Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review
Abstract
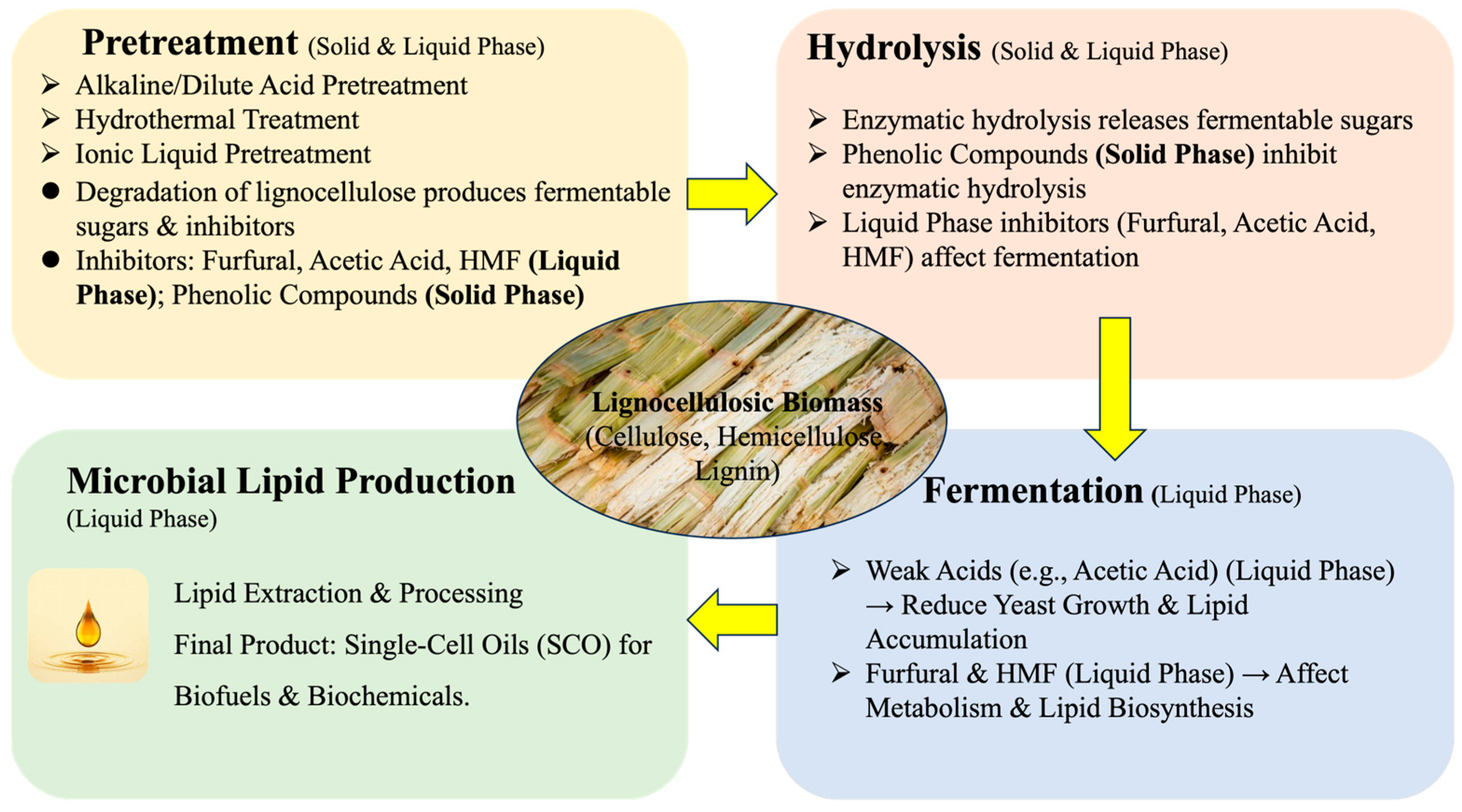
1. Introduction
2. Pretreatment Methods
2.1. Alkaline/Dilute Acid Pretreatment
2.2. Hydrothermal Treatment
2.3. Ionic Liquid Pretreatment
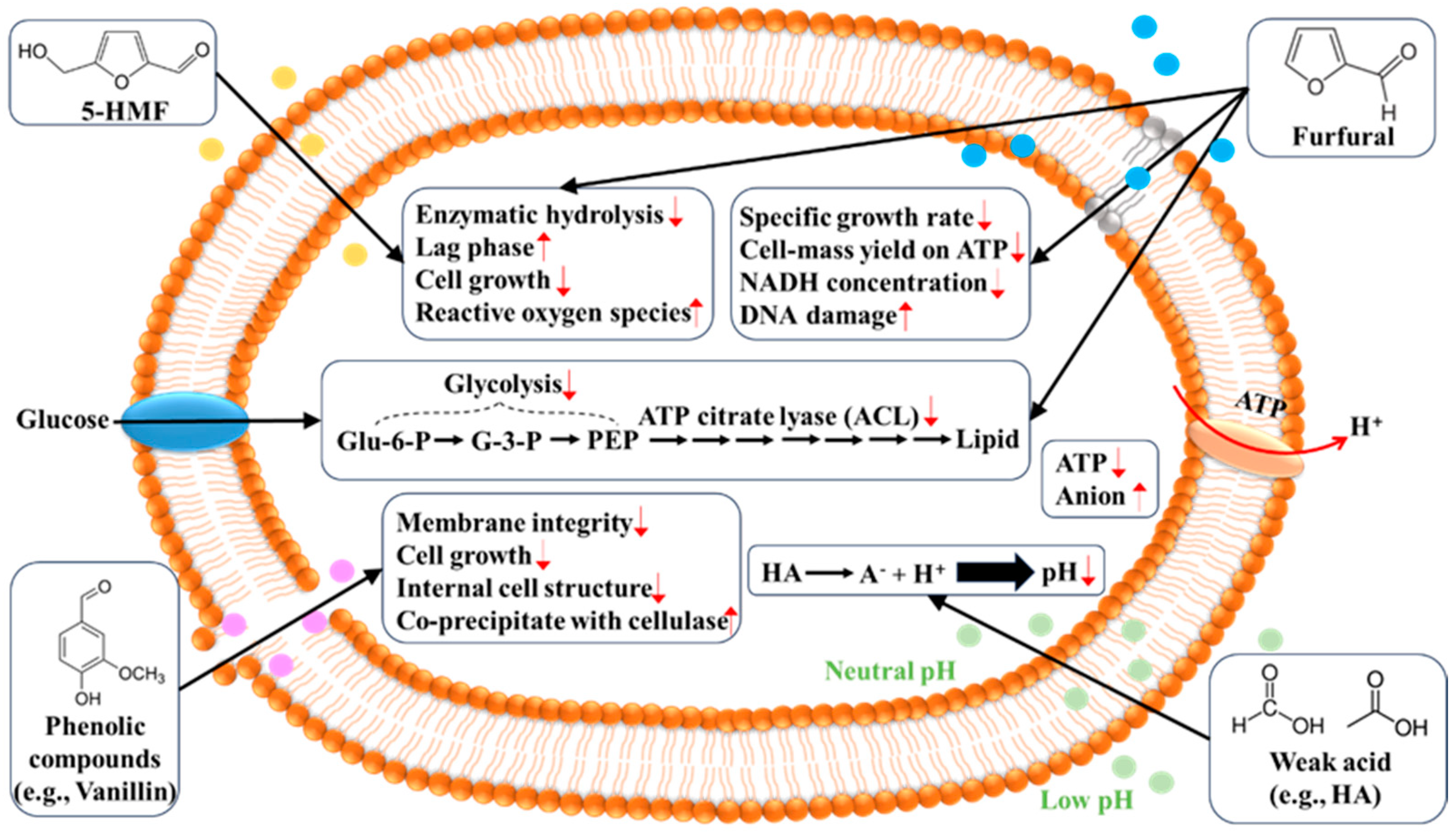
3. Inhibitors and Mechanisms of Inhibition
4. Effects of Inhibitors on Cell Growth and Microbial Oil Production
4.1. Weak Acid
4.2. Furfural and 5-HMF
4.3. Phenols
4.4. Effect of Combinations of Aldehydes on Cell Growth and Lipid Accumulation
5. Strategies for Detoxification of Lignocellulosic Biomass-Derived Inhibitors
5.1. Enzymatic Detoxification
5.2. Combination/Integration of Different Strategies/Measures
5.3. Microbial Genetic Engineering
5.4. Improvement of Fermentation Environment
6. Challenges and Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; da Costa Sousa, L.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.; Fernandes, H.; Lopes, M.; Belo, I. Yarrowia lipolytica produces lipid-rich biomass in medium mimicking lignocellulosic biomass hydrolysate. Appl. Microbiol. Biotechnol. 2023, 107, 3925–3937. [Google Scholar] [CrossRef] [PubMed]
- Juanssilfero, A.B.; Kahar, P.; Amza, R.L.; Sudesh, K.; Ogino, C.; Prasetya, B.; Kondo, A. Lipid production by Lipomyces starkeyi using sap squeezed from felled old oil palm trunks. J. Biosci. Bioeng. 2019, 127, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Dar, R.A.; Tsui, T.-H.; Zhang, L.; Tong, Y.W.; Sharon, S.; Shoseyov, O.; Liu, R. Fermentation of organic wastes through oleaginous microorganisms for lipid production-Challenges and opportunities. Renew. Sustain. Energy Rev. 2024, 195, 114328. [Google Scholar] [CrossRef]
- Yen, H.-W.; Yang, Y.-C.; Yu, Y.-H. Using crude glycerol and thin stillage for the production of microbial lipids through the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2012, 114, 453–456. [Google Scholar] [CrossRef]
- Huang, Q.; Kamal, R.; Shen, H.; Lu, H.; Song, J.; Chu, Y.; Xue, C.; Zhao, Z.K. Pilot-scale conversion of corn stover into lipids by the red yeast Rhodosporidium toruloides. J. Environ. Chem. Eng. 2022, 10, 108858. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Wu, Q. Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol. Bioeng. 2007, 98, 764–771. [Google Scholar] [CrossRef]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. Gcb Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Reyes, P.; Mendonça, R.T.; Aguayo, M.G.; Rodríguez, J.; Vega, B.; Fardim, P. Extraction and characterization of hemicelluloses from Pinus radiata and its feasibility for bioethanol production. Rev. Árvore 2013, 37, 175–180. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G. Biomass pretreatment, biorefineries, and potential products for a bioeconomy development. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–22. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Dar, R.; Parmar, M.; Dar, E.; Sani, R.; Phutela, U. Biomethanation of agricultural residues: Potential, limitations and possible solutions. Renew. Sustain. Energy Rev. 2021, 135, 110217. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Deshavath, N.N.; Dien, B.S.; Slininger, P.J.; Jin, Y.-S.; Singh, V. A chemical-free pretreatment for biosynthesis of bioethanol and lipids from lignocellulosic biomass: An industrially relevant 2G biorefinery approach. Fermentation 2022, 9, 5. [Google Scholar] [CrossRef]
- Abeln, F.; Chuck, C.J. The history, state of the art and future prospects for oleaginous yeast research. Microb. Cell Factories 2021, 20, 1–31. [Google Scholar] [CrossRef]
- Liang, Y.; Jarosz, K.; Wardlow, A.T.; Zhang, J.; Cui, Y. Lipid production by Cryptococcus curvatus on hydrolysates derived from corn fiber and sweet sorghum bagasse following dilute acid pretreatment. Appl. Biochem. Biotechnol. 2014, 173, 2086–2098. [Google Scholar] [CrossRef]
- Kassim, E.; El-Shahed, A. Enzymatic and chemical hydrolysis of certain cellulosic materials. Agric. Wastes 1986, 17, 229–233. [Google Scholar] [CrossRef]
- Šantek, M.I.; Grubišić, M.; Perečinec, M.G.; Beluhan, S.; Šantek, B. Lipid production by Mortierella isabellina from pretreated corn cobs and effect of lignocellulose derived inhibitors on growth and lipid synthesis. Process Biochem. 2021, 109, 46–58. [Google Scholar] [CrossRef]
- Zhang, Z. Waste pretreatment technologies for hydrogen production. In Waste to Renewable Biohydrogen; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–122. [Google Scholar] [CrossRef]
- Gáspár, M.; Kálmán, G.; Réczey, K. Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem. 2007, 42, 1135–1139. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Production of hydrogen and methane from lignocellulose waste by fermentation. A review of chemical pretreatment for enhancing the efficiency of the digestion process. J. Clean. Prod. 2020, 267, 121721. [Google Scholar] [CrossRef]
- Grover, C.; Choudhury, B. Alkaline wet oxidative pretreatment and acid hydrolysis of wheat straw for squalene and monomethyl branched chain fatty acids rich lipid production in Lentibacillus salarius BPIITR. Biocatal. Agric. Biotechnol. 2024, 57, 103062. [Google Scholar] [CrossRef]
- Tahmasebi, Z.; Zilouei, H.; Kot, A.M. Investigating the concomitant production of carotenoids and lipids by the yeast Rhodosporidium babjevae using sugarcane bagasse hydrolysate or corn steep liquor. Renew. Energy 2024, 228, 120618. [Google Scholar] [CrossRef]
- Grubišić, M.; Galić Perečinec, M.; Peremin, I.; Mihajlovski, K.; Beluhan, S.; Šantek, B.; Ivančić Šantek, M. Optimization of pretreatment conditions and enzymatic hydrolysis of corn cobs for production of microbial lipids by Trichosporon oleaginosus. Energies 2022, 15, 3208. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Ezugworie, F.N.; Onyishi, C.L.; Igbokwe, V.C. Lignocellulose biomass pretreatment for efficient hydrolysis and biofuel production. In Advances in Biofuels Production, Optimization and Applications; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–19. [Google Scholar] [CrossRef]
- Hu, F.; Ragauskas, A. Pretreatment and lignocellulosic chemistry. Bioenergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Cheng, J.; Hu, G.; Xie, X.; Peng, C.; Yu, X.; Shen, H.; Zhao, Z.K.; Xie, H. Bio-refining corn stover into microbial lipid and advanced energy material using ionic liquid-based organic electrolyte. Ind. Crops Prod. 2020, 145, 112137. [Google Scholar] [CrossRef]
- Buettner, C.S.; Cognigni, A.; Schröder, C.; Bica-Schröder, K. Surface-active ionic liquids: A review. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Ren, H.; Zong, M.-H.; Wu, H.; Li, N. Utilization of seawater for the biorefinery of lignocellulosic biomass: Ionic liquid pretreatment, enzymatic hydrolysis, and microbial lipid production. ACS Sustain. Chem. Eng. 2016, 4, 5659–5666. [Google Scholar] [CrossRef]
- Franklin, M.S.; Larm, N.E.; Baker, G.A.; Zhao, H. Functionalized ionic liquids for lignite dissolution and treatment. J. Chem. Technol. Biotechnol. 2021, 96, 3273–3281. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, D.; Zhang, T.; Lei, T.; Zhang, H.; Yang, J.; Shui, X.; Li, F.; Zhang, Y.; Zhang, Q. Comparison of three ionic liquids pretreatment of Arundo donax L. For enhanced photo-fermentative hydrogen production. Bioresour. Technol. 2022, 343, 126088. [Google Scholar] [CrossRef]
- Sriariyanun, M.; Kitiborwornkul, N.; Tantayotai, P.; Rattanaporn, K.; Show, P.-L. One-pot ionic liquid-mediated bioprocess for pretreatment and enzymatic hydrolysis of rice straw with ionic liquid-tolerance bacterial cellulase. Bioengineering 2022, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energy Fuels 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Roy, S.; Chundawat, S.P. Ionic liquid–based pretreatment of lignocellulosic biomass for bioconversion: A critical review. BioEnergy Res. 2023, 16, 263–278. [Google Scholar] [CrossRef]
- Swami, S.; Suthar, S.; Singh, R.; Thakur, A.K.; Gupta, L.R.; Sikarwar, V.S. Potential of ionic liquids as emerging green solvent for the pretreatment of lignocellulosic biomass. Environ. Sci. Pollut. Res. 2024, 31, 12871–12891. [Google Scholar] [CrossRef]
- Du, B.; Sharma, L.N.; Becker, C.; Chen, S.F.; Mowery, R.A.; van Walsum, G.P.; Chambliss, C.K. Effect of varying feedstock–pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. Biotechnol. Bioeng. 2010, 107, 430–440. [Google Scholar] [CrossRef]
- van der Pol, E.C.; Bakker, R.R.; Baets, P.; Eggink, G. By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio) chemicals and fuels. Appl. Microbiol. Biotechnol. 2014, 98, 9579–9593. [Google Scholar] [CrossRef]
- Chundawat, S.P.; Vismeh, R.; Sharma, L.N.; Humpula, J.F.; da Costa Sousa, L.; Chambliss, C.K.; Jones, A.D.; Balan, V.; Dale, B.E. Multifaceted characterization of cell wall decomposition products formed during ammonia fiber expansion (AFEX) and dilute acid based pretreatments. Bioresour. Technol. 2010, 101, 8429–8438. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef]
- Klaban, J.; Meile, K.; Godina, D.; Tupciauskas, R.; Berzins, A.; Andze, L.; Sedlarik, V. Evaluation of value-added by-products from steam explosion lignocellulosic biomass (Triticum aestivum, Zea mays, and Phragmites australis). Ind. Crops Prod. 2024, 222, 119443. [Google Scholar] [CrossRef]
- Casella, P.; Loffredo, R.; Rao, M.A.; Balducchi, R.; Liuzzi, F.; De Bari, I.; Molino, A. Inhibitors derived from wheat straw hydrolysate can affect the production of succinic acid by Actinobacillus succinogenes. Process Biochem. 2024, 147, 228–239. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining Innov. A Sustain. Econ. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Uthandi, S.; Kaliyaperumal, A.; Srinivasan, N.; Thangavelu, K.; Muniraj, I.K.; Zhan, X.; Gathergood, N.; Gupta, V.K. Microbial biodiesel production from lignocellulosic biomass: New insights and future challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2197–2225. [Google Scholar] [CrossRef]
- Arantes, V.; Saddler, J.N. Cellulose accessibility limits the effectiveness of minimum cellulase loading on the efficient hydrolysis of pretreated lignocellulosic substrates. Biotechnol. Biofuels 2011, 4, 1–17. [Google Scholar] [CrossRef]
- Wyman, C.E. What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol. 2007, 25, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Zhou, J.; Cen, K. Inhibitory effects of furan derivatives and phenolic compounds on dark hydrogen fermentation. Bioresour. Technol. 2015, 196, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, F.; Du, W.; Liu, C.; Liu, D. Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides and preparation of biodiesel by enzymatic transesterification of the lipid. Bioprocess Biosyst. Eng. 2012, 35, 993–1004. [Google Scholar] [CrossRef]
- Valdés, G.; Mendonça, R.T.; Aggelis, G. Lignocellulosic biomass as a substrate for oleaginous microorganisms: A review. Appl. Sci. 2020, 10, 7698. [Google Scholar] [CrossRef]
- Yu, Z.; Du, Y.; Shang, X.; Zheng, Y.; Zhou, J. Enhancing fermentable sugar yield from cassava residue using a two-step dilute ultra-low acid pretreatment process. Ind. Crops Prod. 2018, 124, 555–562. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Da Silva, S.S.; Singh, O.V. Detoxification of lignocellulose hydrolysates: Biochemical and metabolic engineering toward white biotechnology. BioEnergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Liu, H.; Zhang, J.A. Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Bioresour. Technol. 2015, 180, 32–39. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, B. Microbial lipid production from corn stover via Mortierella isabellina. Appl. Biochem. Biotechnol. 2014, 174, 574–586. [Google Scholar] [CrossRef]
- Wang, L.R.; Zhang, Z.X.; Wang, Y.Z.; Li, Z.J.; Huang, P.W.; Sun, X.M. Assessing the potential of Schizochytrium sp. HX-308 for microbial lipids production from corn stover hydrolysate. Biotechnol. J. 2022, 17, 2100470. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, B.; Korstad, J. Utilization of lignocellulosic biomass by oleaginous yeast and bacteria for production of biodiesel and renewable diesel. Renew. Sustain. Energy Rev. 2017, 73, 654–671. [Google Scholar] [CrossRef]
- Quarterman, J.; Slininger, P.J.; Kurtzman, C.P.; Thompson, S.R.; Dien, B.S. A survey of yeast from the Yarrowia clade for lipid production in dilute acid pretreated lignocellulosic biomass hydrolysate. Appl. Microbiol. Biotechnol. 2017, 101, 3319–3334. [Google Scholar] [CrossRef]
- Wang, B.; Rezenom, Y.H.; Cho, K.-C.; Tran, J.L.; Russell, D.H.; Gill, J.J.; Young, R.; Chu, K.-H. Cultivation of lipid-producing bacteria with lignocellulosic biomass: Effects of inhibitory compounds of lignocellulosic hydrolysates. Bioresour. Technol. 2014, 161, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C. Biochemistry, stoichiometry, substrates and economics. Single Cell Oil 1988, 1, 33–70. [Google Scholar]
- Fakas, S.; Čertik, M.; Papanikolaou, S.; Aggelis, G.; Komaitis, M.; Galiotou-Panayotou, M. γ-Linolenic acid production by Cunninghamella echinulata growing on complex organic nitrogen sources. Bioresour. Technol. 2008, 99, 5986–5990. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Daskalaki, A.; Perdikouli, N.; Aggeli, D.; Aggelis, G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Chen, J.; Imanaka, T.; Bao, J.; Hua, Q. Analysis of metabolic fluxes for better understanding of mechanisms related to lipid accumulation in oleaginous yeast Trichosporon cutaneum. Bioresour. Technol. 2013, 130, 144–151. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, Z.; Chen, S.; Jin, M. Microbial lipid production from dilute acid and dilute alkali pretreated corn stover via Trichosporon dermatis. Bioresour. Technol. 2020, 295, 122253. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem. 2017, 53, 44–60. [Google Scholar] [CrossRef]
- Ulbricht, R.J.; Northup, S.J.; Thomas, J.A. A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Toxicol. Sci. 1984, 4, 843–853. [Google Scholar] [CrossRef]
- Russell, J. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Verduyn, C.; Postma, E.; Scheffers, W.A.; Van Dijken, J.P. Effect of benzoic acid on metabolic fluxes in yeasts: A continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 1992, 8, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Gao, D. Lipid Production from Xylose and Inhibitory Effects from Lignocellulose Hydrolysate Using Mortierella Isabellina. Doctoral Dissertation, Washington State University, Pullman, WA, USA, 2012. [Google Scholar]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of oleaginous yeasts for lipid production using volatile fatty acids as substrate. Biomass Bioenergy 2020, 138, 105553. [Google Scholar] [CrossRef]
- Yu, X.; Zeng, J.; Zheng, Y.; Chen, S. Effect of lignocellulose degradation products on microbial biomass and lipid production by the oleaginous yeast Cryptococcus curvatus. Process Biochem. 2014, 49, 457–465. [Google Scholar] [CrossRef]
- Gong, Z.; Shen, H.; Zhou, W.; Wang, Y.; Yang, X.; Zhao, Z.K. Efficient conversion of acetate into lipids by the oleaginous yeast Cryptococcus curvatus. Biotechnol. Biofuels 2015, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Z.; Hollinshead, W.; Isaguirre, C.; Tang, Y.J.; Liao, W.; Liu, Y. Effects of inhibitory compounds in lignocellulosic hydrolysates on Mortierella isabellina growth and carbon utilization. Bioresour. Technol. 2015, 183, 18–24. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Gustafsson, L.; Niklasson, C.; Lidén, G. Conversion of furfural in aerobic and anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. J. Biosci. Bioeng. 1999, 87, 169–174. [Google Scholar] [CrossRef]
- Fireoved, R.; Mutharasan, R. Effect of furfural and ethanol on the growth and energetics of yeast under microaerobic conditions. Ann. N. Y. Acad. Sci. 1986, 469, 433–446. [Google Scholar] [CrossRef]
- Palmqvist, E.; Almeida, J.S.; Hahn-Hägerdal, B. Influence of furfural on anaerobic glycolytic kinetics of Saccharomyces cerevisiae in batch culture. Biotechnol. Bioeng. 1999, 62, 447–454. [Google Scholar] [CrossRef]
- Laforge, F.B.; Mains, G.H. Furfural from corncobs. Ind. Eng. Chem. 1923, 15, 823–829. [Google Scholar] [CrossRef]
- Sangarunlert, W.; Piumsomboon, P.; Ngamprasertsith, S. Furfural production by acid hydrolysis and supercritical carbon dioxide extraction from rice husk. Korean J. Chem. Eng. 2007, 24, 936–941. [Google Scholar] [CrossRef]
- Almeida, J.R.; Bertilsson, M.; Gorwa-Grauslund, M.F.; Gorsich, S.; Lidén, G. Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl. Microbiol. Biotechnol. 2009, 82, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hahn, J.-S. Roles of the Yap1 transcription factor and antioxidants in Saccharomyces cerevisiae’s tolerance to furfural and 5-hydroxymethylfurfural, which function as thiol-reactive electrophiles generating oxidative stress. Appl. Environ. Microbiol. 2013, 79, 5069–5077. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Karthikeyan, K. Effects of severe pretreatment conditions and lignocellulose-derived furan byproducts on anaerobic digestion of dairy manure. Bioresour. Technol. 2021, 340, 125632. [Google Scholar] [CrossRef]
- Azhar, A.; Bery, M.; Colcord, A.; Roberts, R.; Corbitt, G. Factors affecting alcohol fermentation of wood acid hydrolysate. In Biotechnology and Bioengineering Symposium; Georgia Institute of Technology: Atlanta, GA, USA, 1981; Volume 11. [Google Scholar]
- van der Maas, L.; Driessen, J.L.; Mussatto, S.I. Effects of inhibitory compounds present in lignocellulosic biomass hydrolysates on the growth of Bacillus subtilis. Energies 2021, 14, 8419. [Google Scholar] [CrossRef]
- Huang, C.; Wu, H.; Liu, Q.-p.; Li, Y.-y.; Zong, M.-H. Effects of aldehydes on the growth and lipid accumulation of oleaginous yeast Trichosporon fermentans. J. Agric. Food Chem. 2011, 59, 4606–4613. [Google Scholar] [CrossRef]
- Rahman, S.; Arbter, P.; Popovic, M.; Bajpai, R.; Subramaniam, R. Microbial lipid production from lignocellulosic hydrolyzates: Effect of carbohydrate mixtures and acid-hydrolysis byproducts on cell growth and lipid production by Lipomyces starkeyi. J. Chem. Technol. Biotechnol. 2017, 92, 1980–1989. [Google Scholar] [CrossRef]
- Martín, C.; Galbe, M.; Nilvebrant, N.-O.; Jönsson, L.J. Comparison of the fermentability of enzymatic hydrolyzates of sugarcane bagasse pretreated by steam explosion using different impregnating agents. In Biotechnology for Fuels and Chemicals: The Twenty–Third Symposium; Springer: Berlin/Heidelberg, Germany, 2002; pp. 699–716. [Google Scholar] [CrossRef]
- Larsson, S.; Quintana-Sáinz, A.; Reimann, A.; Nilvebrant, N.-O.; Jönsson, L.J. Influence of lignocellulose-derived aromatic compounds on oxygen-limited growth and ethanolic fermentation by Saccharomyces cerevisiae. In Twenty-First Symposium on Biotechnology for Fuels and Chemicals: Proceedings of the Twenty-First Symposium on Biotechnology for Fuels and Chemicals Held 2–6 May 1999, in Fort Collins, CO, USA; Springer: Berlin/Heidelberg, Germany, 2000; pp. 617–632. [Google Scholar] [CrossRef]
- Ando, S.; Arai, I.; Kiyoto, K.; Hanai, S. Identification of aromatic monomers in steam-exploded poplar and their influences on ethanol fermentation by Saccharomyces cerevisiae. J. Ferment. Technol. 1986, 64, 567–570. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef]
- Klinke, H.B.; Thomsen, A.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Fletcher, E.; Baetz, K. Multi-faceted systems biology approaches present a cellular landscape of phenolic compound inhibition in Saccharomyces cerevisiae. Front. Bioeng. Biotechnol. 2020, 8, 539902. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzym. Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Sampaio, F.C.; Torre, P.; Passos, F.M.L.; De Moraes, C.A.; Perego, P.; Converti, A. Influence of inhibitory compounds and minor sugars on xylitol production by Debaryomyces hansenii. Appl. Biochem. Biotechnol. 2007, 136, 165–181. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Saez, F.; Ballesteros, I.; Manzanares, P.; Gonzalez, A.; Ballesteros, M. Effects of acetic acid, furfural and catechol combinations on ethanol fermentation of Kluyveromyces marxianus. Process Biochem. 2006, 41, 1223–1228. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.K.; Bai, F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzym. Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, X.; Zhao, J.; Wu, S.; Zhao, Z.K. Effects of biomass hydrolysis by-products on oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2009, 100, 4843–4847. [Google Scholar] [CrossRef]
- Zaldivar, J.; Martinez, A.; Ingram, L.O. Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli. Biotechnol. Bioeng. 1999, 65, 24–33. [Google Scholar] [CrossRef]
- Konzock, O.; Zaghen, S.; Norbeck, J. Tolerance of Yarrowia lipolytica to inhibitors commonly found in lignocellulosic hydrolysates. BMC Microbiol. 2021, 21, 77. [Google Scholar] [CrossRef]
- Zeng, J.; Zheng, Y.; Yu, X.; Yu, L.; Gao, D.; Chen, S. Lignocellulosic biomass as a carbohydrate source for lipid production by Mortierella isabellina. Bioresour. Technol. 2013, 128, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Alvira, P.; Tomás-Pejó, E.; Ballesteros, M. A review of biological delignification and detoxification methods for lignocellulosic bioethanol production. Crit. Rev. Biotechnol. 2015, 35, 342–354. [Google Scholar] [CrossRef]
- Fernández-Sandoval, M.; García, A.; Teymennet-Ramírez, K.; Arenas-Olivares, D.; Martínez-Morales, F.; Trejo-Hernández, M. Removal of phenolic inhibitors from lignocellulose hydrolysates using laccases for the production of fuels and chemicals. Biotechnol. Prog. 2024, 40, e3406. [Google Scholar] [CrossRef]
- Saravanakumar, T.; Park, H.-S.; Mo, A.-Y.; Choi, M.-S.; Kim, D.-H.; Park, S.-M. Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J. Mol. Catal. B Enzym. 2016, 134, 196–205. [Google Scholar] [CrossRef]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF–an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, G.; Gong, Z.; Shen, H.; Bai, F.; Zhao, Z.K. Recycling microbial lipid production wastes to cultivate oleaginous yeasts. Bioresour. Technol. 2015, 175, 91–96. [Google Scholar] [CrossRef]
- Lin, J.; Li, S.; Sun, M.; Zhang, C.; Yang, W.; Zhang, Z.; Li, X.; Li, S. Microbial lipid production by oleaginous yeast in D-xylose solution using a two-stage culture mode. RSC Adv. 2014, 4, 34944–34949. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, Q.; Shen, H.; Hu, C.; Jin, G.; Zhao, Z.K. Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour. Technol. 2012, 117, 20–24. [Google Scholar] [CrossRef]
- Justine, I.; Chin, G.J.W.L.; Yong, W.T.L.; Misson, M. Characterization and optimization of Rhodotorula toruloides and Ankistrodesmus falcatus co-culture in palm oil mill effluent for efficient COD removal and lipid production. Biocatal. Agric. Biotechnol. 2023, 51, 102782. [Google Scholar] [CrossRef]
- Mahajan, D.; Sengupta, S.; Sen, S. Strategies to improve microbial lipid production: Optimization techniques. Biocatal. Agric. Biotechnol. 2019, 22, 101321. [Google Scholar] [CrossRef]
- Takaku, H.; Ebina, S.; Kasuga, K.; Sato, R.; Ara, S.; Kazama, H.; Matsuzawa, T.; Yaoi, K.; Araki, H.; Shida, Y. Isolation and characterization of Lipomyces starkeyi mutants with greatly increased lipid productivity following UV irradiation. J. Biosci. Bioeng. 2021, 131, 613–621. [Google Scholar] [CrossRef]
- Sato, R.; Fujii, Y.; Ara, S.; Yamazaki, H.; Aburatani, S.; Ogasawara, W.; Takaku, H. Deletion of LsSNF1 enhances lipid accumulation in the oleaginous yeast Lipomyces starkeyi. J. Biosci. Bioeng. 2024, 137, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Chu, Y.; Zhang, S.; Zhang, Y.; Huang, Q.; Wang, S.; Zhao, Z.K. Engineering the oleaginous yeast Rhodosporidium toruloides for improved resistance against inhibitors in biomass hydrolysates. Front. Bioeng. Biotechnol. 2021, 9, 768934. [Google Scholar] [CrossRef]
- Kang, N.K.; Kim, E.K.; Kim, Y.U.; Lee, B.; Jeong, W.-J.; Jeong, B.-R.; Chang, Y.K. Increased lipid production by heterologous expression of AtWRI1 transcription factor in Nannochloropsis salina. Biotechnol. Biofuels 2017, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Gu, Z.; Zhou, Q.; Sun, H.; Luo, J.; Liu, Z.; Guo, S.; Ren, H.; Zhang, Z.; Strong, P.J. Highly efficient microbial lipid synthesis from co-fermentation of enzymatic hydrolysate of sugarcane bagasse by a Trichosporon dermatis mutant. Ind. Crops Prod. 2021, 171, 113975. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, S.; Elshobary, M.; Qi, W.; Wang, W.; Feng, P.; Wang, Z.; Qin, L. Enhancing detoxification of inhibitors in lignocellulosic pretreatment wastewater by bacterial Action: A pathway to improved biomass utilization. Bioresour. Technol. 2024, 410, 131270. [Google Scholar] [CrossRef]
- Barrick, J.E.; Lenski, R.E. Genome dynamics during experimental evolution. Nat. Rev. Genet. 2013, 14, 827–839. [Google Scholar] [CrossRef]
- Menegon, Y.A.; Gross, J.; Jacobus, A.P. How adaptive laboratory evolution can boost yeast tolerance to lignocellulosic hydrolyses. Curr. Genet. 2022, 68, 319–342. [Google Scholar] [CrossRef]
- Palmqvist, E.; Galbe, M.; Hahn-Hägerdal, B. Evaluation of cell recycling in continuous fermentation of enzymatic hydrolysates of spruce with Saccharomyces cerevisiae and on-line monitoring of glucose and ethanol. Appl. Microbiol. Biotechnol. 1998, 50, 545–551. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, C.; Wu, S.; Shen, H.; Zhao, Z.K. Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J. Ind. Microbiol. Biotechnol. 2011, 38, 627–632. [Google Scholar] [CrossRef]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Ashby, R.D.; Olanya, O.M. Heterologous expression of a yeast lipid transporter promotes accumulation of storage compounds in Chlamydomonas. Biocatal. Agric. Biotechnol. 2023, 52, 102809. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, Y.; Dorgan, K.M.; Chen, S. Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresour. Technol. 2011, 102, 6134–6140. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Zhang, Z. Effects of dissolved oxygen level on cell growth and total lipid accumulation in the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2011, 112, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Shen, H.; Tan, H.; Zhao, X.; Wu, S.; Hu, C.; Zhao, Z.K. Lipid production by Lipomyces starkeyi cells in glucose solution without auxiliary nutrients. J. Biotechnol. 2011, 152, 184–188. [Google Scholar] [CrossRef]
- Li, C.; Chen, K.; Wang, B.; Nges, I.A. Bioreactor design for efficient biofuels production from lignocellulosic biomass. In Biofuels Production from Lignocellulosic Materials; Elsevier: Amsterdam, The Netherlands, 2025; pp. 181–217. [Google Scholar] [CrossRef]
- Anand, V.; Kashyap, M.; Bala, K. Impact of H2O2 on biomass and lipid accumulation in Scenedesmus sp.: Promising species for biofuel production. Biocatal. Agric. Biotechnol. 2023, 51, 102795. [Google Scholar] [CrossRef]
- Banerjee, S.; Singh, V. Economic and environmental bottlenecks in the industrial-scale production of lipid-derived biofuels from oleaginous yeasts: A review of the current trends and future prospects. GCB Bioenergy 2024, 16, e13173. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Verardo, V.; Garcia, B.M.; Oliviero, M.; Masi, P. Sustainable cultivation of Porphyridium cruentum via agro-industrial by-products: A study on biomass and lipid enhancement. Biocatal. Agric. Biotechnol. 2024, 60, 103341. [Google Scholar] [CrossRef]
| Lignocellulose Source | Method | 5-HMF | Furfural | Levulinic Acid | Acetic Acid | Formic Acid | Phenolics | Reference |
|---|---|---|---|---|---|---|---|---|
| Corn stover | Acid hydrolysis | 0.044 g/L | 0.22 g/L | 0.041 g/L | 0.17 g/L | 0.12 g/L | - | [38,39] |
| Corn stover | Acid hydolysis | 15.7 mg/g biomass | 7.94 mg/g biomass | 3.65 mg/g biomass | 34.77 mg/g biomass | 3.17 mg/g biomass | - | [40] |
| maple wood | 230 g/L with hot water at 200 °C for 20 min | 4.1 g/L (5-HMF + Furfural) | 4.1 g/L (5-HMF + Furfural) | - | 13.1 g/L | - | - | [41] |
| Corn stover | Wet oxidation | 0.0028 g/L | 0.0065 g/L | 0.0019 g/L | 0.058 g/L | 0.079 g/L | - | [38] |
| Corn stover | AFEX | 0.642 mg/g biomass | 0 mg/g biomass | 0.024 mg/g biomass | 4.61 mg/g biomass | 0.91 mg/g biomass | - | [40] |
| Wheat straw | Steam explosion | - | 0.30 ± 0.01% | - | 0.72 ± 0.01% | - | 1.49 ± 0.02% | [42] |
| Reed | Steam explosion | - | 1.16 ± 0.04% | - | 1.32 ± 0.00% | - | 1.71 ± 0.04% | [42] |
| Corn stalks | Steam explosion | - | 0.59 ± 0.00% | - | 1.17 ± 0.01% | - | 1.54 ± 0.03% | [42] |
| Pine | Wet oxidation | 0.00064 g/L | 0.0019 g/L | 0.0005 g/L | 0.024 g/L | 0.066 g/L | - | [38] |
| Wheat straw | Catalyzed acid steam explosion treatment | 0.9 ± 0.1 g/L (5-HMF + Furfural) | 0.9 ± 0.1 g/L (5-HMF + Furfural) | - | 1.9 ± 0.2 g/L | - | - | [43] |
| Fermentation Process | Microbial | Inhibitor | Inhibitor Concentration | Biomass Decline (%) | Lipid Concentration Decreased (%) | References |
|---|---|---|---|---|---|---|
| Microbial growth | Yarrowia lipolytica | acetic acid | 75 mM | 100 | - | [107] |
| Yarrowia lipolytica | formic acid | 37.5 mM | 100 | - | [107] | |
| Rhodosporidium fluviale DMKU-SP314 | formic acid | 0.5 g/L | 100 | - | [72] | |
| Rhodosporidium fluviale DMKU-SP314 Rhodosporidium toruloides Rhodosporidium toruloides Rhodosporidium toruloides | acetic acid formic acid acetic acid furfural | 1.0 g/L 2, 4 g/L 5, 10, 20 g/L 1.0 g/L | 72 25, 40 15.6, 50, 100 60 | 97 - - | [53,72] | |
| Rhodosporidium toruloides Y4 Mortierella isabelline DSM 1414 Mortierella isabelline NRRL 1757 Mortierella isabelline NRRL 1757 | furfural furfural furfural 5-HMF | 1 mM 21.8 mM 2.0 g/L 2.0 g/L | 45.5 77 11 25 | 26.5 84 3 23 | [20,105,108] | |
| Lipid accumulation | Trichosporon fermentans CICC 1368 | furfural | 2.1, 4.7 mM | 25, 50 | [92] | |
| Trichosporon fermentans CICC 1368 | HMF | 15.1, 37.7 mM | 25, 50 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Q.; Dar, R.A.; Baganz, F.; Smoliński, A.; Rasmey, A.-H.M.; Liu, R.; Zhang, L. Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review. Fermentation 2025, 11, 121. https://doi.org/10.3390/fermentation11030121
Lyu Q, Dar RA, Baganz F, Smoliński A, Rasmey A-HM, Liu R, Zhang L. Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review. Fermentation. 2025; 11(3):121. https://doi.org/10.3390/fermentation11030121
Chicago/Turabian StyleLyu, Qiwei, Rouf Ahmad Dar, Frank Baganz, Adam Smoliński, Abdel-Hamied Mohamed Rasmey, Ronghou Liu, and Le Zhang. 2025. "Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review" Fermentation 11, no. 3: 121. https://doi.org/10.3390/fermentation11030121
APA StyleLyu, Q., Dar, R. A., Baganz, F., Smoliński, A., Rasmey, A.-H. M., Liu, R., & Zhang, L. (2025). Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review. Fermentation, 11(3), 121. https://doi.org/10.3390/fermentation11030121









