi, E.; De Francesco, G.; Sileoni, V.; Perretti, G.; Galgano, F.; Marconi, O. Brewing By-Product Upcycling Potential: Nutritionally Valuable Compounds and Antioxidant Activity Evaluation. Antioxidants 2021, 10, 165. [Google Scholar] [CrossRef]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; Del Nozal, M.J. Variability of Brewer’s Spent Grain within a Brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Bianco, A.; Budroni, M.; Zara, S.; Mannazzu, I.; Fancello, F.; Zara, G. The Role of Microorganisms on Biotransformation of Brewers’ Spent Grain. Appl. Microbiol. Biotechnol. 2020, 104, 8661–8678. [Google Scholar] [CrossRef]
- Sahin, A.W.; Hardiman, K.; Atzler, J.J.; Vogelsang-O’Dwyer, M.; Valdeperez, D.; Münch, S.; Cattaneo, G.; O’Riordan, P.; Arendt, E.K. Rejuvenated Brewer’s Spent Grain: The Impact of Two BSG-Derived Ingredients on Techno-Functional and Nutritional Characteristics of Fibre-Enriched Pasta. Innov. Food Sci. Emerg. Technol. 2021, 68, 102633. [Google Scholar] [CrossRef]
- Llimós, J.; Martínez-Avila, O.; Marti, E.; Corchado-Lopo, C.; Llenas, L.; Gea, T.; Ponsá, S. Brewer’s Spent Grain Biotransformation to Produce Lignocellulolytic Enzymes and Polyhydroxyalkanoates in a Two-Stage Valorization Scheme. Biomass Convers. Biorefin. 2022, 12, 3921–3932. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Biotechnological Potential of Brewers Spent Grain and Its Recent Applications. Waste Biomass Valorization 2012, 3, 213–232. [Google Scholar] [CrossRef]
- Bernal-Ruiz, M.; Correa-Lozano, A.; Gomez-Sánchez, L.; Quevedo-Hidalgo, B.; Rojas-Pérez, L.C.; García-Castillo, C.; Gutiérrez-Rojas, I.; Narváez-Rincón, P.C. Brewer’s Spent Grain as Substrate for Enzyme and Reducing Sugar Production Using Penicillium sp. HC1. Rev. Acad. Colomb. Cienc. Exactas Fís. Nat. 2021, 45, 850–863. [Google Scholar] [CrossRef]
- Nishida, V.S.; de Oliveira, R.F.; Brugnari, T.; Correa, R.C.G.; Peralta, R.A.; Castoldi, R.; de Souza, C.G.M.; Bracht, A.; Peralta, R.M. Immobilization of Aspergillus awamori β-Glucosidase on Commercial Gelatin: An Inexpensive and Efficient Process. Int. J. Biol. Macromol. 2018, 111, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Su, Y.; Wang, R.; Zhang, H.; Jing, H.; Meng, J.; Zhang, G.; Huang, L.; Guo, L.; Wang, J.; et al. Microbial Production and Applications of β-Glucosidase—A Review. Int. J. Biol. Macromol. 2024, 256, 127915. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme Immobilization: An Update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.Y.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- De Souza, T.S.P.; Kawaguti, H.Y. Cellulases, Hemicellulases, and Pectinases: Applications in the Food and Beverage Industry. Food Bioprocess Technol. 2021, 14, 1446–1477. [Google Scholar] [CrossRef]
- Su, M.; Hu, Y.; Cui, Y.; Wang, Y.; Yu, H.; Liu, J.; Dai, W.; Piao, C. Production of β-Glucosidase from Okara Fermentation Using Kluyveromyces marxianus. J. Food Sci. Technol. 2021, 58, 366–376. [Google Scholar] [CrossRef]
- Zhang, B.; Wendan, Y.; Wang, F.; Omedi, J.O.; Liu, R.; Huang, J.; Zhang, L.; Zou, Q.; Huang, W.; Li, S. Use of Kluyveromyces marxianus Prefermented Wheat Bran as a Source of Enzyme Mixture to Improve Dough Performance and Bread Biochemical Properties. Cereal Chem. 2019, 96, 142–153. [Google Scholar] [CrossRef]
- Magwaza, B.; Amobonye, A.; Pillai, S. Microbial β-glucosidases: Recent advances and applications. Biochimie 2024, 225, 49–67. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).