Expanding Layers of Bacteriocin Applications: From Food Preservation to Human Health Interventions
Abstract
1. Introduction
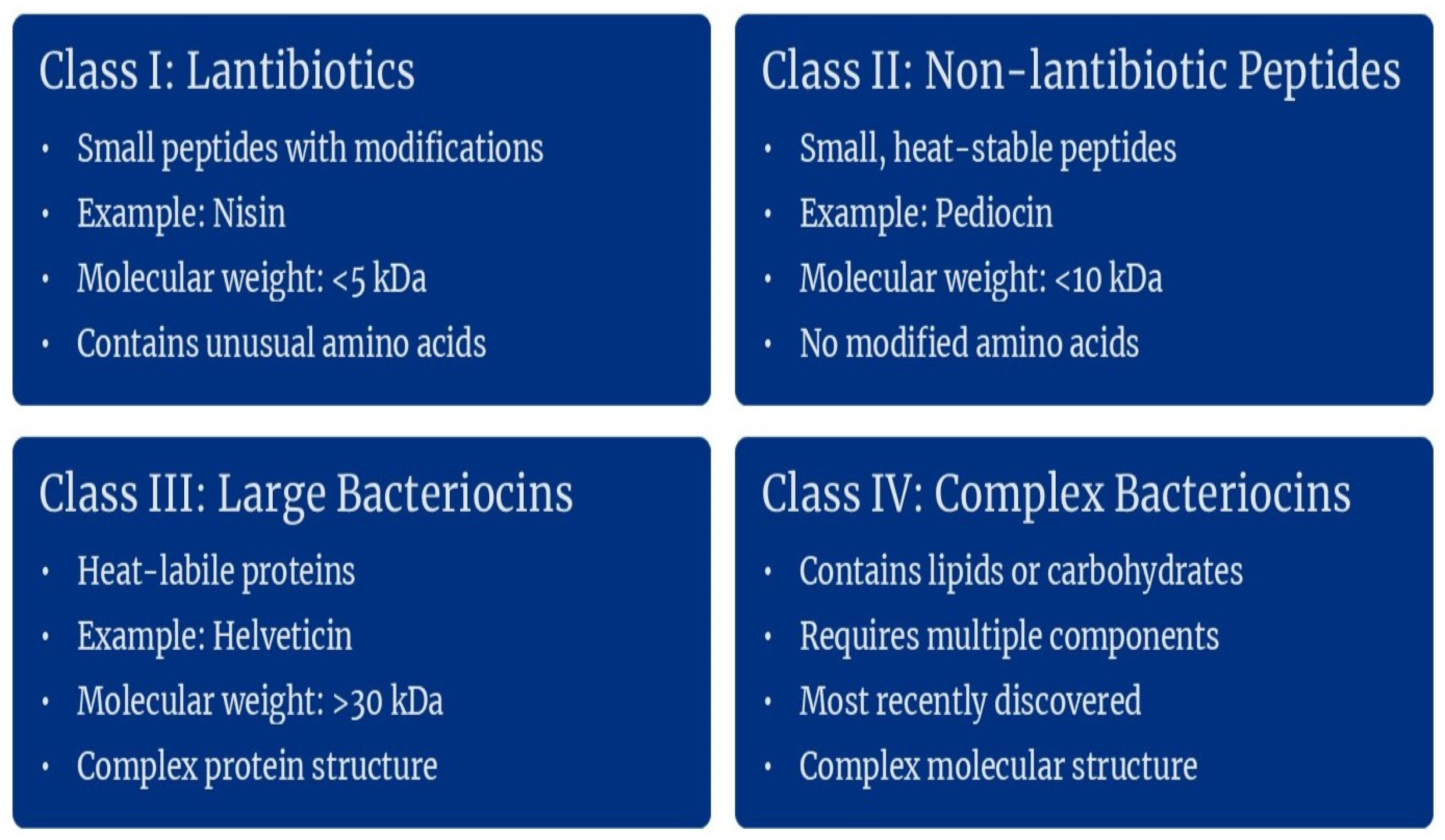
2. Classification of Bacteriocins
2.1. Gram-Positive Bacteriocin Producers and Their Bacteriocins
2.1.1. Class I Bacteriocins
2.1.2. Class II Bacteriocins
2.1.3. Class III Bacteriocins
2.1.4. Class IV Bacteriocins
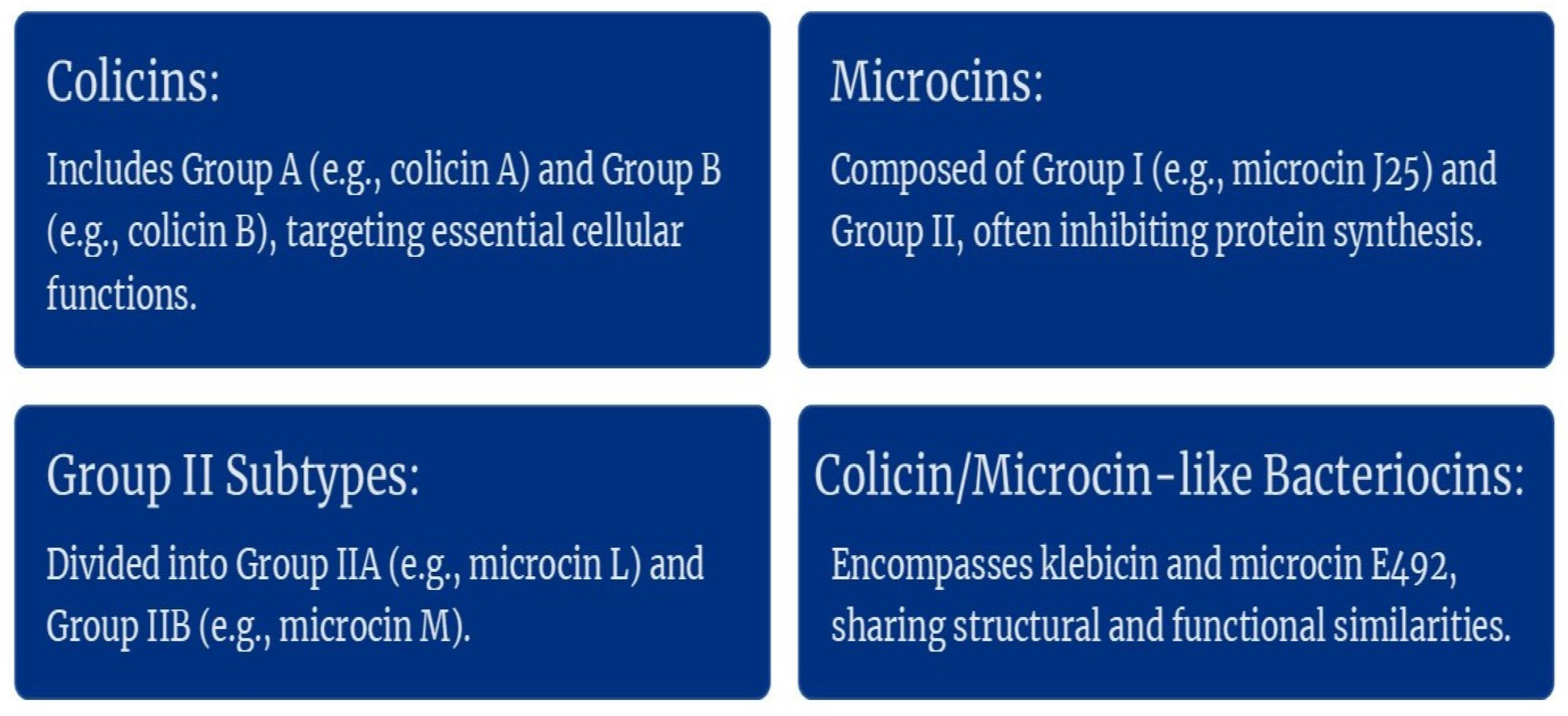
2.2. Gram-Negative Bacteriocin Producers and Their Bacteriocins
3. Mechanisms of Action of Bacteriocins
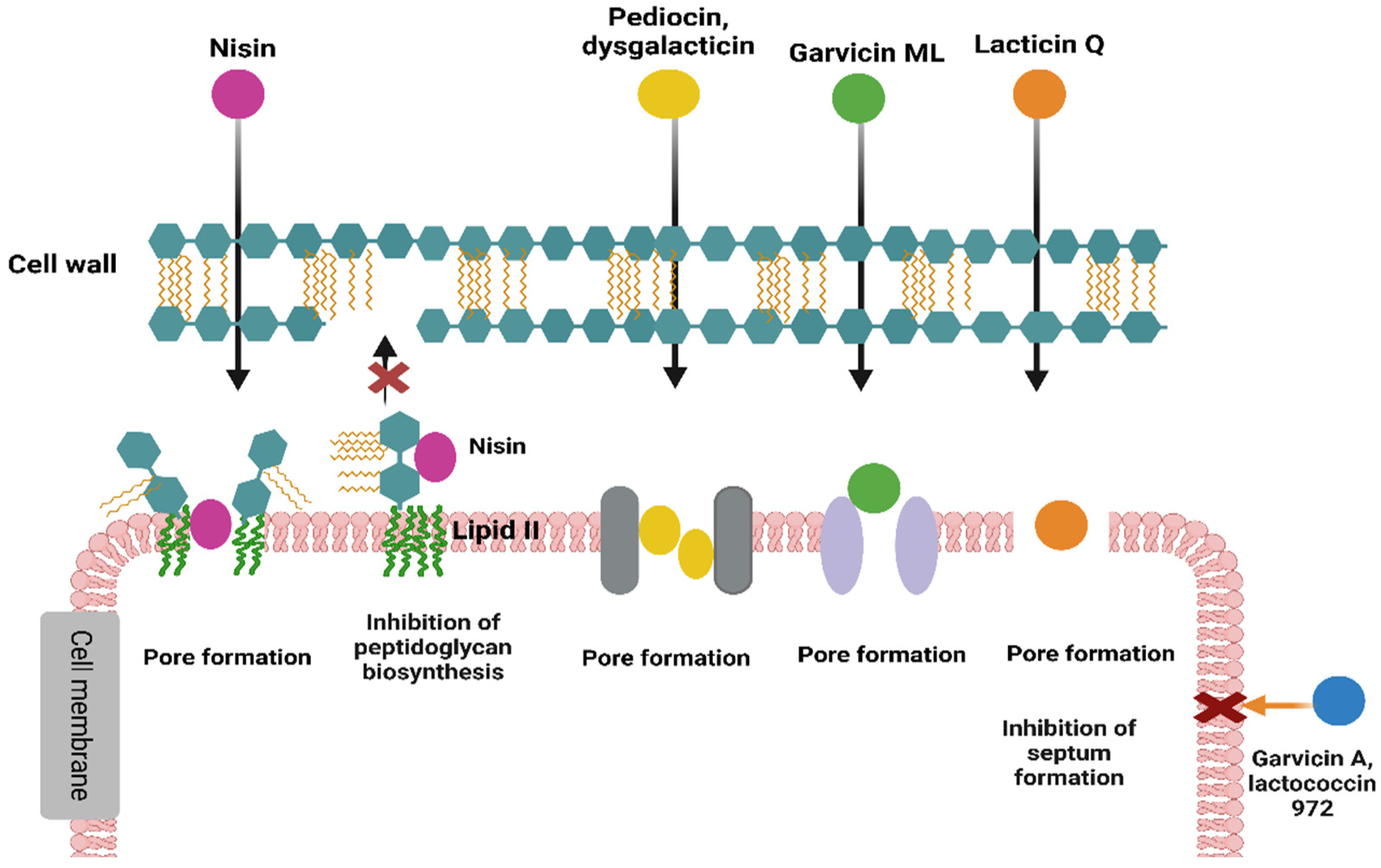
3.1. Mechanisms of Action of Gram-Positive Bacteriocins
3.1.1. Inhibitors of Cell Wall Synthesis
3.1.2. Disruptors of Bacterial Membrane Integrity
3.1.3. Inhibitors of Septum Formation
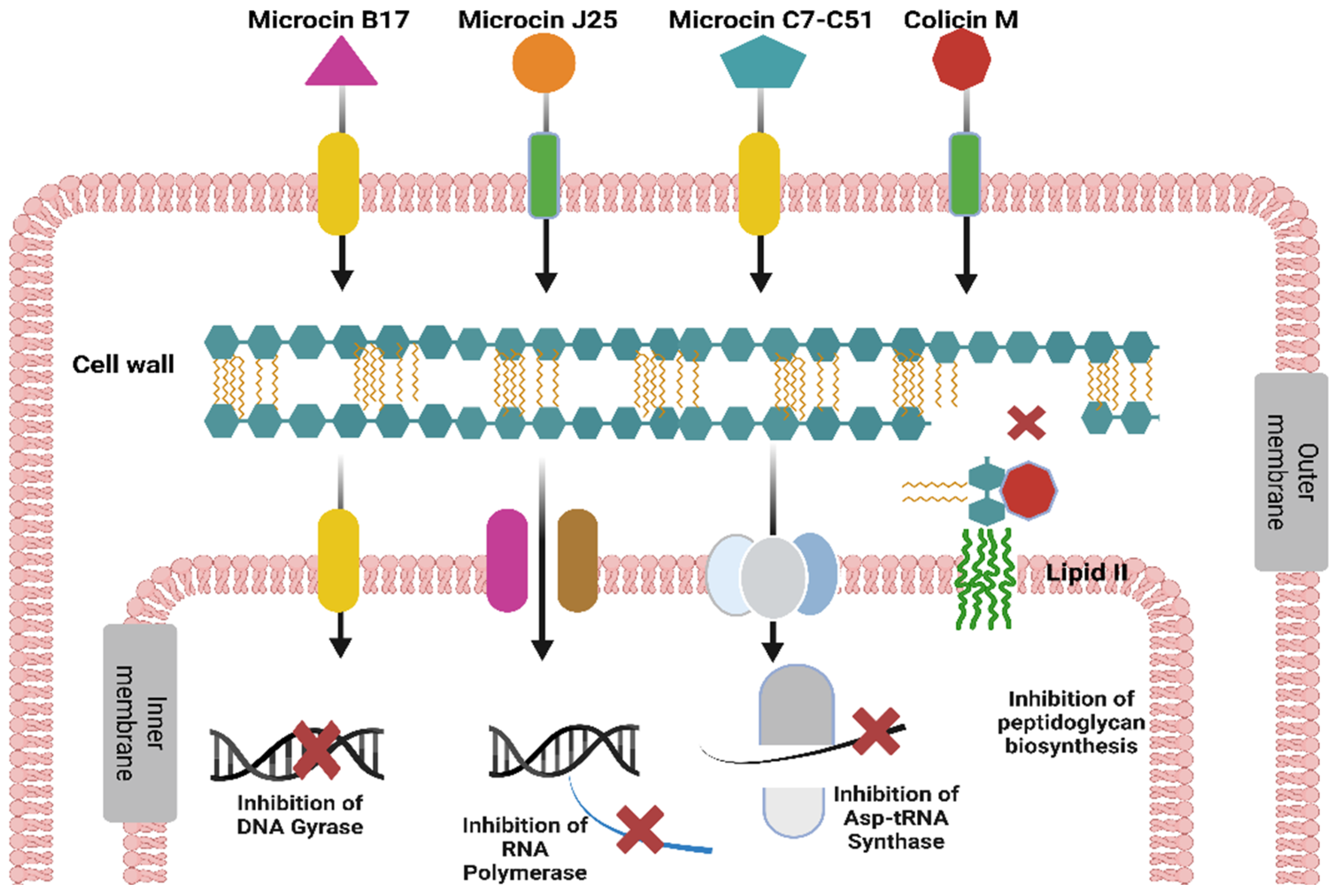
3.2. Mechanisms of Action of Gram-Negative Bacteriocins
3.2.1. Inhibitors of Cell Wall Synthesis
3.2.2. Pore Formers in the Cytoplasmic Membrane
3.2.3. Inhibitors of DNA Replication
3.2.4. Inhibitors of Protein Synthesis
4. Innovative Approaches to the Application of Bacteriocins in Food Systems
4.1. Use of Bacteriocin in Film and Coating Material
4.2. Use of Bacteriocin in Nanotechnology
5. Use of Bacteriocins to Inhibit Biofilm Formation
6. Effects of Bacteriocins on Human Health
6.1. Use in the Treatment of Bacterial Infections
6.2. Use in the Treatment of Viral Infections
6.3. Effect on Cancer Cells
7. Challenges and Limitations
8. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Vasquez-Moscoso, C.A.; Merlano, J.A.R.; Olivera Gálvez, A.; Volcan Almeida, D. Antimicrobial peptides (AMPs) from microalgae as an alternative to conventional antibiotics in aquaculture. Prep. Biochem. Biotechnol. 2025, 55, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, I.; Ross, R.P.; Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat. Rev. Microbiol. 2024, 22, 556–571. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Teixeira, J.A.; Silva, C.C.G. Recent advances in the use of edible films and coatings with probiotic and bacteriocin-producing lactic acid bacteria. Food Biosci. 2023, 56, 103196. [Google Scholar] [CrossRef]
- Zhu, R.; Li, B.; Han, S.; Gu, Q.; Song, D. Purification, characterization, and antimicrobial mechanism of a novel broad-spectrum bacteriocin produced by Lactiplantibacillus plantarum SCA12 from traditional Chinese fermented mustard greens. LWT 2024, 211, 116933. [Google Scholar] [CrossRef]
- Daba, G.M.; Elkhateeb, W.A. Ribosomally synthesized bacteriocins of lactic acid bacteria: Simplicity yet having wide potentials—A review. Int. J. Biol. Macromol. 2024, 256, 128325. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Sun, T.; Xu, J. Bacteriocins in cancer treatment: Mechanisms and clinical potentials. Biomolecules 2024, 14, 831. [Google Scholar] [CrossRef]
- Aggarwal, R.; Mahajan, P.; Pandiya, S.; Bajaj, A.; Verma, S.K.; Yadav, P.; Kharat, A.S.; Khan, A.U.; Dua, M.; Johri, A.K. Antibiotic resistance: A global crisis, problems and solutions. Crit. Rev. Microbiol. 2024, 50, 896–921. [Google Scholar] [CrossRef]
- Cheruvari, A.; Kammara, R. Bacteriocins future perspectives: Substitutes to antibiotics. Food Control 2025, 168, 110834. [Google Scholar] [CrossRef]
- Hassan, M.; Kjos, M.; Nes, I.F.; Diep, D.B.; Lotfipour, F. Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 2012, 113, 723–736. [Google Scholar] [CrossRef]
- Qiao, Z.; Guo, X.; Wang, T.; Wei, J.; Liu, Y.; Ma, Y.; Lü, X. Effects of sub-minimum inhibitory concentrations of bacteriocin BM173 on Listeria monocytogenes biofilm formation. Probiotics Antimicrob. Proteins 2024, 16, 2305–2315. [Google Scholar] [CrossRef]
- Shafique, B.; Ranjha, M.M.A.N.; Murtaza, M.A.; Walayat, N.; Nawaz, A.; Khalid, W.; Mahmood, S.; Nadeem, M.; Manzoor, M.F.; Ameer, K.; et al. Recent trends and applications of nanoencapsulated bacteriocins against microbes in food quality and safety. Microorganisms 2023, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.P.M.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C.G. Application of an alginate-based edible coating with bacteriocin-producing Lactococcus strains in fresh cheese preservation. LWT 2022, 153, 112486. [Google Scholar] [CrossRef]
- Li, Q.; Liang, W.; Lv, L.; Fang, Z.; Xu, D.; Liao, J.; Liu, Y. Preparation of PCL/lecithin/bacteriocin CAMT6 antimicrobial and antioxidant nanofiber films using emulsion electrospinning: Characteristics and application in chilled salmon preservation. Food Res. Int. 2024, 175, 113747. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, N.; Kaushal, N. Utilization of novel bacteriocin-synthesized silver nanoparticles (AgNPs) for their application in antimicrobial packaging for preservation of tomato fruit. Front. Sustain. Food Syst. 2023, 7, 1072738. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Wirawan, R.E.; Klesse, N.A.; Jack, R.W.; Tagg, J.R. Molecular and genetic characterization of a novel nisin variant produced by Streptococcus uberis. Appl. Environ. Microbiol. 2006, 72, 1148–1156. [Google Scholar] [CrossRef]
- Nes, I.F.; Yoon, S.S.; Diep, D.B. Ribosomally synthesized antimicrobial peptides (bacteriocins) in lactic acid bacteria: A review. Food Sci. Biotechnol. 2007, 16, 675–690. [Google Scholar]
- Angelopoulou, A.; Warda, A.K.; O’Connor, P.M.; Stockdale, S.R.; Shkoporov, A.N.; Field, D.; Draper, L.A.; Stanton, C.; Hill, C.; Ross, R.P. Diverse bacteriocins produced by strains from the human milk microbiota. Front. Microbiol. 2020, 11, 788. [Google Scholar] [CrossRef]
- Martin-Visscher, L.A.; Gong, X.; Duszyk, M.; Vederas, J.C. The three-dimensional structure of carnocyclin A reveals that many circular bacteriocins share a common structural motif. J. Biol. Chem. 2009, 284, 28674–28681. [Google Scholar] [CrossRef]
- Jiménez, M.A.; Barrachi-Saccilotto, A.C.; Valdivia, E.; Maqueda, M.; Rico, M. Design, NMR characterization and activity of a 21-residue peptide fragment of bacteriocin AS-48 containing its putative membrane interacting region. J. Pept. Sci. 2005, 11, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yang, F.; Huang, J.; Yu, H.; Qiao, S. Microcin C7 as a potential antibacterial-immunomodulatory agent in the postantibiotic era: Overview of its bioactivity aspects and applications. Int. J. Mol. Sci. 2024, 25, 7213. [Google Scholar] [CrossRef] [PubMed]
- Wolden, R.; Ovchinnikov, K.V.; Venter, H.J.; Oftedal, T.F.; Diep, D.B.; Cavanagh, J.P. The novel bacteriocin romsacin from Staphylococcus haemolyticus inhibits Gram-positive WHO priority pathogens. Microbiol. Spectr. 2023, 11, e0086923. [Google Scholar] [CrossRef]
- Kawulka, K.; Sprules, T.; McKay, R.T.; Mercier, P.; Diaper, C.M.; Zuber, P.; Vederas, J.C. Structure of subtilosin A, an antimicrobial peptide from Bacillus subtilis with unusual posttranslational modifications linking cysteine sulfurs to α-carbons of phenylalanine and threonine. J. Am. Chem. Soc. 2003, 125, 4726–4727. [Google Scholar] [CrossRef]
- Rea, M.C.; Ross, R.P.; Cotter, P.D.; Hill, C. Classification of bacteriocins from Gram-positive bacteria. In Prokaryotic Antimicrobial Peptides; Drider, D., Rebuffat, S., Eds.; Springer: New York, NY, USA, 2011; pp. 29–53. [Google Scholar]
- Molloy, E.M.; Casjens, S.R.; Cox, C.L.; Maxson, T.; Ethridge, N.A.; Margos, G.; Fingerle, V.; Mitchell, D.A. Identification of the minimal cytolytic unit for streptolysin S and an expansion of the toxin family. BMC Microbiol. 2015, 15, 141. [Google Scholar] [CrossRef]
- Amso, Z.; Bisset, S.W.; Yang, S.H.; Harris, P.W.R.; Wright, T.H.; Navo, C.D.; Patchett, M.L.; Norris, G.E.; Brimble, M.A. Total chemical synthesis of glycocin F and analogues: S-glycosylation confers improved antimicrobial activity. Chem. Sci. 2018, 9, 1686–1691. [Google Scholar] [CrossRef]
- Acedo, J.Z.; Chiorean, S.; Vederas, J.C.; van Belkum, M.J. The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiol. Rev. 2018, 42, 805–828. [Google Scholar] [CrossRef]
- Henderson, J.T.; Chopko, A.L.; van Wassenaar, P.D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch. Biochem. Biophys. 1992, 295, 5–12. [Google Scholar] [CrossRef]
- Aymerich, T.; Holo, H.; Håvarstein, L.S.; Hugas, M.; Garriga, M.; Nes, I.F. Biochemical and genetic characterization of enterocin A from Enterococcus faecium, a new antilisterial bacteriocin in the pediocin family of bacteriocins. Appl. Environ. Microbiol. 1996, 62, 1676–1682. [Google Scholar] [CrossRef]
- Hastings, J.W.; Sailer, M.; Johnson, K.; Roy, K.L.; Vederas, J.C.; Stiles, M.E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J. Bacteriol. 1991, 173, 7491–7500. [Google Scholar] [CrossRef] [PubMed]
- Héchard, Y.; Dérijard, B.; Letellier, F.; Cenatiempo, Y. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J. Gen. Microbiol. 1992, 138, 2725–2731. [Google Scholar] [CrossRef] [PubMed]
- Tichaczek, P.S.; Nissen-Meyer, J.; Nes, I.F.; Vogel, R.F.; Hammes, W.P. Characterization of the bacteriocins curvacin A from Lactobacillus curvatus LTH1174 and sakacin P from L. sake LTH673. Syst. Appl. Microbiol. 1992, 15, 460–468. [Google Scholar] [CrossRef]
- Heng, N.C.K.; Wescombe, P.A.; Burton, J.P.; Jack, R.W.; Tagg, J.R. The diversity of bacteriocins in Gram-positive bacteria. In Bacteriocins; Riley, M.A., Chavan, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nissen-Meyer, J.; Rogne, P.; Oppegard, C.; Haugen, H.S.; Kristiansen, P.E. Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by Gram-positive bacteria. Curr. Pharm. Biotechnol. 2009, 10, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Towle, K.M.; Vederas, J.C. Structural features of many circular and leaderless bacteriocins are similar to those in saposins and saposin-like peptides. Med. Chem. Commun. 2017, 8, 276–285. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Antimicrobial antagonists against food pathogens: A bacteriocin perspective. Curr. Opin. Food Sci. 2015, 2, 51–57. [Google Scholar] [CrossRef]
- Joerger, M.C.; Klaenhammer, T.R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J. Bacteriol. 1986, 167, 439–446. [Google Scholar] [CrossRef]
- Valdés-Stauber, N.; Scherer, S. Isolation and characterization of linocin M18, a bacteriocin produced by Brevibacterium linens. Appl. Environ. Microbiol. 1994, 60, 3809–3814. [Google Scholar] [CrossRef]
- Beukes, M.; Bierbaum, G.; Sahl, H.G.; Hastings, J. Purification and partial characterization of a murein hydrolase, millericin B, produced by Streptococcus milleri NMSCC 061. Appl. Environ. Microbiol. 2000, 66, 23–28. [Google Scholar] [CrossRef]
- Simmonds, R.S.; Simpson, W.J.; Tagg, J.R. Cloning and sequence analysis of zooA, a Streptococcus zooepidemicus gene encoding a bacteriocin-like inhibitory substance having a domain structure similar to that of lysostaphin. Gene 1997, 189, 255–261. [Google Scholar] [CrossRef]
- Bastos, M.D.C.D.F.; Coutinho, B.G.; Coelho, M.L.V. Lysostaphin: A staphylococcal bacteriolysin with potential clinical applications. Pharmaceuticals 2010, 3, 1139–1161. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, A.B.; Al-Hejin, A.M.; Shori, A.B. Bacteriocins as promising antimicrobial peptides, definition, classification, and their potential applications in cheeses. Food Sci. Technol. 2023, 43, e118021. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: Resistance is futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, antimicrobial peptides from bacterial origin: Overview of their biology and their impact against multidrug-resistant bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Ari, M.M.; Ohadi, E.; Talebi, M.; Zadeh, M.H.; Emamie, A.D.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2021, 36, e24093. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Valyshev, A.V. Pore-forming bacteriocins: Structural–functional relationships. Arch. Microbiol. 2019, 201, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Smith, K.; McCaughey, L.; Walker, D. Colicin-like bacteriocins as novel therapeutic agents for the treatment of chronic biofilm-mediated infection. Biochem. Soc. Trans. 2012, 40, 1549–1552. [Google Scholar] [CrossRef]
- Behrens, H.M.; Six, A.; Walker, D.; Kleanthous, C. The therapeutic potential of bacteriocins as protein antibiotics. Emerg. Top. Life Sci. 2017, 1, 65–74. [Google Scholar]
- Le, M.N.; Nguyen, T.H.; Trinh, V.M.; Nguyen, T.P.; Kawada-Matsuo, M.; Kayama, S.; Sugai, M.; Komatsuzawa, H. Comprehensive analysis of bacteriocins produced by the hypermucoviscous Klebsiella pneumoniae species complex. Microbiol. Spectr. 2023, 11, e00863-23. [Google Scholar] [CrossRef]
- Wertz, J.E.; Margaret, A.R. Chimeric nature of two plasmids of Hafnia alvei encoding the bacteriocins alveicins A and B. J. Bacteriol. 2004, 186, 1598–1605. [Google Scholar] [CrossRef]
- Patzer, S.I.; Albrecht, R.; Braun, V.; Zeth, K. Structural and mechanistic studies of pesticin, a bacterial homolog of phage lysozymes. J. Biol. Chem. 2012, 287, 23381–23396. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Kopel, J.; Hamood, A. The in vivo and in vitro assessment of pyocins in treating Pseudomonas aeruginosa infections. Antibiotics 2022, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents. 2017, 49, 1–11. [Google Scholar] [CrossRef]
- Cavera, V.L.; Arthur, T.D.; Kashtanov, D.; Chikindas, M.L. Bacteriocins and their position in the next wave of conventional antibiotics. Int. J. Antimicrob. Agents. 2015, 46, 494–501. [Google Scholar] [CrossRef]
- Kaya, H.İ.; Özel, B.; Şimşek, Ö. A natural way of food preservation: Bacteriocins and their applications. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Dimov, S.; Ivanova, P.; Harizanova, N. Genetics of bacteriocins biosynthesis by lactic acid bacteria. Biotechnol. Biotechnol. Equip. 2005, 19, 4–10. [Google Scholar] [CrossRef]
- Jack, R.W.; Tagg, J.R.; Ray, B. Bacteriocins of Gram-positive bacteria. Microbiol. Rev. 1995, 59, 171–200. [Google Scholar] [CrossRef]
- Mouloud, G.; Daoud, H.; Bassem, J.; Laribi Atef, I.; Hani, B. New bacteriocin from Bacillus clausii strain GM17: Purification, characterization, and biological activity. Appl. Biochem. Biotechnol. 2013, 171, 2186–2200. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Beyond biopreservatives, bacteriocins biotechnological applications: History, current status, and promising potentials. Biocatal. Agric. Biotechnol. 2022, 39, 102248. [Google Scholar] [CrossRef]
- Oscáriz, J.C.; Pisabarro, A.G. Classification and mode of action of membrane-active bacteriocins produced by Gram-positive bacteria. Int. Microbiol. 2001, 4, 13–19. [Google Scholar] [CrossRef]
- Sharma, K.; Kaur, S.; Singh, R.; Kumar, N. Classification and mechanism of bacteriocin induced cell death: A review: Bacteriocin classification and their mode action. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e3733. [Google Scholar] [CrossRef]
- Cascales, E.; Buchanan, S.K.; Duché, D.; Kleanthous, C.; Lloubès, R.; Postle, K.; Riley, M.; Slatin, S.; Cavard, D. Colicin Biology. Microbiol. Mol. Biol. Rev. 2007, 71, 158–229. [Google Scholar] [CrossRef] [PubMed]
- Lagos, R.; Tello, M.; Mercado, G.; García, V.; Monasterio, O. Antibacterial and antitumorigenic properties of microcin E492, a pore-forming bacteriocin. Curr. Pharm. Biotechnol. 2009, 10, 74–85. [Google Scholar] [CrossRef] [PubMed]
- de Zamaroczy, M.; Mora, L. Hijacking cellular functions for processing and delivery of colicins E3 and D into the cytoplasm. Biochem. Soc. Trans. 2012, 40, 1486–1491. [Google Scholar] [CrossRef]
- Kim, Y.C.; Tarr, A.W.; Penfold, C.N. Colicin import into E. coli cells: A model system for insights into the import mechanisms of bacteriocins. Biochim. Biophys. Acta Mol. Cell Res. 2014, 1843, 1717–1731. [Google Scholar]
- Duquesne, S.; Destoumieux-Garzón, D.; Peduzzi, J.; Rebuffat, S. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat. Prod. Rep. 2007, 24, 708–734. [Google Scholar] [CrossRef]
- Masaki, H.; Ogawa, T. The modes of action of colicins E5 and D, and related cytotoxic tRNases. Biochimie 2002, 84, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.A.; Vincent, P.A.; Farías, R.N.; Salomón, R.A. YojI of Escherichia coli functions as a microcin J25 efflux pump. J. Bacteriol. 2005, 187, 3465–3470. [Google Scholar] [CrossRef]
- Favaro, L.; Penna, A.L.B.; Todorov, S.D. Bacteriocinogenic LAB from cheeses—Application in biopreservation? Trends Food Sci. Technol. 2015, 41, 37–48. [Google Scholar] [CrossRef]
- Hassan, H.; St-Gelais, D.; Gomaa, A.; Fliss, I. Impact of nisin and nisin-producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and bacterial ecosystem of cheese matrices. Foods 2021, 10, 898. [Google Scholar]
- Todorov, S.D.; Popov, I.; Weeks, R.; Chikindas, M.L. Use of bacteriocins and bacteriocinogenic beneficial organisms in food products: Benefits, challenges, concerns. Foods 2022, 11, 3145. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Hashempour-Baltork, F.; Alizadeh-Sani, M.; Maleki, M.; Azizi-Lalabadi, M.; Khosravi-Darani, K. Inhibition of Clostridium botulinum and its toxins by probiotic bacteria and their metabolites: An updated review. Qual. Assur. Saf. Crops Foods 2020, 12, 59–68. [Google Scholar] [CrossRef]
- Camargo, A.C.; Todorov, S.D.; Chihib, N.E.; Drider, D.; Nero, L.A. Lactic acid bacteria (LAB) and their bacteriocins as alternative biotechnological tools to control Listeria monocytogenes biofilms in food processing facilities. Mol. Biotechnol. 2018, 60, 712–726. [Google Scholar] [CrossRef]
- Morgan, S.M.; Galvin, M.; Ross, R.P.; Hill, C. Evaluation of a spray-dried lacticin 3147 powder for the control of Listeria monocytogenes and Bacillus cereus in a range of food systems. Lett. Appl. Microbiol. 2001, 33, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.I.; Simsek, O. Characterization of pathogen-specific bacteriocins from lactic acid bacteria and their application within a cocktail against pathogens in milk. LWT 2019, 115, 108464. [Google Scholar] [CrossRef]
- Muñoz, A.; Ananou, S.; Gálvez, A.; Martínez-Bueno, M.; Rodríguez, A.; Maqueda, M.; Valdivia, E. Inhibition of Staphylococcus aureus in dairy products by enterocin AS-48 produced in situ and ex situ: Bactericidal synergism with heat. Int. Dairy J. 2007, 17, 760–769. [Google Scholar] [CrossRef]
- Rodriguez, E.; Arques, J.L.; Nunez, M.; Gaya, P.; Medina, M. Combined effect of high-pressure treatments and bacteriocin-producing lactic acid bacteria on inactivation of Escherichia coli O157:H7 in raw-milk cheese. Appl. Environ. Microbiol. 2005, 71, 3399–3404. [Google Scholar] [CrossRef]
- McNamee, C.; Noci, F.; Cronin, D.A.; Lyng, J.G.; Morgan, D.J.; Scannell, A.G.M. PEF based hurdle strategy to control Pichia fermentans, Listeria innocua and Escherichia coli K12 in orange juice. Int. J. Food Microbiol. 2010, 138, 13–18. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, Y.; Liu, H.; Jin, J.; Duan, H.; Zhang, H. Effects of two application methods of plantaricin BM-1 on control of Listeria monocytogenes and background spoilage bacteria in sliced vacuum-packaged cooked ham stored at 48 °C. J. Food Prot. 2015, 78, 1835–1841. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; Van Tassell, M.L.; Miller, M.J. Antimicrobial behavior of phage endolysin PlyP100 and its synergy with nisin to control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2018, 72, 128–134. [Google Scholar] [CrossRef]
- Norhana, M.N.W.; Poole, S.E.; Deeth, H.C.; Dykes, G.A. Effects of nisin, EDTA and salts of organic acids on Listeria monocytogenes, Salmonella and native microflora on fresh vacuum packaged shrimps stored at 4 °C. Food Microbiol. 2012, 31, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M.; Toselli, M.; Sabia, C.; Messi, P.; de Niederhäusern, S.; Bondi, M.; Iseppi, R. Effectiveness of polymeric coated films containing bacteriocin-producer living bacteria for Listeria monocytogenes control under simulated cold chain break. Food Microbiol. 2018, 76, 173–179. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Souza, B.W.S.; Carmo Avides, M.D.; Vicente, A.A. Shelf life extension of ricotta cheese using coatings of galactomannans from nonconventional sources incorporating nisin against Listeria monocytogenes. J. Agric. Food Chem. 2010, 58, 1884–1891. [Google Scholar] [CrossRef]
- Guitián, M.V.; Ibarguren, C.; Soria, M.C.; Hovanyecz, P.; Banchio, C.; Audisio, M.C. Anti-Listeria monocytogenes effect of bacteriocin-incorporated agar edible coatings applied on cheese. Int. Dairy J. 2019, 97, 92–98. [Google Scholar] [CrossRef]
- Contessa, C.R.; da Rosa, G.S.; Moraes, C.C. New active packaging based on biopolymeric mixture added with bacteriocin as active compound. Int. J. Mol. Sci. 2021, 22, 10628. [Google Scholar] [CrossRef]
- La Storia, A.; Di Giuseppe, F.A.; Volpe, S.; Oliviero, V.; Villani, F.; Torrieri, E. Physical properties and antimicrobial activity of bioactive film based on whey protein and Lactobacillus curvatus 54M16 producer of bacteriocins. Food Hydrocoll. 2020, 108, 105959. [Google Scholar] [CrossRef]
- Rashid, M.; Sharma, S.; Kaur, A.; Kaur, A.; Kaur, S. Biopreservative efficacy of Enterococcus faecium-immobilised film and its enterocin against Salmonella enterica. AMB Express 2023, 13, 11. [Google Scholar] [CrossRef]
- Chandrakasan, G.; Rodríguez-Hernández, A.I.; del Rocío López-Cuellar, M.; Palma-Rodríguez, H.M.; Chavarría-Hernández, N. Bacteriocin encapsulation for food and pharmaceutical applications: Advances in the past 20 years. Biotechnol. Lett. 2019, 41, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Zohri, M.; Alavidjeh, M.S.; Mirdamadi, S.S.; Behmadi, H.; Nasr, S.M.H.; Gonbaki, S.E.; Ardestani, M.S.; Arabzadeh, A.J. Nisin loaded chitosan/alginate nanoparticles: A hopeful hybrid biopreservative. J. Food Saf. 2013, 33, 40–49. [Google Scholar] [CrossRef]
- Pandit, R.; Rai, M.; Santos, C.A. Enhanced antimicrobial activity of the food-protecting nisin peptide by bioconjugation with silver nanoparticles. Environ. Chem. Lett. 2017, 15, 443–452. [Google Scholar] [CrossRef]
- Ahire, J.J.; Neveling, D.P.; Dicks, L.M.T. Co-spinning of silver nanoparticles with nisin increases the antimicrobial spectrum of PDLLA: PEO nanofibers. Curr. Microbiol. 2015, 71, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K-s. Potential novel food-related and biomedical applications of nanomaterials combined with bacteriocins. Pharmaceutics 2021, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.K.; Sapra, M.; Chopra, A.; Sharma, R.; Patil, S.D.; Malik, R.K.; Pathania, R.; Navani, N.K. Interaction of bacteriocin-capped silver nanoparticles with food pathogens and their antibacterial effect. Int. J. Green Nanotechnol. 2012, 4, 93–110. [Google Scholar] [CrossRef]
- Correia, L.F.; da Silva Pinho, G.; da Cruz Neves, T.J.; de Oliveira Vieira, K.C.; Maddela, N.R.; Prasad, R.; Winkelstroter, L.K. Nanotechnology innovation combined with bacteriocins as emerging strategy for the development of active and intelligent food packaging. Sustain. Chem. Pharm. 2024, 39, 101551. [Google Scholar] [CrossRef]
- de Mello, M.B.; da Silva Malheiros, P.; Brandelli, A.; Pesce da Silveira, N.; Jantzen, M.M.; de Souza da Motta, A. Characterization and antilisterial effect of phosphatidylcholine nanovesicles containing the antimicrobial peptide pediocin. Probiotics Antimicrob. Proteins 2013, 5, 43–50. [Google Scholar] [CrossRef]
- Malheiros, P.S.; Cuccovia, I.M.; Franco, B.D.G.M. Inhibition of Listeria monocytogenes in vitro and in goat milk by liposomal nanovesicles containing bacteriocins produced by Lactobacillus sakei subsp. sakei 2a. Food Control 2016, 63, 158–164. [Google Scholar] [CrossRef]
- Kokare, C.R.; Chakraborty, S.; Khopade, A.N.; Mahadik, K.R. Biofilm: Importance and applications. Indian J. Biotechnol. 2009, 8, 159–168. [Google Scholar]
- Tilahun, A.; Haddis, S.; Teshale, A.; Hadush, T. Review on biofilm and microbial adhesion. Int. J. Microbiol. Res. 2016, 7, 63–73. [Google Scholar]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Fighting biofilms with lantibiotics and other groups of bacteriocins. NPJ Biofilms Microbiomes 2018, 4, 9. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, C.; Bao, X.; Chen, F.; Guo, X. Strategies for controlling biofilm formation in the food industry. Grain Oil Sci. Technol. 2022, 5, 179–186. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Effect of small chain N acyl homoserine lactone quorum sensing signals on biofilms of food-borne pathogens. J. Food. Sci. Technol. 2016, 53, 3609–3614. [Google Scholar]
- da Silva, E.P.; De Martinis, E.C.P. Current knowledge and perspectives on biofilm formation: The case of Listeria monocytogenes. Appl. Microbiol. Biotechnol. 2013, 97, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Silva-de-Jesus, A.C.; Ferrari, R.G.; Panzenhagen, P.; Conte-Junior, C.A. Staphylococcus aureus biofilm: The role in disseminating antimicrobial resistance over the meat chain. Microbiology 2022, 168, 001245. [Google Scholar] [CrossRef]
- Li, X.; Gu, N.; Huang, T.Y.; Zhong, F.; Peng, G. Pseudomonas aeruginosa: A typical biofilm forming pathogen and an emerging but underestimated pathogen in food processing. Front. Microbiol. 2023, 13, 1114199. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa biofilm: A review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef]
- Kukhtyn, M.; Berhilevych, O.; Kravcheniuk, K.; Shynkaruk, O.; Horyuk, Y. Formation of biofilms on dairy equipment and the influence of disinfectants on them. East.-Eur. J. Enterp. Technol. 2017, 5, 26–33. [Google Scholar] [CrossRef][Green Version]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540. [Google Scholar] [CrossRef]
- Kumari, S.; Sarkar, P.K. Bacillus cereus hazard and control in industrial dairy processing environment. Food Control 2016, 69, 20–29. [Google Scholar] [CrossRef]
- Pang, X.; Song, X.; Chen, M.; Tian, S.; Lu, Z.; Sun, J.; Li, X.; Lu, Y.; Yuk, H.G. Combating biofilms of foodborne pathogens with bacteriocins by lactic acid bacteria in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1657–1676. [Google Scholar] [CrossRef]
- Zgheib, H.; Drider, D.; Belguesmia, Y. Broadening and enhancing bacteriocins activities by association with bioactive substances. Int. J. Environ. Res. Public Health 2020, 17, 7835. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Field, D.; Pérez-Ibarreche, M.; Warda, A.K.; Hill, C.; Ross, R.P. Vancomycin and nisin A are effective against biofilms of multi-drug resistant Staphylococcus aureus isolates from human milk. PLoS ONE 2020, 15, e0233284. [Google Scholar] [CrossRef] [PubMed]
- Al Atya, A.K.; Belguesmia, Y.; Chataigne, G.; Ravallec, R.; Vachée, A.; Szunerits, S.; Boukherroub, R.; Drider, D. Anti-MRSA activities of enterocins DD28 and DD93 and evidences on their role in the inhibition of biofilm formation. Front. Microbiol. 2016, 7, 817. [Google Scholar] [CrossRef]
- Sharma, P.; Yadav, M. Enhancing antibacterial properties of bacteriocins using combination therapy. J. Appl. Biol. Biotechnol. 2023, 11, 232–243. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, H.; Lee, D.; Lee, H.; Kim, W.J. Antibiofilm activity of crude bacteriocin JM01 produced by Pediococcus acidilactici against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Food Sci. Technol. 2023, 58, 2580–2589. [Google Scholar] [CrossRef]
- Diep, D.B.; Skaugen, M.; Salehian, Z.; Holo, H.; Nes, I.F. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. USA 2007, 104, 2384–2389. [Google Scholar] [CrossRef]
- Kjos, M.; Borrero, J.; Opsata, M.; Birri, D.J.; Holo, H.; Cintas, L.M.; Snipen, L.; Hernández, P.E.; Nes, I.F.; Diep, D.B. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology 2011, 157, 3256–3267. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of antimicrobial peptides against bacterial biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed]
- Ghapanvari, P.; Taheri, M.; Jalilian, F.A.; Dehbashi, S.; Dezfuli, A.A.Z.; Arabestani, M.R. The effect of nisin on the biofilm production, antimicrobial susceptibility and biofilm formation of Staphylococcus aureus and Pseudomonas aeruginosa. Eur. J. Med. Res. 2022, 27, 173. [Google Scholar] [CrossRef]
- Kranjec, C.; Ovchinnikov, K.V.; Grønseth, T.; Ebineshan, K.; Srikantam, A.; Diep, D.B. A bacteriocin-based antimicrobial formulation to effectively disrupt the cell viability of methicillin-resistant Staphylococcus aureus (MRSA) biofilms. NPJ Biofilms Microbiomes 2020, 6, 58. [Google Scholar] [CrossRef]
- Algburi, A.; Zehm, S.; Netrebov, V.; Bren, A.B.; Chistyakov, V.; Chikindas, M.L. Subtilosin prevents biofilm formation by inhibiting bacterial quorum sensing. Probiotics Antimicrob. Proteins 2017, 9, 81–90. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Ding, X.; Wang, J.; Zhan, X. Inhibitory effects of reuterin on biofilm formation, quorum sensing and virulence genes of Clostridium perfringens. LWT 2022, 162, 113421. [Google Scholar] [CrossRef]
- Melian, C.; Segli, F.; Gonzalez, R.; Vignolo, G.; Castellano, P. Lactocin AL705 as quorum sensing inhibitor to control Listeria monocytogenes biofilm formation. J. Appl. Microbiol. 2019, 127, 911–920. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Pimentel-Filho, N.; de Freitas Martins, M.C.; Nogueira, G.B.; Mantovani, H.C.; Vanetti, M.C.D. Bovicin HC5 and nisin reduce Staphylococcus aureus adhesion to polystyrene and change the hydrophobicity profile and Gibbs free energy of adhesion. Int. J. Food Microbiol. 2014, 190, 1–8. [Google Scholar] [CrossRef]
- Okuda, K.; Zendo, T.; Sugimoto, S.; Iwase, T.; Tajima, A.; Yamada, S.; Sonomoto, K.; Mizunoe, Y. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2013, 57, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, A.; Madi-Moussa, D.; Coucheney, F.; Drider, D. Current knowledge of the mode of action and immunity mechanisms of LAB-bacteriocins. Microorganisms 2021, 9, 2107. [Google Scholar] [CrossRef]
- Qiao, Z.; Chen, J.; Zhou, Q.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Purification, characterization, and mode of action of a novel bacteriocin BM173 from Lactobacillus crustorum MN047 and its effect on biofilm formation of Escherichia coli and Staphylococcus aureus. J. Dairy Sci. 2021, 104, 1474–1483. [Google Scholar] [CrossRef]
- Perez-Ibarreche, M.; Castellano, P.; Leclercq, A.; Vignolo, G. Control of Listeria monocytogenes biofilms on industrial surfaces by the bacteriocin-producing Lactobacillus sakei CRL1862. FEMS Microbiol. Lett. 2016, 363, fnw118. [Google Scholar] [CrossRef]
- Kim, N.N.; Kim, W.J.; Kang, S.S. Anti-biofilm effect of crude bacteriocin derived from Lactobacillus brevis DF01 on Escherichia coli and Salmonella Typhimurium. Food Control 2019, 98, 274–280. [Google Scholar] [CrossRef]
- Krishna, A.R.; Jayalekshmi, S.K.; Pandipilly Antony, T.M.; Ramasamy, S. Effect of bacteriocin (ALC102) of Enterococcus faecium GRD AA on biofilm forming Listeria monocytogenes MTCC 657. J. Pure Appl. Microbiol. 2022, 16, 481–493. [Google Scholar] [CrossRef]
- Howlader, M.; Das, S.C.; Gupta, S.D.; Barman, D.N.; Islam, M.M.; Mia, E.; Salahuddin; Hossain, M. An insight into bacteriocins for combating microbial infections and ensuring food safety. Int. J. Pept. Res. Ther. 2025, 31, 31. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Davies, S.C.; Marcelin, J.R. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet 2020, 396, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Umu, Ö.C.; Bäuerl, C.; Oostindjer, M.; Pope, P.B.; Hernández, P.E.; Pérez-Martínez, G.; Diep, D.B. The potential of class II bacteriocins to modify gut microbiota to improve host health. PLoS ONE 2016, 11, e0164036. [Google Scholar] [CrossRef]
- Anjana; Tiwari, S.K. Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 851140. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Fact. 2014, 13, S3. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Rea, M.C.; Dobson, A.; O’Sullivan, O.; Crispie, F.; Fouhy, F.; Cotter, P.D.; Shanahan, F.; Kiely, B.; Hill, C.; Ross, R.P. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc. Natl. Acad. Sci. USA 2011, 108, 4639–4644. [Google Scholar] [CrossRef]
- Lopez, F.E.; Vincent, P.A.; Zenoff, A.M.; Salomón, R.A.; Farías, R.N. Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J. Antimicrob. Chemother. 2007, 59, 676–680. [Google Scholar] [CrossRef]
- Stern, N.J.; Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Pokhilenko, V.D.; Levchuk, V.P.; Svetoch, O.E.; Seal, B.S. Isolation of a Lactobacillus salivarius strain and purification of its bacteriocin, which is inhibitory to Campylobacter jejuni in the chicken gastrointestinal system. Antimicrob. Agents Chemother. 2006, 50, 3111–3116. [Google Scholar] [CrossRef]
- Van Kuijk, S.; Noll, K.S.; Chikindas, M.L. The species-specific mode of action of the antimicrobial peptide subtilosin against Listeria monocytogenes Scott A. Lett. Appl. Microbiol. 2012, 54, 52–58. [Google Scholar] [CrossRef]
- Dabour, N.; Zihler, A.; Kheadr, E.; Lacroix, C.; Fliss, I. In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int. J. Food Microbiol. 2009, 133, 225–233. [Google Scholar] [CrossRef]
- Corr, S.C.; Li, Y.; Riedel, C.U.; O’Toole, P.W.; Hill, C.; Gahan, C.G. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl. Acad. Sci. USA 2007, 104, 7617–7621. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.S.; Hur, J.W.; Yu, M.A.; Cheigh, C.I.; Kim, K.N.; Hwang, J.K.; Pyun, Y.R. Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J. Food Prot. 2003, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.P.; Wei, J.; Greenberg, K.; Novick, R. Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J. Antimicrob. Chemother. 1998, 42, 277–278. [Google Scholar] [CrossRef][Green Version]
- De Kwaadsteniet, M.; Doeschate, K.T.; Dicks, L.M.T. Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett. Appl. Microbiol. 2009, 48, 65–70. [Google Scholar] [CrossRef]
- Sosunov, V.; Mischenko, V.; Eruslanov, B.; Svetoch, E.; Shakina, Y.; Stern, N.; Majorov, K.; Sorokoumova, G.; Selishcheva, A.; Apt, A. Antimycobacterial activity of bacteriocins and their complexes with liposomes. J. Antimicrob. Chemother. 2007, 59, 919–925. [Google Scholar] [CrossRef]
- Tong, Z.; Dong, L.; Zhou, L.; Tao, R.; Ni, L. Nisin inhibits dental caries-associated microorganism in vitro. Peptides 2010, 31, 2003–2008. [Google Scholar] [CrossRef]
- Shin, J.M.; Ateia, I.; Paulus, J.R.; Liu, H.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front. Microbiol. 2015, 6, 617. [Google Scholar] [CrossRef] [PubMed]
- Sutyak Noll, K.; Prichard, M.N.; Khaykin, A.; Sinko, P.J.; Chikindas, M.L. The natural antimicrobial peptide subtilosin acts synergistically with glycerol monolaurate, lauric arginate, and ε-poly-L-lysine against bacterial vaginosis. Antimicrob. Agents Chemother. 2012, 56, 1756–1761. [Google Scholar] [CrossRef]
- Oh, S.; Kim, S.H.; Ko, Y.; Sim, J.H.; Kim, K.S.; Lee, S.H.; Park, S.; Kim, Y.J. Effect of bacteriocin produced by Lactococcus sp. HY 449 on skin-inflammatory bacteria. Food Chem. Toxicol. 2006, 44, 552–559. [Google Scholar] [CrossRef]
- Kang, B.S.; Seo, J.G.; Lee, G.S.; Kim, J.H.; Kim, S.Y.; Han, Y.W.; Kang, H.; Kim, H.O.; Rhee, J.H.; Chung, M.J.; et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J. Microbiol. 2009, 47, 101–109. [Google Scholar] [CrossRef]
- Hanchi, H.; Hammami, R.; Gingras, H.; Kourda, R.; Bergeron, M.G.; Ben Hamida, J.; Ouellette, M.; Fliss, I. Inhibition of MRSA and of Clostridium difficile by Durancin 61A: Synergy with bacteriocins and antibiotics. Future Microbiol. 2017, 12, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Piper, C.; Draper, L.A.; Cotter, P.D.; Ross, R.P.; Hill, C. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J. Antimicrob. Chemother. 2009, 64, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Teng, K.; Liu, Y.; Cao, Y.; Wang, T.; Ma, C.; Zhang, J.; Zhong, J. Bacteriocins: Potential for human health. Oxid. Med. Cell. Longev. 2021, 2021, 5518825. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; de Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.; Tomé, E.; Dousset, X.; Destro, M.T.; Dicks, L.M.T.; de Melo Franco, B.D.G.; Vaz-Velho, M.; Drider, D. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010, 27, 869–879. [Google Scholar] [CrossRef]
- Torres, N.I.; Noll, K.S.; Xu, S.; Li, J.; Huang, Q.; Sinko, P.J.; Wachsman, M.B.; Chikindas, M.L. Safety, formulation and in vitro antiviral activity of the antimicrobial peptide subtilosin against herpes simplex virus type 1. Probiot. Antimicrob. Proteins. 2013, 5, 26–35. [Google Scholar] [CrossRef]
- Quintana, V.M.; Torres, N.I.; Wachsman, M.B.; Sinko, P.J.; Castilla, V.; Chikindas, M. Antiherpes simplex virus type 2 activity of the antimicrobial peptide subtilosin. J. Appl. Microbiol. 2014, 117, 1253–1259. [Google Scholar] [CrossRef]
- Ermolenko, E.I.; Desheva, Y.A.; Kolobov, A.A.; Kotyleva, M.P.; Sychev, I.A.; Suvorov, A.N. Anti–influenza activity of enterocin B in vitro and protective effect of bacteriocinogenic enterococcal probiotic strain on influenza infection in mouse model. Probiotics Antimicrob. Proteins 2019, 11, 705–712. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; de Carvalho, O.V.; de Paiva, J.C.; Todorov, S.D.; Júnior, A.S.; Nero, L.A. Inhibition of herpes simplex virus 1 (HSV-1) and poliovirus (PV-1) by bacteriocins from Lactococcus lactis subsp. lactis and Enterococcus durans strains isolated from goat milk. Int. J. Antimicrob. Agents 2018, 51, 33–37. [Google Scholar] [CrossRef]
- Lange-Starke, A.; Petereit, A.; Truyen, U.; Braun, P.G.; Fehlhaber, K.; Albert, T. Antiviral potential of selected starter cultures, bacteriocins and D, L-lactic acid. Food Environ. Virol. 2014, 6, 42–47. [Google Scholar] [CrossRef]
- Badani, H.; Garry, R.F.; Wimley, W.C. Peptide entry inhibitors of enveloped viruses: The importance of interfacial hydrophobicity. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2180–2197. [Google Scholar] [CrossRef]
- Goh, K.S.; Ng, Z.J.; Halim, M.; Oslan, S.N.; Oslan, S.N.H.; Tan, J.S. A comprehensive review on the anticancer potential of bacteriocin: Preclinical and clinical studies. Int. J. Pept. Res. Ther. 2022, 28, 75. [Google Scholar] [CrossRef]
- Baindara, P.; Korpole, S.; Grover, V. Bacteriocins: Perspective for the development of novel anticancer drugs. Appl. Microbiol. Biotechnol. 2018, 102, 10393–10408. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC 1. Cancer Med. 2012, 1, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, P.; Hayami, T.; Matte, B.; Liu, Y.; Danciu, T.; Ramamoorthy, A.; Worden, F.; Kapila, S.; Kapila, Y. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS ONE 2015, 10, e0131008. [Google Scholar] [CrossRef]
- Ahmadi, S.; Ghollasi, M.; Hosseini, H.M. The apoptotic impact of nisin as a potent bacteriocin on the colon cancer cells. Microb. Pathog. 2017, 111, 193–197. [Google Scholar] [CrossRef]
- Villarante, K.I.; Elegado, F.B.; Iwatani, S.; Zendo, T.; Sonomoto, K.; de Guzman, E.E. Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2-3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells. World J. Microbiol. Biotechnol. 2011, 27, 975–980. [Google Scholar] [CrossRef]
- Varas, M.A.; Muñoz-Montecinos, C.; Kallens, V.; Simon, V.; Allende, M.L.; Marcoleta, A.E.; Lagos, R. Exploiting zebrafish xenografts for testing the in vivo antitumorigenic activity of microcin E492 against human colorectal cancer cells. Front. Microbiol. 2020, 11, 405. [Google Scholar] [CrossRef]
- Arunmanee, W.; Ecoy, G.A.U.; Khine, H.E.E.; Duangkaew, M.; Prompetchara, E.; Chanvorachote, P.; Chaotham, C. Colicin N mediates apoptosis and suppresses integrin-modulated survival in human lung cancer cells. Molecules 2020, 25, 816. [Google Scholar] [CrossRef]
- Cursino, L.; Šmarda, J.; Chartone-Souza, E.; Nascimento, A. Recent updated aspects of colicins of Enterobacteriaceae. Braz. J. Microbiol. 2002, 33, 185–195. [Google Scholar] [CrossRef]
- Punj, V.; Bhattacharyya, S.; Saint-Dic, D.; Vasu, C.; Cunningham, E.A.; Graves, J.; Yamada, T.; Constantinou, A.I.; Christov, K.; White, B.; et al. Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene 2004, 23, 2367–2378. [Google Scholar] [CrossRef]
- Niamah, A.K.; Al-Sahlany, S.T.G.; Verma, D.K.; Shukla, R.M.; Patel, A.R.; Tripathy, S.; Singh, S.; Baranwal, D.; Singh, A.K.; Utama, G.L.; et al. Emerging lactic acid bacteria bacteriocins as anti-cancer and anti-tumor agents for human health. Heliyon 2024, 10, e37054. [Google Scholar] [CrossRef] [PubMed]
- Musatti, A.; Cavicchioli, D.; Mapelli, C.; Bertoni, D.; Hogenboom, J.A.; Pellegrino, L.; Rollini, M. From cheese whey permeate to sakacin A: A circular economy approach for the food-grade biotechnological production of an Anti-Listeria bacteriocin. Biomolecules 2020, 10, 597. [Google Scholar] [CrossRef] [PubMed]
- Isaac, S.L.; Mohd Hashim, A.; Faizal Wong, F.W.; Mohamed Akbar, M.A.; Wan Ahmad Kamil, W.N.I. A review on bacteriocin extraction techniques from lactic acid bacteria. Probiotics Antimicrob. Proteins, 2024; online ahead of print. [Google Scholar] [CrossRef]
- Inglis, R.F.; Scanlan, P.; Buckling, A. Iron availability shapes the evolution of bacteriocin resistance in Pseudomonas aeruginosa. ISME J. 2016, 10, 2060–2066. [Google Scholar] [CrossRef]
- Guryanova, S.V. Immunomodulation, Bioavailability and Safety of Bacteriocins. Life 2023, 13, 1521. [Google Scholar] [CrossRef]
- Thanjavur, N.; Sangubotla, R.; Lakshmi, B.A.; Rayi, R.; Mekala, C.D.; Reddy, A.S.; Viswanath, B. Evaluating the antimicrobial and apoptogenic properties of bacteriocin (nisin) produced by Lactococcus lactis. Process Biochem. 2022, 122, 76–86. [Google Scholar] [CrossRef]
- Cox, C.R.; Coburn, P.S.; Gilmore, M.S. Enterococcal cytolysin: A novel two-component peptide system that serves as a bacterial defense against eukaryotic and prokaryotic cells. Curr. Protein Pept. Sci. 2005, 6, 77–84. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; Omar, N.B.; Lucas, R. Food applications and regulation. In Prokaryotic Antimicrobial Peptides; Drider, D., Rebuffat, S., Eds.; Springer: New York, NY, USA, 2011; pp. 353–390. [Google Scholar]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a new generation of antimicrobials: Toxicity aspects and regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]

| Biofilm-Forming Isolates | Common Locations in Food Industry | References |
|---|---|---|
| L. monocytogenes | Stainless steel surfaces, food processing equipment | [105] |
| S. aureus | Dairy equipment, meat processing plants, food contact surfaces | [106] |
| P. aeruginosa | Food processing equipment, water systems, fresh produce surfaces | [107,108] |
| E. coli | Food contact surfaces, stainless steel surfaces, dairy processing environments | [109] |
| Salmonella spp. | Food processing equipment, poultry processing environments, food contact surfaces | [110] |
| B. cereus | Dairy processing equipment, stainless steel surfaces | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirgül, F.; Kaya, H.İ.; Ucar, R.A.; Mitaf, N.A.; Şimşek, Ö. Expanding Layers of Bacteriocin Applications: From Food Preservation to Human Health Interventions. Fermentation 2025, 11, 142. https://doi.org/10.3390/fermentation11030142
Demirgül F, Kaya Hİ, Ucar RA, Mitaf NA, Şimşek Ö. Expanding Layers of Bacteriocin Applications: From Food Preservation to Human Health Interventions. Fermentation. 2025; 11(3):142. https://doi.org/10.3390/fermentation11030142
Chicago/Turabian StyleDemirgül, Furkan, Halil İbrahim Kaya, Redife Aslıhan Ucar, Naciye Afranur Mitaf, and Ömer Şimşek. 2025. "Expanding Layers of Bacteriocin Applications: From Food Preservation to Human Health Interventions" Fermentation 11, no. 3: 142. https://doi.org/10.3390/fermentation11030142
APA StyleDemirgül, F., Kaya, H. İ., Ucar, R. A., Mitaf, N. A., & Şimşek, Ö. (2025). Expanding Layers of Bacteriocin Applications: From Food Preservation to Human Health Interventions. Fermentation, 11(3), 142. https://doi.org/10.3390/fermentation11030142






