Fermentation of Light and Dark Bean Coffee Brews with SCOBY and Lactic Acid Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fermented Coffee Beverages
2.3. pH, Titratable Acidity (TA%), Organic Acid Determination
2.4. Enumeration of Lactic Acid Bacteria (LAB), Acetic Acid Bacteria (AAB), and Yeast
2.5. Bioactive Compound Analysis
2.5.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.5.2. Antioxidant Activity Assessment
2.5.3. HPLC Analysis of Phenolic Compounds
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
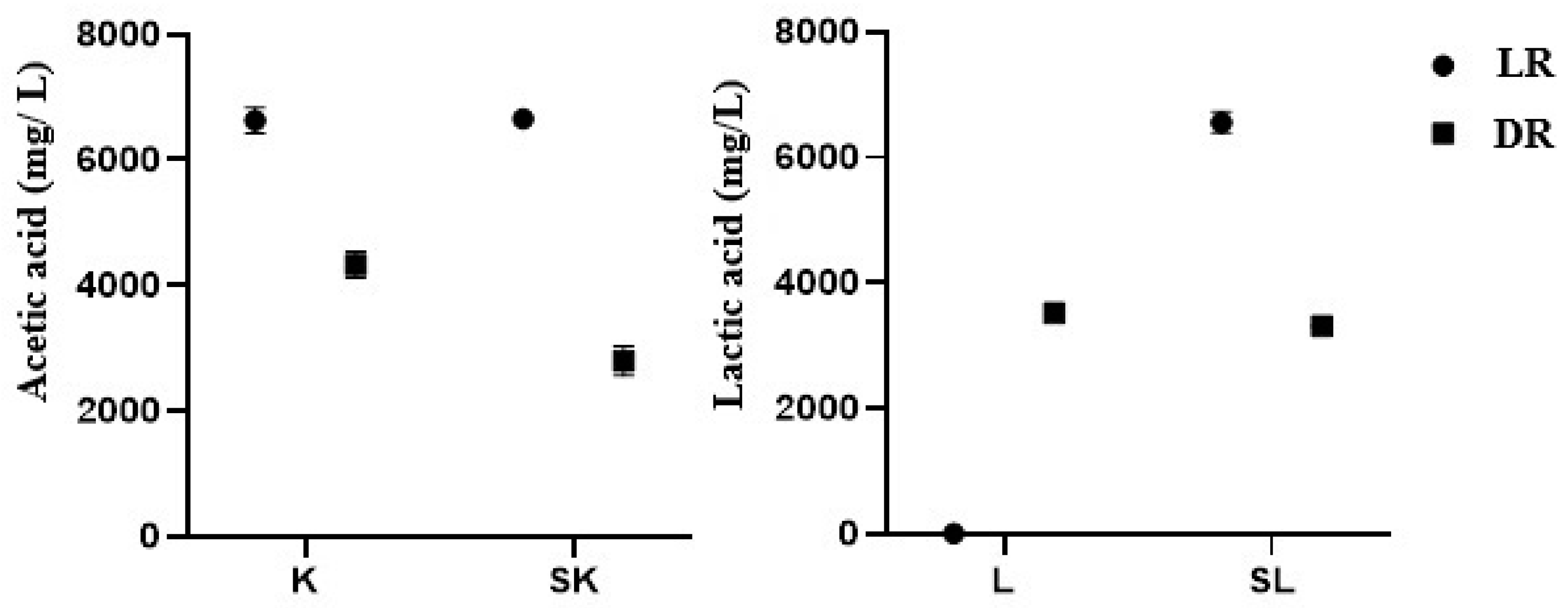
3.1. pH, Titratable Acidity (TA%), Organic Acid Contents of the Coffee Samples
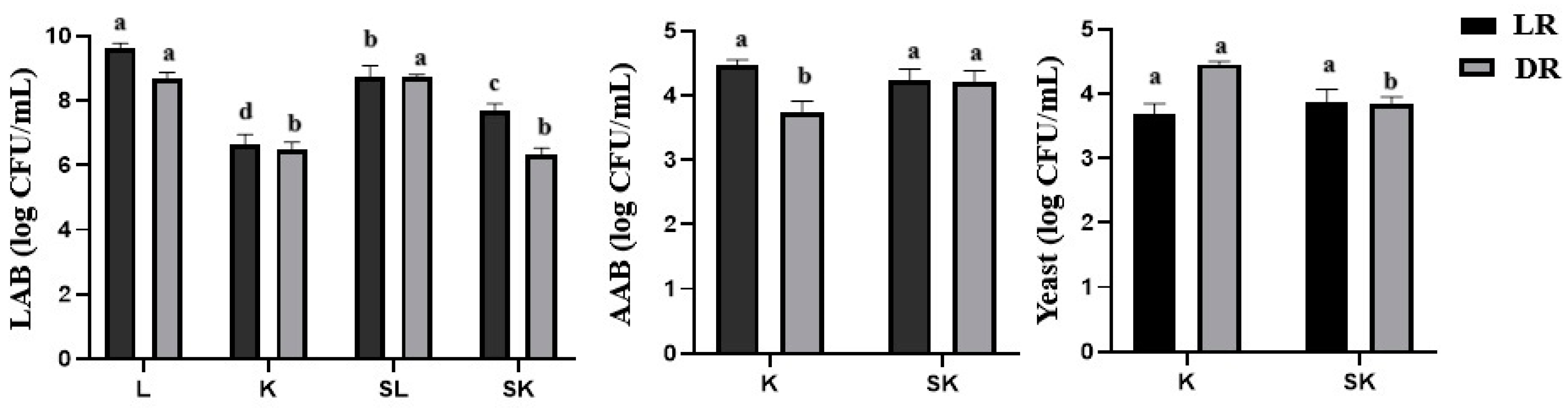
3.2. Cell Counts of Lactic Acid Bacteria (LAB), Acetic Acid Bacteria (AAB), and Yeast
3.3. TPC, TFC, Antioxidant Activities, Caffeine, and Phenolic Constituents of Samples
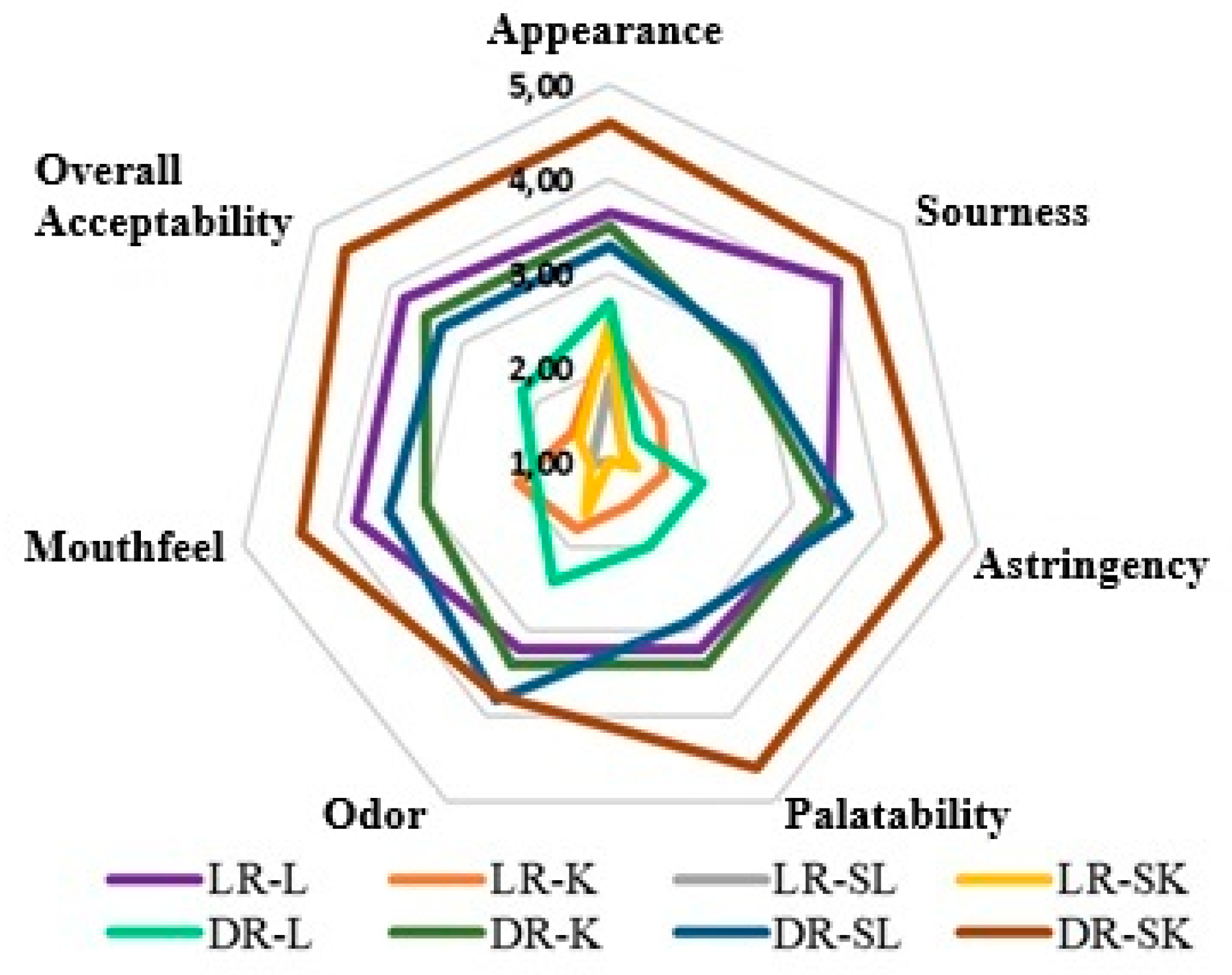
3.4. Sensory Evaluation of Coffee Samples
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and Potential Impact on Health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [PubMed]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of Coffee Roasting on the Incorporation of Phenolic Compounds into Melanoidins and Their Relationship with Antioxidant Activity of the Brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant Activity, Polyphenols, Caffeine and Melanoidins in Soluble Coffee: The Influence of Processing Conditions and Raw Material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of Lactic Acid Bacteria Fermentation on Phenolic Compounds and Antioxidant Activity of Avocado Leaf Extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of Phenolic Compounds by Lactobacillus spp. during Fermentation of Cherry Juice and Broccoli Puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Lei, H. Phenolics Profile, Antioxidant Activity and Flavor Volatiles of Pear Juice: Influence of Lactic Acid Fermentation Using Three Lactobacillus Strains in Monoculture and Binary Mixture. Foods 2022, 11, 11. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Łopusiewicz, Ł.; Kika, J.; Janda-Milczarek, K.; Skonieczna-Żydecka, K. Fermented Tea as a Food with Functional Value—Its Microbiological Profile, Antioxidant Potential and Phytochemical Composition. Foods 2024, 13, 50. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable Management of Coffee Industry By-Products and Value Addition—A Review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioproc. Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Bueno, F.; Chouljenko, A.; Sathivel, S. Development of Coffee Kombucha Containing Lactobacillus rhamnosus and Lactobacillus casei: Gastrointestinal Simulations and DNA Microbial Analysis. LWT 2021, 142, 110980. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Enhancement of the Functional Properties of Coffee Through Fermentation by “Tea Fungus” (Kombucha). J. Food Process Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Pavlović, M.O.; Stajić, M.; Gašić, U.; Duletić-Laušević, S.; Ćilerdžić, J. The Chemical Profiling and Assessment of Antioxidative, Antidiabetic and Antineurodegenerative Potential of Kombucha Fermented Camellia sinensis, Coffea arabica and Ganoderma lucidum Extracts. Food Funct. 2022, 14, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, R.M.; Arici, M. Effect of the Fermentation Temperature on the Degradation of Phytic Acid in Whole-Wheat Sourdough Bread. LWT 2019, 112, 108224. [Google Scholar] [CrossRef]
- Karadag, A.; Bozkurt, F.; Bekiroglu, H.; Sagdic, O. Use of Principal Component Analysis and Cluster Analysis for Differentiation of Traditionally-Manufactured Vinegars Based on Phenolic and Volatile Profiles, and Antioxidant Activity. Pol. J. Food Nutr. Sci. 2020, 70, 347–360. [Google Scholar] [CrossRef]
- Karadag, A.; Ozkan, K.; Sagdic, O. Development of Microencapsulated Grape Juice Powders Using Black ‘Isabel’ Grape Peel Pectin and Application in Jelly Formulation with Enhanced in Vitro Bioaccessibility of Anthocyanins. J. Food Sci. 2024, 89, 2067–2083. [Google Scholar] [CrossRef]
- Kim, D.H.; Chon, J.W.; Kim, H.; Seo, K.H. Development of a Novel Selective Medium for the Isolation and Enumeration of Acetic Acid Bacteria from Various Foods. Food Control 2019, 106, 106717. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Yener, E.; Saroglu, O.; Sagdic, O.; Karadag, A. The Effects of Different Drying Methods on the In Vitro Bioaccessibility of Phenolics, Antioxidant Capacity, and Morphology of European Plums (Prunes domestica L.). ACS Omega 2023, 9, 12711–12724. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Yang, Z.; Qin, C.; Weng, P.; Zhang, X.; Xia, Q.; Wu, Z.; Liu, L.; Xiao, J. In Vitro Evaluation of Digestive Enzyme Inhibition and Antioxidant Effects of Naked Oat Phenolic Acid Compound (OPC). Int. J. Food Sci. Technol. 2020, 55, 2531–2540. [Google Scholar] [CrossRef]
- Zhi, R.; Zhao, L.; Shi, J. Improving the Sensory Quality of Flavored Liquid Milk by Engaging Sensory Analysis and Consumer Preference. J. Dairy Sci. 2016, 99, 5305–5317. [Google Scholar] [CrossRef]
- Fernando dos Santos, D.; Leonarski, E.; Araújo Rossoni, M.; Alves, V.; Tavares dos Passos Francisco, C.; Zanella Pinto, V.; Bergler Bitencourt, T. Honey-Kombucha Beverage with Yerba Maté Infusion: Development, Polyphenols Profile, and Sensory Acceptance. Int. J. Gastron. Food Sci. 2024, 36, 100909. [Google Scholar] [CrossRef]
- Milić, M.D.; Buntić, A.V.; Mihajlovski, K.R.; Ilić, N.V.; Davidović, S.Z.; Dimitrijević-Branković, S.I. The Development of a Combined Enzymatic and Microbial Fermentation as a Viable Technology for the Spent Coffee Ground Full Utilization. Biomass Convers. Biorefin. 2023, 13, 6747–6759. [Google Scholar] [CrossRef]
- Freitas, A.; Sousa, P.; Wurlitzer, N. Alternative Raw Materials in Kombucha Production. Int. J. Gastron. Food Sci. 2022, 30, 100594. [Google Scholar] [CrossRef]
- Ojo, A.O.; de Smidt, O. Microbial Composition, Bioactive Compounds, Potential Benefits and Risks Associated with Kombucha: A Concise Review. Fermentation 2023, 9, 472. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Aung, T.; Eun, J.B. Production and Characterization of a Novel Beverage from Laver (Porphyra Dentata) through Fermentation with Kombucha Consortium. Food Chem. 2021, 350, 129274. [Google Scholar] [CrossRef]
- Malbaša, R.; Lončar, E.; Djurić, M.; Došenović, I. Effect of Sucrose Concentration on the Products of Kombucha Fermentation on Molasses. Food Chem. 2008, 108, 926–932. [Google Scholar] [CrossRef]
- Kallel, L.; Desseaux, V.; Hamdi, M.; Stocker, P.; Ajandouz, E.H. Insights into the Fermentation Biochemistry of Kombucha Teas and Potential Impacts of Kombucha Drinking on Starch Digestion. Food Res. Int. 2012, 49, 226–232. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-Based Analysis of the Bacterial and Fungal Compositions of Multiple Kombucha (Tea Fungus) Samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of Vegetables and Fruits through Lactic Acid Fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oosterveld, A.; Voragen, A.G.J.; Schols, H.A. Effect of Roasting on the Carbohydrate Composition of Coffea arabica Beans. Carbohydr. Polym. 2003, 54, 183–192. [Google Scholar] [CrossRef]
- Grassi, A.; Cristani, C.; Palla, M.; Di Giorgi, R.; Giovannetti, M.; Agnolucci, M. Storage Time and Temperature Affect Microbial Dynamics of Yeasts and Acetic Acid Bacteria in a Kombucha Beverage. Int. J. Food Microbiol. 2022, 382, 109934. [Google Scholar] [CrossRef]
- Kahraman-Ilıkkan, Ö. Microbiome Composition of Kombucha Tea from Türkiye Using High-Throughput Sequencing. J. Food Sci. Technol. 2023, 60, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Jayabalan, R.; Malini, K.; Sathishkumar, M.; Swaminathan, K.; Yun, S.E. Biochemical characteristics of tea fungus produced during kombucha fermentation. Food Sci. Biotechnol. 2010, 19, 843–847. [Google Scholar] [CrossRef]
- Tian, T.; Freeman, S.; Corey, M.; German, J.B.; Barile, D. Effect of Roasting on Oligosaccharide Abundance in Arabica Coffee Beans. J. Agric. Food Chem. 2018, 66, 10067–10076. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C. Antioxidant Properties of Ready-to-Drink Coffee Brews. J. Agric. Food Chem. 2003, 51, 92–96. [Google Scholar] [CrossRef]
- Linhares Sabino, N.; Maziero Fogarin, H.; Lucia Murillo-Franco, S.; Oliviera Bérgamo, M.; Vicente Moreno, L.; Danielle Virginio da Silva, D.; Soleo Funari, C.; Johana Dussán, K. Investigating the Influence of Solvents and Extraction Methods on the Efficacy of Phenolic Compound Recovery from Spent Coffee Grounds. Sep. Purif. Technol. 2025, 362, 131793. [Google Scholar] [CrossRef]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of Spent Coffee Grounds as Food Ingredient in Bakery Products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bekedam, E.K.; Loots, M.J.; Schols, H.A.; Van Boekel, M.A.J.S.; Smit, G. Roasting Effects on Formation Mechanisms of Coffee Brew Melanoidins. J. Agric. Food Chem. 2008, 56, 7138–7145. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha Tea Fermentation: Microbial and Biochemical Dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Sales, A.L.; Iriondo-DeHond, A.; DePaula, J.; Ribeiro, M.; Ferreira, I.M.P.L.V.O.; Miguel, M.A.L.; del Castillo, M.D.; Farah, A. Intracellular Antioxidant and Anti-Inflammatory Effects and Bioactive Profiles of Coffee Cascara and Black Tea Kombucha Beverages. Foods 2023, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, Y.; Sun, Y.; Lu, T.; Cao, Q.; Chen, X. Characterization of Kombucha Prepared from Black Tea and Coffee Leaves: A Comparative Analysis of Physiochemical Properties, Bioactive Components, and Bioactivities. J. Food Sci. 2024, 89, 3430–3444. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Review of Methods for the Reduction of Dietary Content and Toxicity of Acrylamide. J. Agric. Food Chem. 2008, 56, 6113–6140. [Google Scholar]
- Albedwawi, A.S.; Al Sakkaf, R.; Osaili, T.M.; Yusuf, A.; Olaimat, A.; Liu, S.Q.; Palmisano, G.; Shah, N.P.; Ayyash, M.M. Investigating Acrylamide Mitigation by Potential Probiotics Bifidobacterium breve and Lactiplantibacillus plantarum: Optimization, In Vitro Gastrointestinal Conditions, and Mechanism. LWT 2022, 163, 113553. [Google Scholar] [CrossRef]
- Akillioglu, H.G.; Gökmen, V. Mitigation of Acrylamide and Hydroxymethyl Furfural in Instant Coffee by Yeast Fermentation. Food Res. Int. 2014, 61, 252–256. [Google Scholar] [CrossRef]
- Aguirre Santos, E.A.; Schieber, A.; Weber, F. Site-Specific Hydrolysis of Chlorogenic Acids by Selected Lactobacillus Species. Food Res. Int. 2018, 109, 426–432. [Google Scholar] [CrossRef]
- Bel-Rhlid, R.; Thapa, D.; Kraehenbuehl, K.; Hansen, C.E.; Fischer, L. Biotransformation of Caffeoyl Quinic Acids from Green Coffee Extracts by Lactobacillus johnsonii NCC 533. AMB Express 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Barthelmebs, L.; Diviès, C.; Cavin, J.-F.; Barthelmebs, L. Molecular Characterization of the Phenolic Acid Metabolism in the Lactic Acid Bacteria Lactobacillus plantarum. Le Lait 2001, 81, 161–171. [Google Scholar] [CrossRef]
- Kraehenbuehl, K.; Page-Zoerkler, N.; Mauroux, O.; Gartenmann, K.; Blank, I.; Bel-Rhlid, R. Selective Enzymatic Hydrolysis of Chlorogenic Acid Lactones in a Model System and in a Coffee Extract. Application to Reduction of Coffee Bitterness. Food Chem. 2017, 218, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Verde, C.L.; Pacioles, C.T.; Paterson, N.; Chin, J.; Owens, C.P.; Senger, L.W. Hydrolysis of Chlorogenic Acid in Sunflower Flour Increases Consumer Acceptability of Sunflower Flour Cookies by Improving Cookie Color. J. Food Sci. 2023, 88, 3538–3550. [Google Scholar] [CrossRef]
- Mehaya, F.M.; Mohammad, A.A. Thermostability of Bioactive Compounds during Roasting Process of Coffee Beans. Heliyon 2020, 6, e05508. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kitts, D.D. Confirmation That the Maillard Reaction Is the Principle Contributor to the Antioxidant Capacity of Coffee Brews. Food Res. Int. 2011, 44, 2418–2424. [Google Scholar] [CrossRef]
- Campa, C.; Doulbeau, S.; Dussert, S.; Hamon, S.; Noirot, M. Qualitative Relationship between Caffeine and Chlorogenic Acid Contents among Wild Coffea Species. Food Chem. 2005, 93, 135–139. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Y.; Dong, L.; Shi, R.; Wu, Z.; Liu, L.; Zhang, J.; Wu, Z.; Pan, D. Quantitative Determination of Nε-(Carboxymethyl)Lysine in Sterilized Milk by Isotope Dilution UPLC-MS/MS Method without Derivatization and Ion Pair Reagents. Food Chem. 2022, 385, 132697. [Google Scholar] [CrossRef]
- Roldan, M.; Loebner, J.; Degen, J.; Henle, T.; Antequera, T.; Ruiz-Carrascal, J. Advanced Glycation End Products, Physico-Chemical and Sensory Characteristics of Cooked Lamb Loins Affected by Cooking Method and Addition of Flavour Precursors. Food Chem. 2015, 168, 487–495. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Gazzani, G. Free α-Dicarbonyl Compounds in Coffee, Barley Coffee and Soy Sauce and Effects of in Vitro Digestion. Food Chem. 2014, 164, 259–265. [Google Scholar] [CrossRef]


| Day | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 4 | 6 | 8 | 11 | 14 | ||
| LR | L | 5.10 ± 0.01 a | 4.89 ± 0.01 b | 4.68 ± 0.03 c | 4.66 ± 0.04 cd | 4.67 ± 0.02 cd | 4.60 ± 0.01 e | 4.62 ± 0.01 de |
| K | 5.10 ± 0.01 a | 4.20 ± 0.01 b | 4.13 ± 0.02 c | 3.90 ± 0.01 d | 3.79 ± 0.03 e | 3.50 ± 0.02 f | 3.40 ± 0.02 g | |
| SL | 5.19 ± 0.01 a | 4.75 ± 0.01 b | 4.20 ± 0.01 c | 3.95 ± 0.01 d | 3.87 ± 0.03 e | 3.77 ± 0.04 f | 3.66 ± 0.02 g | |
| SK | 5.19 ± 0.02 a | 4.48 ± 0.02 b | 4.39 ± 0.01 c | 4.10 ± 0.02 d | 4.00 ± 0.03 e | 3.58 ± 0.02 f | 3.39 ± 0.03 g | |
| DR | L | 5.70 ± 0.01 a | 5.70 ± 0.02 a | 5.50 ± 0.01 b | 5.38 ± 0.02 c | 5.38 ± 0.02 c | 5.28 ± 0.02 d | 5.25 ± 0.05 d |
| K | 5.70 ± 0.01 a | 4.45 ± 0.05 b | 4.37 ± 0.03 bc | 4.28 ± 0.02 c | 4.12 ± 0.01 d | 3.59 ± 0.05 e | 3.29 ± 0.05 f | |
| SL | 5.86 ± 0.04 a | 5.68 ± 0.02 b | 5.47 ± 0.03 c | 5.28 ± 0.03 d | 5.16 ± 0.04 e | 4.78 ± 0.02 f | 4.47 ± 0.04 g | |
| SK | 5.86 ± 0.04 a | 4.65 ± 0.05 bc | 4.67 ± 0.04 b | 4.58 ± 0.03 c | 4.48 ± 0.02 d | 4.69 ± 0.01 b | 4.63 ± 0.02 bc | |
| Day | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 6 | 8 | 11 | 14 | ||
| LR | L | 0.7 ± 0.05 c | 1.0 ± 0.04 b | 1.0 ± 0.05 b | 0.9 ± 0.05 b | 0.9 ± 0.05 b | 1.2 ± 0.06 a |
| K | 0.8 ± 0.04 f | 2.2 ± 0.05 e | 3.0 ± 0.06 d | 3.1 ± 0.12 c | 4.3 ± 0.10 b | 5.6 ± 0.05 a | |
| SL | 0.7 ± 0.05 d | 2.4 ± 0.10 c | 2.5 ± 0.08 c | 2.5 ± 0.05 c | 3.2 ± 0.06 b | 4.2 ± 0.10 a | |
| SK | 0.9 ± 0.04 f | 2.2 ± 0.05 e | 3.1 ± 0.05 d | 3.4 ± 0.08 c | 6.9 ± 0.10 b | 7.3 ± 0.10 a | |
| DR | L | 0.5 ± 0.05 c | 0.5 ± 0.04 c | 0.5 ± 0.05 c | 0.6 ± 0.04 b | 0.7 ± 0.04 a | 0.7 ± 0.05 a |
| K | 0.8 ± 0.04 d | 1.7 ± 0.06 c | 1.7 ± 0.06 c | 1.6 ± 0.10 c | 3.3 ± 0.05 b | 5.2 ± 0.15 a | |
| SL | 0.6 ± 0.05 d | 0.9 ± 0.05 c | 0.8 ± 0.05 c | 0.8 ± 0.04 c | 1.0 ± 0.06 b | 1.4 ± 0.06 a | |
| SK | 0.7 ± 0.05 b | 1.5 ± 0.05 a | 1.5 ± 0.04 a | 1.5 ± 0.04 a | 1.4 ± 0.10 a | 1.5 ± 0.09 a | |
| TPC mg GAE/mL | TFC mg CE/mL | DPPH mg TE/mL | FRAP mg TE/mL | ||
|---|---|---|---|---|---|
| LR | C | 2.74 ± 0.70 c | 6.78 ± 0.39 a | 2.97 ± 0.06 b | 31.79 ± 4.20 b |
| L | 5.75 ± 0.46 b | 1.74 ± 0.19 b | 3.21 ± 0.06 b | 4.63 ± 1.20 c | |
| K | 5.94 ± 0.69 b | 1.19 ± 0.67 b | 2.98 ± 0.17 b | 4.66 ± 0.25 c | |
| SC | 9.22 ± 1.25 a | 6.43 ± 0.49 a | 4.28 ± 0.12 a | 47.79 ± 8.09 a | |
| SL | 6.21 ± 0.66 b | 1.21 ± 0.04 b | 3.30 ± 0.54 b | 5.27 ± 1.40 c | |
| SK | 5.30 ± 1.07 b | 0.78 ± 0.04 b | 2.62 ± 0.15 b | 3.30 ± 1.24 c | |
| DR | C | 0.52 ± 0.45 b | 1.87 ± 0.14 b | 2.31 ± 0.09 a | 21.01 ± 2.99 b |
| L | 4.31 ± 0.63 a | 1.29 ± 0.31 bc | 2.33 ± 0.49 a | 2.52 ± 1.09 c | |
| K | 3.93 ± 0.77 a | 0.54 ± 0.06 c | 1.98 ± 0.22 a | 3.48 ± 0.54 c | |
| SC | 5.12 ± 1.53 a | 5.90 ± 0.98 a | 2.51 ± 0.43 a | 28.30 ± 4.98 a | |
| SL | 3.83 ± 0.42 a | 1.27 ± 0.16 bc | 2.65 ± 0.24 a | 3.22 ± 0.83 c | |
| SK | 4.34 ± 0.41 a | 0.80 ± 0.09 bc | 2.47 ± 0.22 a | 4.06 ± 1.54 c |
| Caffeine | Ferulic Acid | ρ-Coumaric Acid | Caffeic Acid | 5-CQA | 4-CQA | 3-CQA | Total CQA | ||
|---|---|---|---|---|---|---|---|---|---|
| LR | C | 873.84 ± 0.36 d | 1.66 ± 0.31 d | 18.36 ± 0.15 b | 7.47 ± 0.53 c | 681.02 ± 0.08 d | 446.07 ± 1.17 d | 285.23 ± 17.30 d | 1412.32 ± 18.38 e |
| L | 1033.48 ± 0.57 b | 7.55 ± 0.45 b | 11.74 ± 0.07 cd | 36.17 ± 0.39 a | 754.72 ± 0.70 b | 526.56 ± 4.25 c | 320.83 ± 1.16 c | 1602.11 ± 4.71 c | |
| K | 788.97 ± 5.15 f | 1.80 ± 0.21 d | 13.09 ± 2.66 c | 3.56 ± 0.23 d | 709.71 ± 7.44 c | 459.85 ± 4.16 d | 294.57 ± 4.17 d | 1464.14 ± 15.77 d | |
| SC | 1345.71 ± 1.90 a | 9.96 ± 1.95 a | 31.51 ± 0.22 a | 15.01 ± 1.39 b | 1177.48 ± 11.67 a | 806.43 ± 10.99 a | 474.97 ± 0.72 a | 2458.87 ± 0.04 a | |
| SL | 998.28 ± 0.29 c | 5.20 ± 0.22 c | 9.18 ± 0.16 d | 0.74 ± 0.26 e | 762.34 ± 3.44 b | 550.57 ± 2.36 b | 359.37 ± 0.25 b | 1672.28 ± 1.33 b | |
| SK | 866.57 ± 0.20 e | 1.65 ± 0.36 d | 12.54 ± 0.55 c | 1.53 ± 0.05 e | 771.19 ± 6.44 b | 544.36 ± 7.89 b | 360.44 ± 0.83 b | 1675.99 ± 2.27 b | |
| DR | C | 900.49 ± 2.38 d | 4.71 ± 0.53 a | 15.82 ± 0.41 a | 6.93 ± 0.40 b | 232.64 ± 0.12 d | 181.10 ± 2.66 f | 106.04 ± 1.21 f | 519.77 ± 3.75 e |
| L | 991.58 ± 1.31 b | 2.78 ± 0.28 c | 6.20 ± 0.56 d | 11.27 ± 0.51 a | 232.66 ± 0.61 d | 222.49 ± 0.26 c | 137.56 ± 0.39 c | 592.71 ± 0.74 c | |
| K | 938.55 ± 0.17 c | 4.32 ± 0.01 ab | 13.59 ± 0.55 b | 6.74 ± 0.03 b | 240.39 ± 0.11 c | 208.82 ± 1.82 d | 128.31 ± 1.82 d | 577.52 ± 3.53 d | |
| SC | 904.14 ± 1.37 d | 4.83 ± 0.22 a | 15.27 ± 0.16 a | 7.11 ± 0.21 b | 265.27 ± 0.00 b | 201.42 ± 0.94 e | 119.68 ± 0.38 e | 586.37 ± 1.32 c | |
| SL | 1022.13 ± 3.86 a | 1.47 ± 0.15 d | 7.54 ± 0.36 c | 2.64 ± 0.28 c | 262.67 ± 5.73 b | 235.78 ± 3.10 b | 156.51 ± 0.12 b | 654.95 ± 2.75 b | |
| SK | 934.18 ± 7.44 c | 3.63 ± 0.56 bc | 8.30 ± 0.52 c | 3.45 ± 0.54 c | 343.57 ± 1.21 a | 263.94 ± 0.46 a | 173.10 ± 0.62 a | 780.61 ± 2.29 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, R.M. Fermentation of Light and Dark Bean Coffee Brews with SCOBY and Lactic Acid Bacteria. Fermentation 2025, 11, 158. https://doi.org/10.3390/fermentation11030158
Yildirim RM. Fermentation of Light and Dark Bean Coffee Brews with SCOBY and Lactic Acid Bacteria. Fermentation. 2025; 11(3):158. https://doi.org/10.3390/fermentation11030158
Chicago/Turabian StyleYildirim, Rusen Metin. 2025. "Fermentation of Light and Dark Bean Coffee Brews with SCOBY and Lactic Acid Bacteria" Fermentation 11, no. 3: 158. https://doi.org/10.3390/fermentation11030158
APA StyleYildirim, R. M. (2025). Fermentation of Light and Dark Bean Coffee Brews with SCOBY and Lactic Acid Bacteria. Fermentation, 11(3), 158. https://doi.org/10.3390/fermentation11030158






