Abstract
Lycopene is a natural carotenoid with antioxidation properties. The objective of the present study was to investigate the roles of glutamate and proline in lycopene biosynthesis in a newly isolated purple non-sulfur bacterium Cereibacter sphaeroides DT.1, under aerobic conditions. This strain contained a distinct CrtI4 capable of converting phytoene to lycopene via four-step desaturation. In order to enhance lycopene production, a crtC knockout mutant was constructed via homologous recombination. Supplementation with glutamate or proline to fermentative medium significantly enhanced intracellular lycopene accumulation in wildtype strain by a respective 99.40 ± 0.54% and 101.70 ± 0.49% and in a ΔcrtC mutant strain by 38.13 ± 0.15% and 39.83 ± 0.27%, respectively. Differential transcriptomic and metabolomic analyses showed that these promoting effects were associated with downregulation of the expression of the acyclic carotenoid 1,2-hydratase gene, and increased accumulation of lycopene precursors such as pyruvate and acetyl-CoA. The fermentation conditions for lycopene production were optimized through shake flask experiments. Feasibility for lycopene production was confirmed in a fed-batch cultivation process and a high yield of 151.10 ± 0.13 mg/L was achieved. This ΔcrtC mutant strain exhibited advantages, such as relatively lower oxygen demand and no need for illumination, making it a potentially useful strain for lycopene production under aerobic conditions.
1. Introduction
Lycopene, a naturally occurring carotenoid predominantly found in red or yellow pigmented edible plants such as tomatoes [1], watermelons [2], pomegranates [3], and papayas [4], exhibits significant antioxidant properties through its capacity to neutralize reactive oxygen species and scavenge free radicals [5,6]. Given these potent bioactivities, global market demand for lycopene has surged substantially [7], driving intensified research efforts toward optimizing its biosynthesis pathways [8]. Extracting lycopene from plants, especially from tomatoes, is a traditional and commonly used method, often involving various extraction techniques such as organic solvent extraction, ultrasound-assisted extraction, and supercritical fluid extraction [8]. However, the lycopene content in tomatoes and other plants is relatively low [9], and there are issues with inefficient resource utilization [10]. Chemical synthesis of lycopene poses potential risks to food safety due to chemical residues. On the other hand, microbial production of lycopene, as a more efficient and safer production method, has been widely adopted [8].
Photosynthetic bacteria, especially purple non-sulfur bacteria (PNSB), demonstrate remarkable adaptability to diverse environmental conditions. They can grow chemoheterotrophically under aerobic conditions or photoheterotrophically under anaerobic light conditions. These bacteria naturally synthesize carotenoids, which makes them ideal candidates for lycopene biosynthesis [10]. Among the PNSBs, potential lycopene producers reported in the literatures include Rhodospirillum rubrum, Rhodopseudomonas palustris, Rhodopseudomonas faecalis, and Cereibacter sphaeroides (formally Rhodobacter sphaeroides) [10,11,12,13]. However, the accumulation of lycopene in wildtype strains is typically low (0~3.24 mg/g DCW), and metabolic engineering strategies are often required to achieve higher yields, usually by means of enhancing precursor synthesis or eliminating competing pathways. For instance, Li et al. increased the lycopene yield from 3.24 mg/g DCW to 32.06 mg/g DCW in the wildtype Rhodopseudomonas palustris by knocking out the acyclic carotenoid 1,2-hydratase gene (crtC) [13]. Similarly, Qu et al. introduced the crtI4 gene from Rhodospirillum rubrum into the wildtype C. sphaeroides and knocked out the crtC gene, thereby boosting the yield to 10.32 mg/g DCW [14].
In PNSBs, dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP), the precursors of lycopene, are primarily synthesized through the methylerythritol 4-phosphate (MEP) pathway [15]. Within this synthetic pathway, phytoene desaturase (CrtI) is a key enzyme that catalyzes the four-step dehydrogenation of phytoene to form lycopene [15]. Therefore, the activity and functionality of CrtI significantly influence the bacteria’s capacity of synthesizing lycopene [16,17,18,19]. Currently, the production of lycopene using PNSBs is predominantly carried out under anaerobic light conditions. However, this strategy will be limited by the insufficient illumination that is caused by high cell density in large-scale fermentation. The purpose of this study is to explore the feasibility of synthesizing lycopene under aerobic conditions, investigate the effects of amino acids on bacterial lycopene synthesis, and delve into the underlying mechanisms.
2. Materials and Methods
2.1. Isolation and Identification of PNSB
The PNSB was isolated from the sediment of a fish pond in Dongtai City, Jiangsu Province, China. Enrichment culture was carried out in 120 mL serum bottles containing 60 mL of enrichment medium (C2H3O2Na 3 g/L, MgSO4·7H2O 0.5 g/L, NH4Cl 0.5 g/L, NaCl 0.5 g/L, K2HPO4 0.5 g/L, NaHCO3 1 g/L, yeast extract 0.1 g/L, pH 7.0) under anaerobic light conditions (30 °C, 3000 lux) provided by an incandescent lamp. When the culture turned brownish red, the double-layer plate method was employed for bacterial isolation and purification [20]. The morphological characteristics of the isolated strain were observed using a transmission electron microscope Hitachi H7650 (Hitachi, Tokyo, Japan).
The isolated PNSB strain was cultured in a shake flask under a temperature of 30 °C containing 100 mL yeast extract-peptone (YP) medium (yeast extract 3 g/L, peptone 3 g/L, MgSO4·7H2O 0.5 g/L, NH4Cl 0.5 g/L, NaCl 0.5 g/L, K2HPO4 0.5 g/L, pH 7.0) until the logarithmic growth was reached (OD660 = 1.0). A 50 mL sample was centrifuged (12,000 rpm, 5 min) to collect the bacterial cells, which were immediately frozen in liquid nitrogen and sent with sufficient dry ice to Wuhan BGI Biotechnology Co., Ltd. (Wuhan, China). DNA extraction and library construction were performed using the Illumina TruSeq™ Nano DNA Kit, followed by whole-genome sequencing using PacBio technology. The full-length 16S rRNA gene sequence was obtained from the bacterial genome and submitted to NCBI (GenBank number: PQ416756). It was then compared with those of related type species, and a phylogenetic tree was constructed using the neighbor-joining method. The whole-genome sequence was uploaded to the JSpeciesWS website and compared with the type strain Cereibacter sphaeroides ATH 2.4.1 to calculate the average nucleotide identity (ANI) value.
2.2. Lycopene Accumulation Capacity in the PNSB Isolate
The isolated strain was activated and cultured separately in three types of media, namely YP medium, yeast extract medium (peptone omitted in YP) and peptone medium (yeast extract omitted in YP). Anaerobic cultivation was conducted in 120 mL serum bottles containing 60 mL of medium, at 30 °C and 3000 lux. Aerobic cultivation was carried out in 250 mL shake flasks containing 100 mL of medium at 30 °C and 160 rpm. Samples were taken after 24 h or 48 h of cultivation to measure OD660 and lycopene production. The linear fitting relationship between OD660 and dry cell weight was obtained (DCW = OD660 × 738.78 − 3.3896) through preliminary experiments.
The influence of 19 amino acids on the growth and intracellular lycopene accumulation of the isolated strain was investigated under shake flask conditions. Each of the amino acids was individually added to the yeast extract medium at a fixed concentration of 200 mg/L and a control flask was set up using the same medium without addition of any amino acid. Each treatment was performed in triplicate. After culturing at 30 °C and 160 rpm for 24 h, a 50 mL sample of the bacterial culture was centrifuged at 12,000 rpm for 5 min. The resulting cell pellet was lyophilized at −40 °C by Thermo LL3000 (Wilmington, DE, USA) prior to lycopene extraction and quantification. Based on these results, the amino acids that significantly promoted lycopene accumulation were identified. Further experiments were conducted to optimize the concentrations for enhancing the growth and lycopene accumulation of the isolated strain.
2.3. Transcriptomic and Metabolomic Analysis
The isolated strain was cultured in yeast extract media supplemented with 400 mg/L of glutamate or proline under aerobic dark conditions (30 °C, 160 rpm) for 24 h. Subsequently, 50 mL of the bacterial culture was centrifuged at 4 °C, 12,000 rpm for 1 min. The supernatant was discarded, and the cell pellet was washed twice with pre-chilled PBS at 4 °C. The cell pellet was then flash-frozen in liquid nitrogen and shipped with sufficient dry ice to Genedenovo Biotechnology Co., Ltd. (Shenzhen, China) for transcriptomic and metabolomic analysis. Three independent samples were prepared for each treatment. The expression levels of each gene across different samples were compared using a negative binomial statistical model to obtain p-values. The Benjamini–Hochberg (BH) method was then applied to adjust the p-values, resulting in a false discovery rate (FDR) by which to identify significant differences between genes. Genes with |logFC| ≥ 1 and FDR ≤ 0.05 were considered significantly differentially expressed. These differentially expressed genes were subsequently classified and analyzed based on annotations from the GO and KEGG databases. Differential metabolites were analyzed under the positive ion mode (POS) and the negative ion mode (NEG) and a threshold of p-value < 0.05 and variable importance in projection (VIP) > 1 were used to identify significant changes.
2.4. Real-Time Quantitative PCR
Total bacterial RNA was extracted using the RNA-easy isolation reagent kit (Vazyme, Nanjing, China), and its purity and concentration were measured using a Thermo Scientific NanoDrop One spectrophotometer (Wilmington, DE, USA). The 16S rRNA gene was used as the internal reference gene. RNA reverse transcription and real-time quantitative PCR were performed according to the method described by Caetano et al. [21]. Quantitative PCR was conducted using SYBR Green (Vazyme, Nanjing, China), employing the 2−ΔΔCt method for data analysis. The RT-qPCR mixture consisted of 1 µL of primer, 4 µL of cDNA, and 5 µL of 2× SYBR Green Master Mix. The primers used for qPCR analysis are listed in Table 1.

Table 1.
Primers used for RT-qPCR in this work.
2.5. Extraction of Lycopene and Measurement of Pigment Absorption Spectra
To extract lycopene, 20 mL of the culture was centrifuged to collect the bacterial cells (12,000 rpm, 4 °C, 5 min). The cells were washed twice with pre-chilled 0.01 mol/L PBS (pH 7.5) and then lyophilized [22,23,24,25,26]. Subsequently, 5 mL of pre-chilled acetone: methanol (1:1, v/v) was added to the centrifuge tube, which was wrapped in aluminum foil to protect it from light. The mixture was vortexed in the dark for 10 min until the cell pellet became colorless [16]. The mixture was then centrifuged at 12,000 rpm for 2 min at 4 °C. The supernatant was filtered through a 0.22 µm membrane to obtain the extract, which was stored in a brown chromatography vial at −20 °C and analyzed within 2 days. The absorption spectrum of the pigment extract was measured by scanning from 320 nm to 1000 nm on microplate reader MD iD5 (Rockville, MD, USA).
2.6. Quantitative Analysis of Lycopene
The content of lycopene was determined using a high-performance liquid chromatography (HPLC) system CTO-20A (Shimadzu, Tokyo, Japan). A C18 reverse-phase column (Zorbax SB-Aq 4.6 × 250 mm, 5 μm) was used, and the detection wavelength was set at 450 nm. The mobile phase consisted of acetonitrile, isopropanol, and methanol (V:V:V = 5:2:3), with a flow rate of 1.0 mL/min. The column temperature was maintained at 40 °C, and the injection volume was 10 μL, with a run time of 8 min. A standard curve including 8 points was prepared by dissolving lycopene (purity ≥ 98%, Aladdin, Shanghai, China) in an acetone: methanol (1:1, v/v) solution at concentrations from 1 to 100 mg/L. This standard curve was plotted to calculate the lycopene content in the pigment extract.
2.7. Gene Knockout of crtC
Gene knockout of the isolated strain was performed using the procedure by Li et al. [13]. The primers utilized for suicide plasmid construction and mutant strain verification are listed in Table 2. The upstream (545 bp) and downstream (526 bp) fragments of crtC gene (840 bp) were amplified from the genome of C. sphaeroides DT.1 using primers crtC U-F/crtC U-R and crtC D-F/crtC D-R, respectively. Then it was ligated into the Xbal I/Sac I site of pJQ200SK (Miaoling, Wuhan, China). The conjugation strain E. coli S17-1 with pJQ200SK-ΔcrtC was mixed with C. sphaeroides DT.1 in a 1:5 ratio. After incubating the mixture on an LB plate for 48 h, it was suspended in sterile water and vortexed to stop the conjugation process. The mixture was then diluted and spread onto an LB plate containing 20 mg/L gentamicin and incubated at 30 °C to allow colony development. Red colonies were selected and streaked onto an LB plate containing 10% sucrose for further PCR validation.

Table 2.
Primers used for gene knockout.
2.8. Optimization of Fermentative Conditions for Lycopene Production
The effects of various factors, including temperature (15, 20, 25, 30, 35, 40 °C), pH (5.0, 6.0, 7.0, 7.5, 8.0, 9.0), shaking speed (120, 140,160, 180, 200 rpm), medium volume (50, 100, 200, 300, 400 mL in 500 mL conical flasks), inoculation volume (1, 2.5, 5, 10, 20%), and different carbon sources (glucose, acetic acid, pyruvic acid, lactic acid, glycerol) on the growth and lycopene production of the ΔcrtC mutant strain were investigated in a series of shake flask experiments. The flasks containing 100 mL liquid medium and 5% (V/V) seed culture were incubated at 30 °C and 160 rpm unless otherwise specified. Samples were taken every 24 h to measure OD660 and lycopene content. The optimal fermentation conditions were determined by comparison on intracellular lycopene accumulation under different conditions.
Once the fermentation conditions were optimized, a fed-batch fermentation experiment was initiated to further explore the potential of the ΔcrtC mutant strain for lycopene production in large-scale fermentation processes. The activated seed culture was inoculated into shake flasks containing 400 mL liquid medium and incubated at 30 °C and 160 rpm. The shake flasks were fed 3 times by replacing 40 mL of culture with fresh medium at 4 N, 8 N, and 16 N concentrations. Feeding stopped once the bacterial growth expressed as OD660 did not show a significant increase. At each stage of the fed-batch fermentation process the lycopene content (mg/g DCW) and the lycopene titer (mg/L) were determined to evaluate the performance of the newly constructed ΔcrtC mutant strain.
2.9. Data Statistics and Analysis
Experiments were performed in triplicate, with results presented as mean ± standard deviation (SD). Statistical analysis was performed using Microsoft Excel 2016, and Duncan’s multiple range test was used to measure the specific differences between pairs of means. Differences were considered as significant at the p < 0.05 level. The graphs were generated using GraphPad Prism 9.0.
3. Results
3.1. Isolation and Identification of PNSB Strain
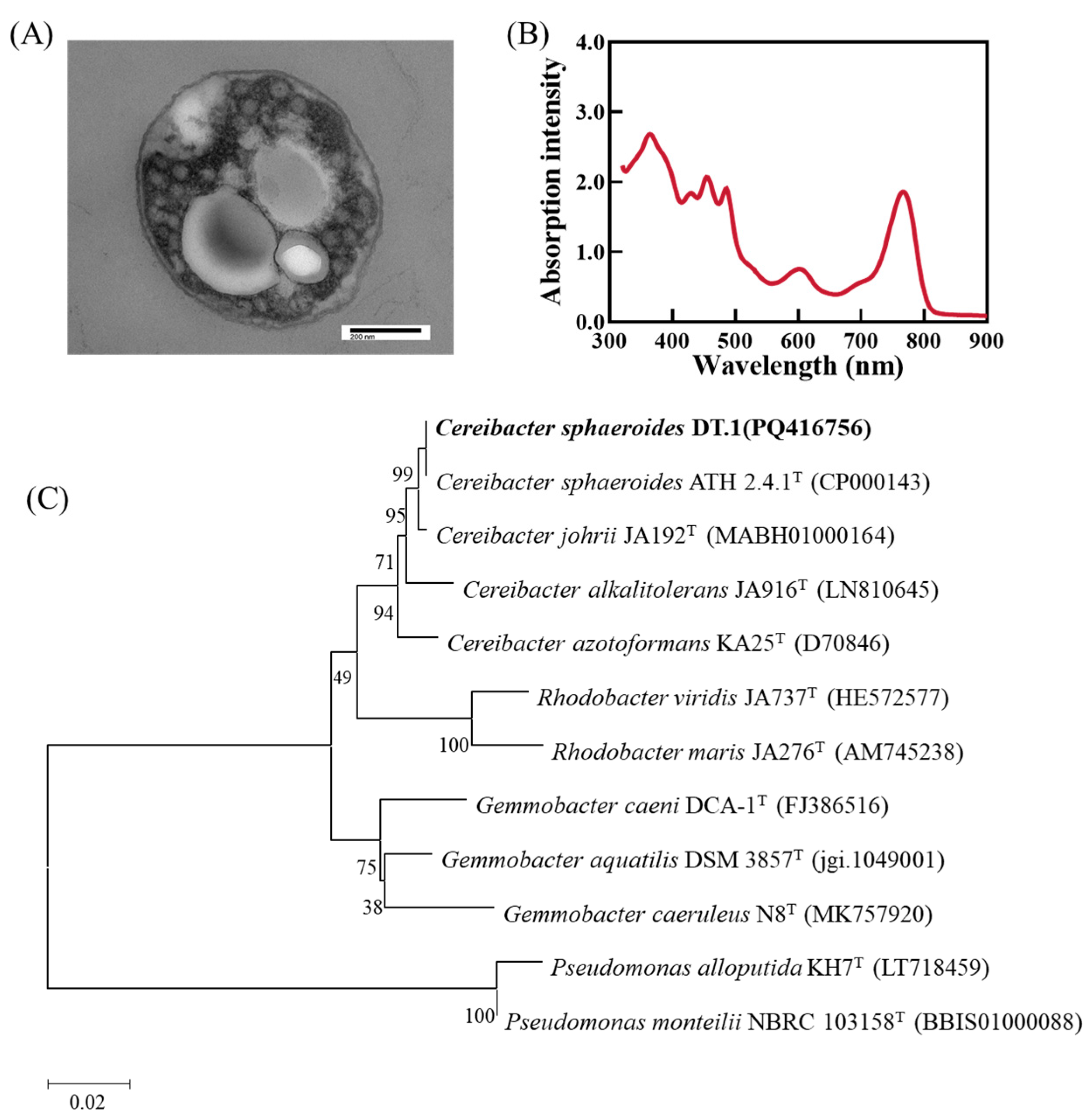
A PNSB strain, DT.1, was successfully isolated from the sediment sample from a local fish pond at a freshwater aquaculture base in Dongtai City, Jiangsu Province. This bacterium forms brownish red, moist, and smooth colonies on YP plates, with diameters ranging from 2.5 to 3.8 mm. Cells are Gram negative and oval shaped. Transmission electron microscopy revealed vesicular photosynthetic internal membranes, with polyhydroxyalkanoates (PHA) granules distributed in the cytoplasmic matrix (Figure 1A). The absorption spectrum of its pigment extract shows the typical three-finger peaks of carotenoids at 415 nm, 450 nm and 475 nm. The formation of this characteristic spectrum is mainly related to the structure and electronic transition characteristics of carotenoids, which correspond to the observation by Li et al. [13] (Figure 1B). Whole-genome sequencing yielded a full-length 16S rRNA gene of 1455 bp. Comparison with related type species in the NCBI database showed that strain DT.1 has the highest 16S rRNA gene similarity of 100% with Cereibacter sphaeroides 2.4.1 (formally Rhodobacter sphaeroides 2.4.1).
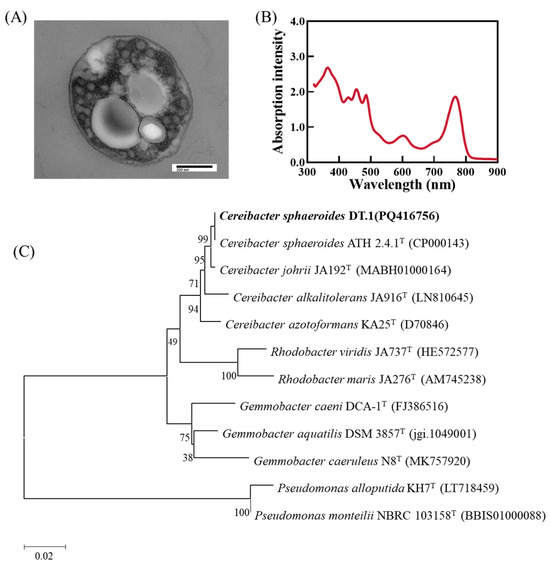
Figure 1.
(A) Cellular morphology and photosynthetic membranes of strain DT.1. (B) The pigment absorption spectrum of strain DT.1. (C) The phylogenetic tree of strain DT.1 constructed based on the 16S rRNA gene sequence.
The genomic sequencing results reveal that the isolated strain DT.1 contains one circular chromosome and three plasmids, with a total length of 4,364,336 bp and a G + C content of 69.35%. The genome comprises 4257 coding genes, with a total sequence length of 3,866,193 bp, accounting for 86.73% of the entire genome sequence (Table 3). The whole genome sequence was uploaded to the JSpeciesWS website, and the ANI value between strain DT.1 and type species C. sphaeroides ATH 2.4.1 was calculated to be 98.14%, surpassing the threshold (95–96%) suggested by Yoon et al. for determining bacterial species [26]. Therefore, the isolated strain DT.1 was identified as a strain of the species Cereibacter sphaeroides. It was noticed that Cereibacter sphaeroides was formally named as Rhodobacter sphaeroides and in 2000 reclassified to the genus Cereibacter according to Oren et al. [25].

Table 3.
Characteristics of genome of strain DT.1.
3.2. Deduced Biosynthetic Pathway of Lycopene in Cereibacter sphaeroides DT.1
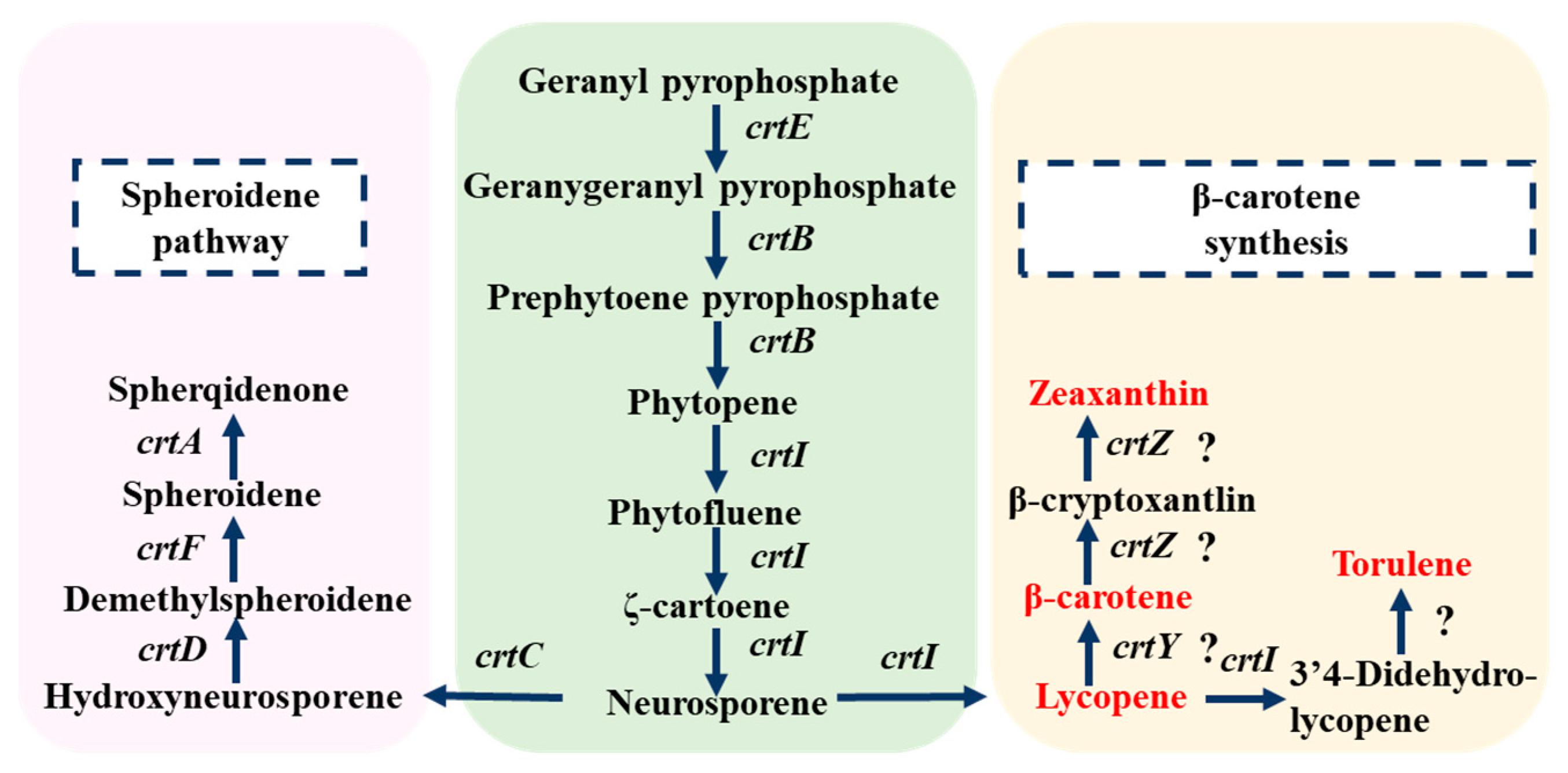
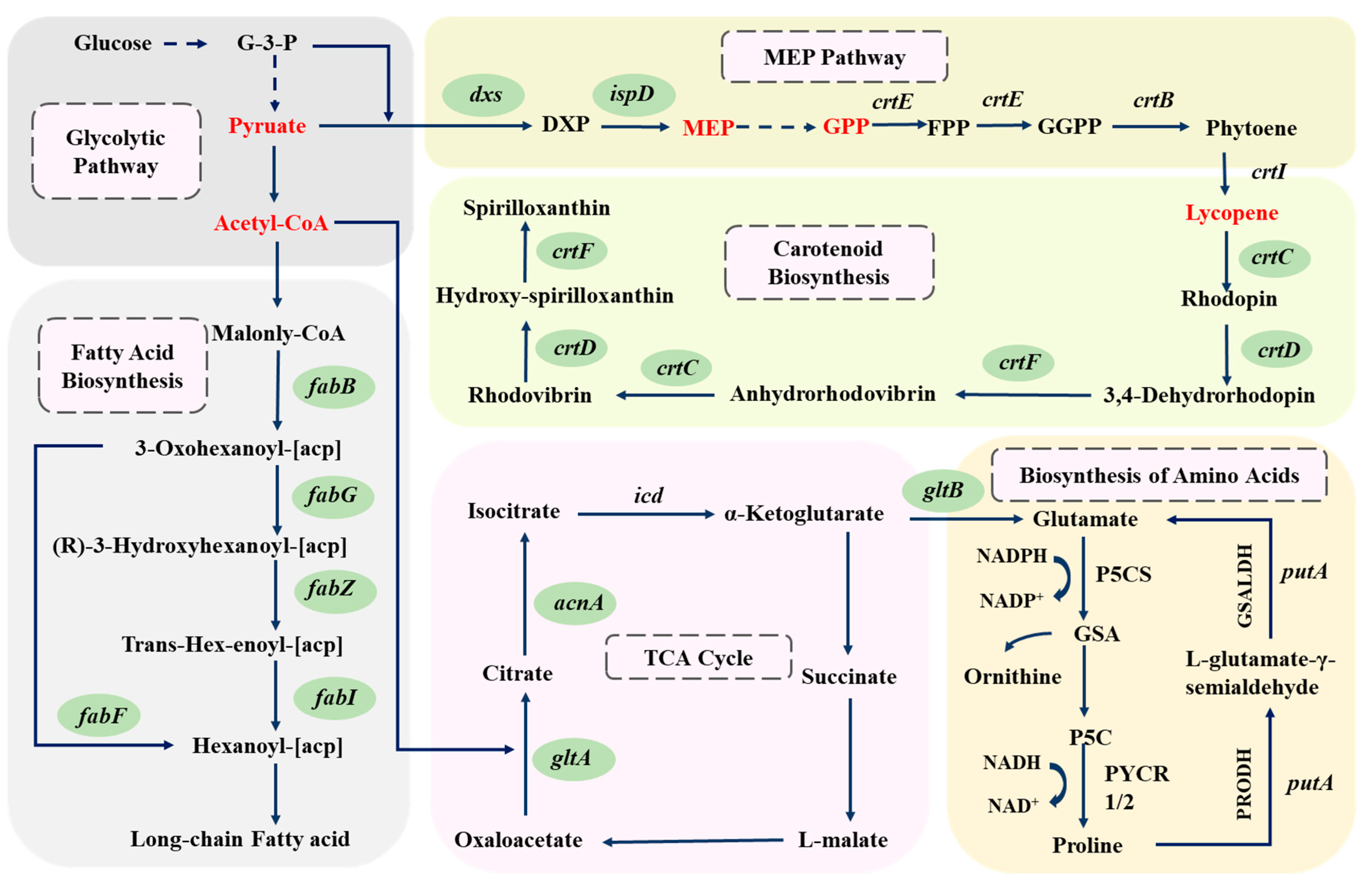
Previous studies have shown that most prokaryotes synthesize lycopene through the MEP pathway, which differs from the MVA pathway in eukaryotes [15]. Comparision of the whole genome sequence with the KEGG database revealed the existence of a complete carotenoid biosynthesis pathway in strain DT.1, including gene clusters related to lycopene metabolism such as dxs, ispD, crtIDCEF, ppsR and prrA. However, key enzymes of the MVA pathway, such as Mvd and PmK, were not detected, indicating that this strain synthesizes lycopene through the MEP pathway, consistent with early findings reported in the literature [13]. Additionally, metabolomic data detected precursor substances related to lycopene synthesis, such as IPP and GPP, as well as downstream metabolites derived from further conversion of lycopene, including β-carotene, zeaxanthin, lutein, canthaxanthin, astaxanthin, spheroidenone, and torulene (Table 4), further confirming the existence of the MEP pathway. Based on the integrated analysis of genomic and metabolomic data, the inferred lycopene synthesis pathway is illustrated in Figure 2.

Table 4.
Metabolites related to lycopene synthesis in strain DT.1.
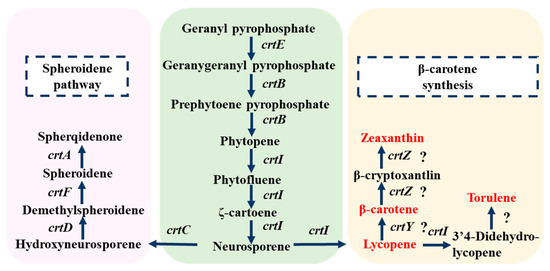
Figure 2.
Deduced metabolic pathway for lycopene synthesis in strain DT.1 (? indicates that the gene was not annotated in the genome, but that the relevant transformation product was detected).
It has been generally accepted that phytoene desaturase (CrtI) is a key enzyme in the MEP pathway, respnsible for catalyzing the four-step desaturation of GPP to form lycopene [26]. However, in the wildtype strains of C. sphaeroides, CrtI only possesses three-step desaturation activity and is incapable of fulfilling the fourth step desaturation reaction to convert neurosporene to lycopene [14]. Therefore, introducing the four-step desaturation gene (crtI4) is a common practice for constructing an efficient lycopene synthesis pathway. For instance, Hunter et al. [27] succeeded in introducing the crtI4 gene from Erwinia herbicola into Escherichia coli to construct a lycopene synthesis pathway. Nevertheless, in the newly isolated wildtype strain DT.1 of C. sphaeroides, lycopene accumulation has been detected in HPLC analysis (Table 5), and several downstream metabolites, such as β-carotene, zeaxanthin and torulene, have been identified through metabolomic analysis (Table 4). Based on these findings, we hypothesize that the CrtI encoded by the crtI gene in strain DT.1 is a four-step desaturase capable of catalyzing the conversion of GPP to lycopene via four-step desaturation, making the newly isolate strain DT.1 an ideal platform for lycopene synthesis.

Table 5.
Growth and lycopene content of strain DT.1 under different culture conditions.
3.3. Conditions for Lycopene Accumulation in Cereibacter sphaeroides DT.1
As reported in the literature, most PNSB, such as Rhodospirillum rubrum, Rhodopseudomonas palustris, Rhodopseudomonas faecalis, and Rhodobacter sphaeroides, prefer to grow and accumulate lycopene under anaerobic light conditions [11,13]. However, the results from the present study show that the lycopene accumulation was below the detection limit (<0.10 mg/g DCW) under anaerobic light conditions, although bacterial biomass expressed as OD660 was significantly higher than that under aerobic dark conditions. The strain DT.1 grew better and tended to accumulate lycopene in nutrient rich medium under aerobic dark conditions. For example, when it was cultured in yeast extract medium, accumulation of 0.82 ± 0.49 mg/g DCW of lycopene was recorded. When cultured in YP medium, both bacterial growth and lycopene production significantly increased, with lycopene accumulation rising to 1.55 ± 0.27 mg/g DCW. It appears that peptone had promoting effects on both the growth and lycopene accululation, as growth of this bacterium became very weak in peptone medium, and no lycopene accumulation was detected (Table 5).
3.4. Effects of Glutamate and Proline on Lycopene Accumulation
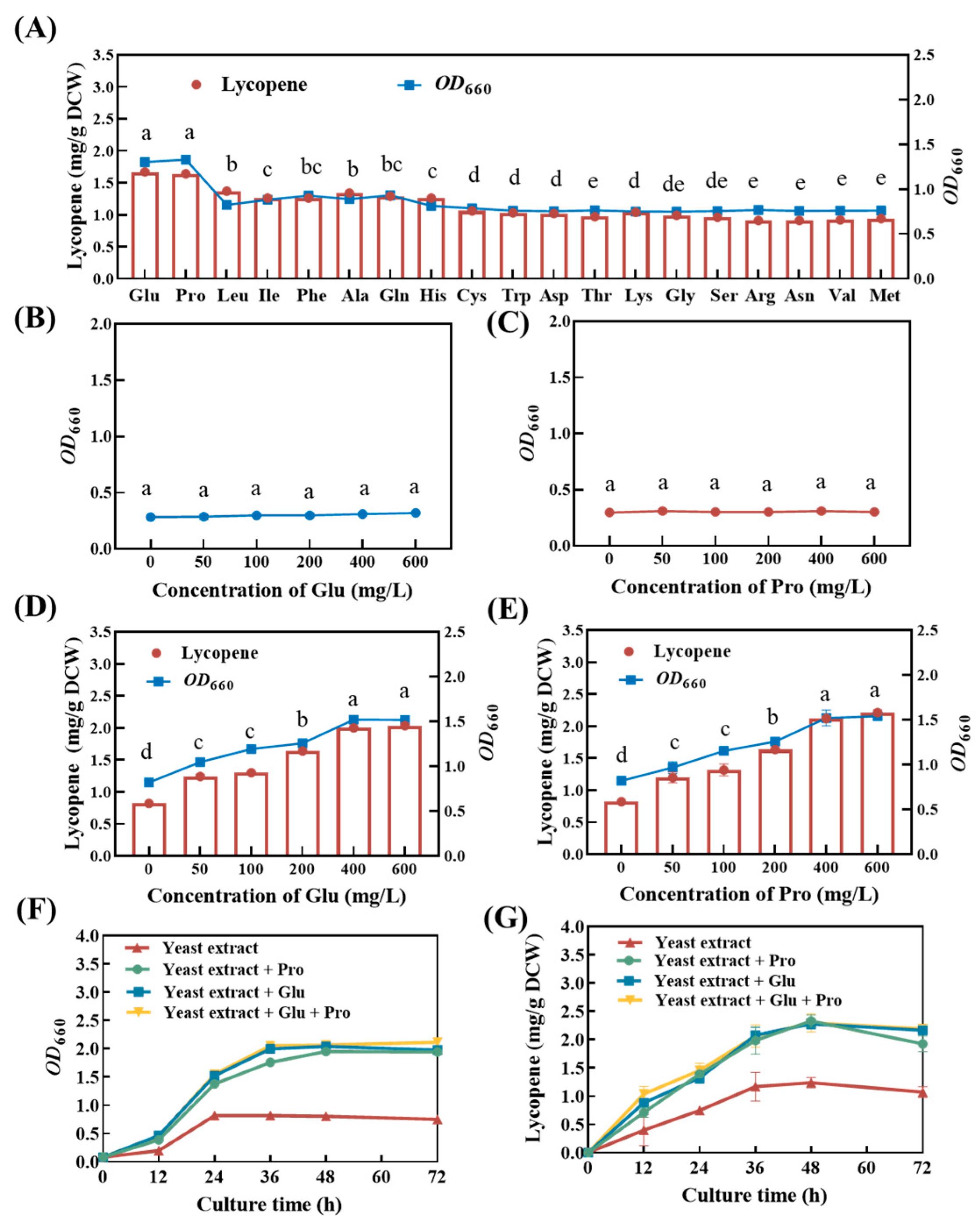
Based on the aforementioned results, we hypothesized that certain amino acids in peptone may have had promoting effects on the growth of and lycopene synthesis in strain DT.1. To validate this hypothesis, the effects of 19 amino acids were tested under shake flask conditions and the results reveal that a total of 8 amino acids significantly promoted the growth and lycopene synthesis of strain DT.1 (Figure 3A). Among these, sodium glutamate and proline exhibited the strongest promoting effects on both the growth and lycopene synthesis of strain DT.1, with OD660 increased by 51.97 ± 0.11% and 52.84 ± 0.14%, and lycopene yield increased by 45.10 ± 0.15% and 63.40 ± 0.26%, respectively, as compared with the control treatment without the addition of any amino acid at 24 h. This was followed by the other six species of amino acids (namely leucine, isoleucine, phenylalanine, alanine, glutamine and histone), which increased bacterial growth by 18.30 ± 0.15–26.80 ± 0.21% and lycopene yield by 10.17 ± 0.33–24.79 ± 0.18% compared with the control at 24 h (Figure 3A).
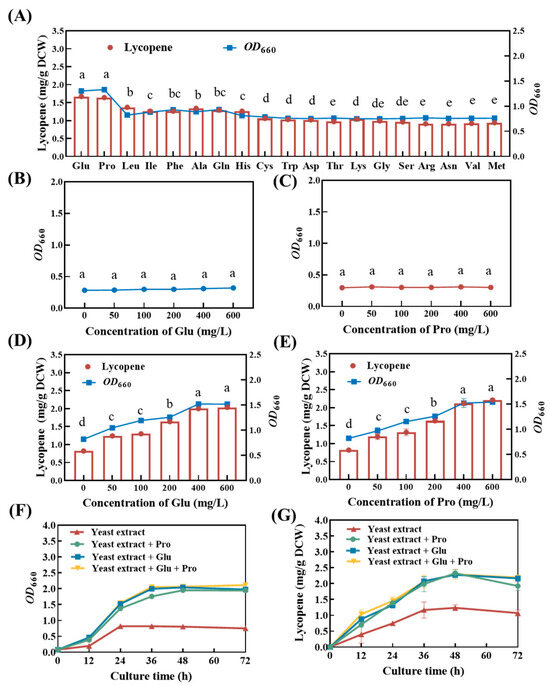
Figure 3.
Lycopene content of strain DT.1 cultured in different media. (A) Effects of different amino acids on the growth and lycopene content of strain DT.1. (B,C) Growth of strain DT.1 in the media with glutamate or proline as the sole carbon and nitrogen source. (D,E) Growth and lycopene content of strain DT.1 in yeast extract media containing different concentrations of glutamic acid or proline. (F,G) Growth curves and lycopene content of strain DT.1 in yeast extract media containing 400 mg/L glutamic acid or/and proline. The data with different lower-case letters in the figures are statistically different in the content of lycopene (p < 0.05).
Glutamate and proline act as growth factors for Cereibacter sphaeroides growth and lycopene synthesis as neither of them sustained the growth of strain DT.1 (Figure 3B,C). Increasing trends in bacterial growth and lycopene yield were observed in the treatments with the addition of glutamate and proline in the range of 0–600 mg/L. Additionally, both bacterial growth and lycopene accumulation reached their maximum values in the treatment receiving glutamate and proline addition at the concentration of ≥400 mg/L, and this corresponded to an increase in OD660 by 140.24 ± 0.32% and 161.25 ± 0.51% and lycopene yield by 99.40 ± 0.54% and 101.70 ± 0.49%, respectively, as compared with the control without addition of any kind of amino acid at 48 h (Figure 3D,E). In addition, simultaneous supplementation with both amino acids did not show further promoting effects, implying that a similar mechanism may be involved in the enhanced growth and lycopene accumulation.
3.5. Mechanisms for Enhanced Lycopene Accumulation by Glutamate and Proline
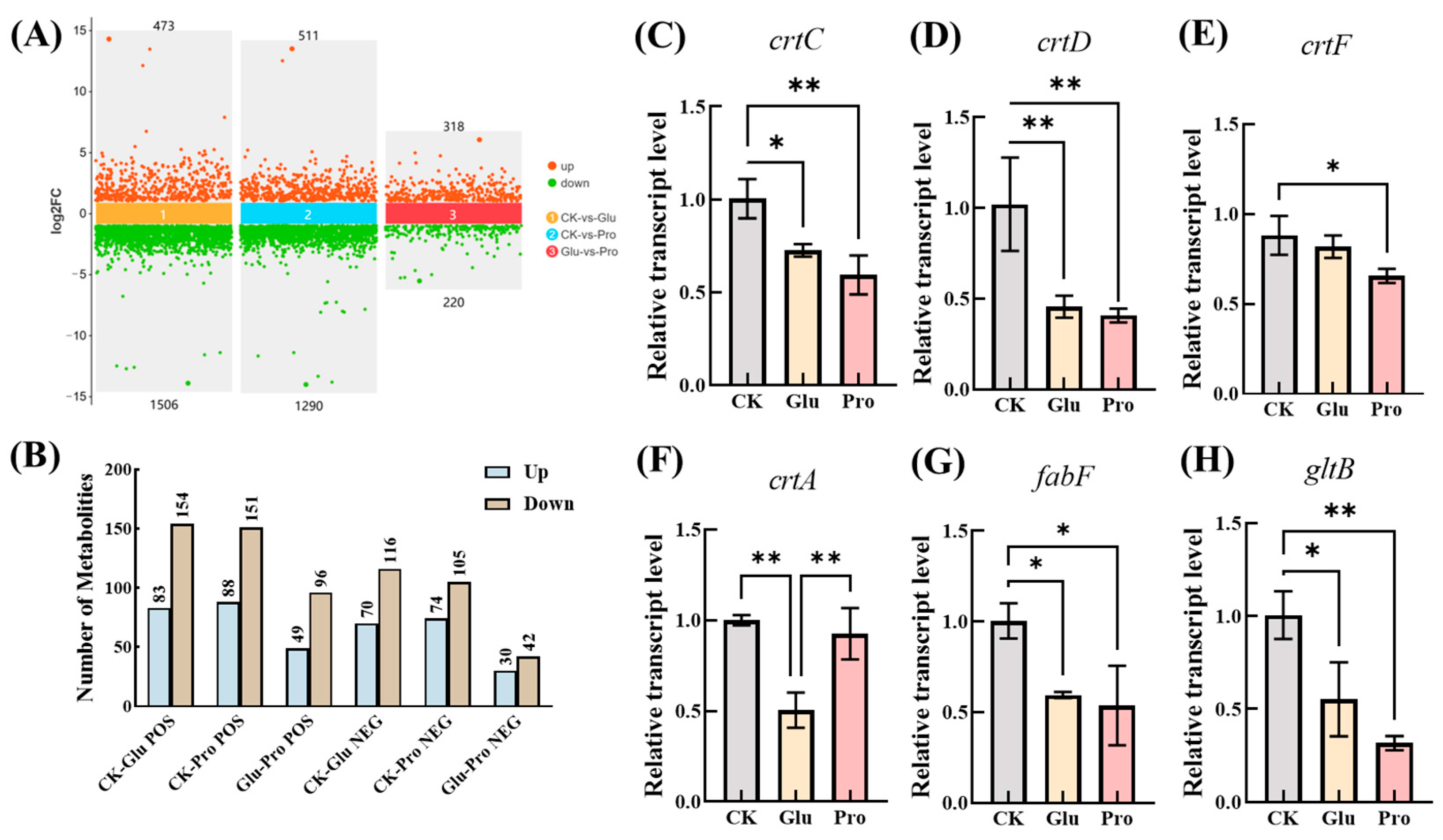
No reports were found to be available on enhanced growth and lycopene accumulation in PNSB by glutamate and proline in the literature. To elucidate the mechanisms, differential expression analysis and differential metabolomic analysis were performed in the present study. The results show that, compared with the control, the addition of glutamate to the culture medium led to the upregulation of 1506 genes and the downregulation of 473 genes in the bacterial cells. Similarly, the addition of proline resulted in the upregulation of 1290 genes and the downregulation of 511 genes (Figure 4A). The differentially expressed genes were involved in energy metabolism, the TCA cycle, amino acid metabolism, fatty acid metabolism, and carotenoid metabolism, among others. Notably, genes closely related to lycopene synthesis were predominantly downregulated rather than upregulated. For instance, the expression levels of genes related to the conversion of lycopene, such as crtC, crtD, and crtF, were significantly lower than those in the control group. In contrast, no significant changes were observed in the expression levels of genes related to lycopene synthesis, such as crtI, crtB, and crtE (Figure 4C–E). This suggests that reducing the conversion of lycopene represents the first mechanism by which glutamate or proline promotes lycopene accumulation in PNSB strain DT.1
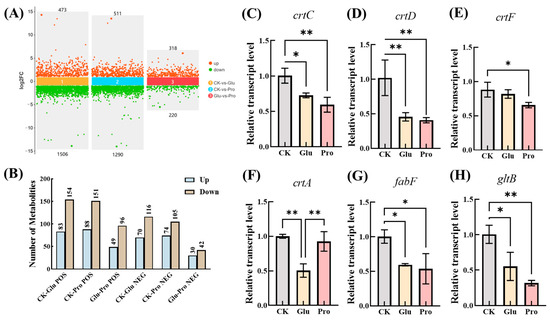
Figure 4.
Transcriptome and metabolome analysis and RT-qPCR validation. (A) Number of differentially expressed genes. (B) Number of differential metabolites (C–H). RT-qPCR validation of differentially expressed genes, where * denotes p < 0.05 and ** denotes p < 0.01.
On the other hand, the expression of five genes involved in fatty acid synthesis—fabB, fabF, fabG, fabI, and fabZ—was also significantly downregulated (Figure 4F,G and Figure 5). These genes encode enzymes responsible for synthesizing long-chain fatty acids using acetyl-CoA as the substrate. The downregulation of expression of these genes implies a reduction in the consumption of acetyl-CoA within the cells, which is evidently conducive to increasing intracellular acetyl-CoA levels. Concurrently, the expression of genes in the TCA cycle, such as gltA and acnA, as well as the gene gltB in amino acid metabolism, was also significantly downregulated. These genes encode enzymes that catalyze the stepwise conversion of acetyl-CoA, ultimately leading to the synthesis of glutamate. Therefore, the downregulation of these genes also contributes to an increase in intracellular acetyl-CoA levels [28,29]. The accumulation of acetyl-CoA can inhibit the activity of the pyruvate dehydrogenase complex, leading to the buildup of pyruvate, thereby providing more precursors for lycopene synthesis. Metabolomic results confirm that acetyl-CoA and pyruvate were significantly upregulated in treatments supplemented with glutamate or proline. Based on the above results, a second mechanism is proposed in which the downregulation of genes encoding enzymes that consume acetyl-CoA results in increased intracellular levels of acetyl-CoA and pyruvate, which, in turn, enhances the availability of the precursor substances required for lycopene synthesis.
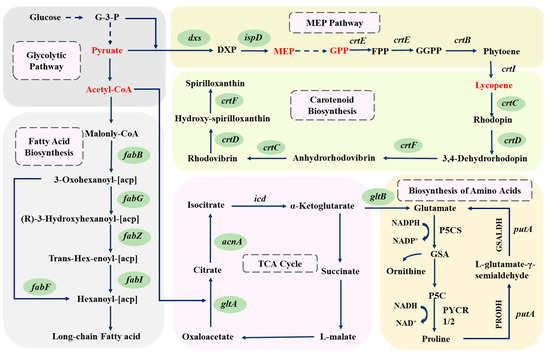
Figure 5.
Regulation of lycopene synthesis-related gene expression and metabolites in strain DT.1 by glutamic acid or proline (downregulated genes are indicated by green circles, while upregulated metabolites are indicated in red text).
Differential metabolomic analysis showed, that compared with the control, 83 metabolites were upregulated and 154 metabolites were downregulated (POS), 70 metabolites were upregulated and 116 metabolites were downregulated (NEG) in the DT.1 cells cultured in the medium supplemented with glutamate culture. Additionally, 74 metabolites were upregulated and 151 metabolites were downregulated for POS and 88 metabolites were upregulated and 105 were downregulated for NEG in the DT.1 cells cultured in the medium supplemented with proline (Figure 4B). Both glutamic acid and proline supplementation led to significant upregulation of pyruvate (Figure 5), which is a raw material for lycopene synthesis, giving a reasonable explanation for the enhanced lycopene accumulation. Meanwhile, MEP and GPP, the precursors of intracellular lycopene synthesis, were also upregulated (Figure 5), further confirmed the second mechanism proposed above.
3.6. Knocking Out of the crtC Gene Promotes the Accumulation of Lycopene
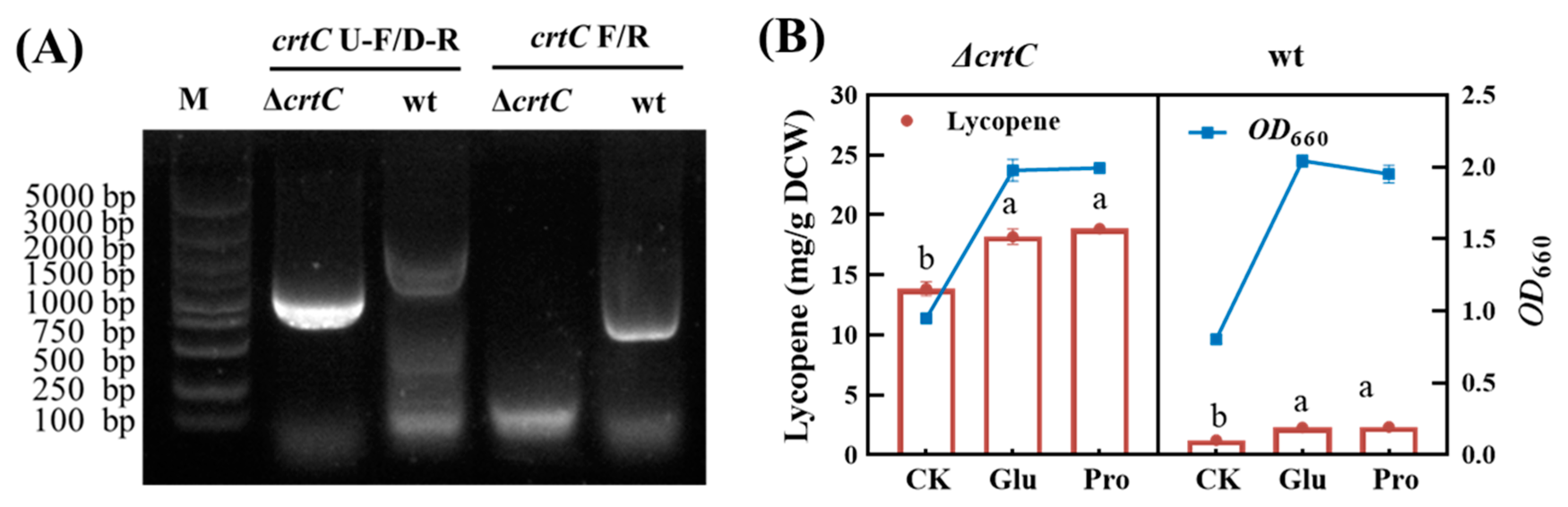
In this study, a crtC gene knockout mutant was constructed by using homologous recombination technology. The agarose electrophoresis of PCR products from the colonies of mutant and wildtype strains is shown in Figure 6A When using crtC U-F and crtC D-R as primers, a band of 1000 bp was amplified from the mutant strain, which is smaller than the 1800 bp band amplified from the wildtype strain (lanes 1 and 2). This is because the crtC coding frame is absent in the mutant strain. When crtC F and crtC R were used as primers, an expected band of 850 bp (equal to crtC gene) was amplified from the wildtype strain, while the target band was not amplified from the mutant strain (lanes 3 and 4). This confirmed the successful construction of the ΔcrtC knockout mutant.
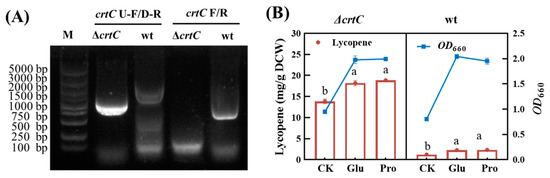
Figure 6.
Comparison of the crtC knockout mutant and wildtype strains. (A) PCR identification results. (B) Growth and lycopene accumulation of wt and ΔcrtC strains in the presence or absence of amino acid. The data with different lower-case letters in the figures are statistically different in the content of lycopene (p < 0.05).
In fact, no significant difference in the growth rate and the final biomass was observed between the crtC knockout mutant strain and the wildtype strain, indicating that crtC deletion had no effect on bacterial growth. Nevertheless, the intracellular lycopene yield reached 13.48 ± 0.32, 18.60 ± 0.65 and 18.85 ± 0.31 mg/g DCW, respectively for the mutant cells cultured in yeast extract, YE + Glu, and YE + Pro mediums at 48 h. The averages of these values were respectively 8.70, 8.09 and 7.93 times higher than those of the wildtype strain at 48 h. In the MEP pathway acyclic carotenoid 1,2-hydratase encoded by crtC gene has multiple functions, including converting lycopene to rhodopin. Therefore, the above results could be explained by the significant reduction in downstream conversion of lycopene due to the knockout of the crtC gene and this is in good agreement with the first mechanism proposed above. It was noted that the promoting effects of glutamate and proline were reconfirmed in the crtC knockout mutant, as indicated by an increase in lycopene yield by 38.13 ± 0.15% and 39.83 ± 0.27%, respectively, as compared with the control without the addition of any kind of amino acid at 48 h (Figure 6B).
3.7. Optimization of Fermentative Conditions for Lycopene Production in ΔcrtC Mutant
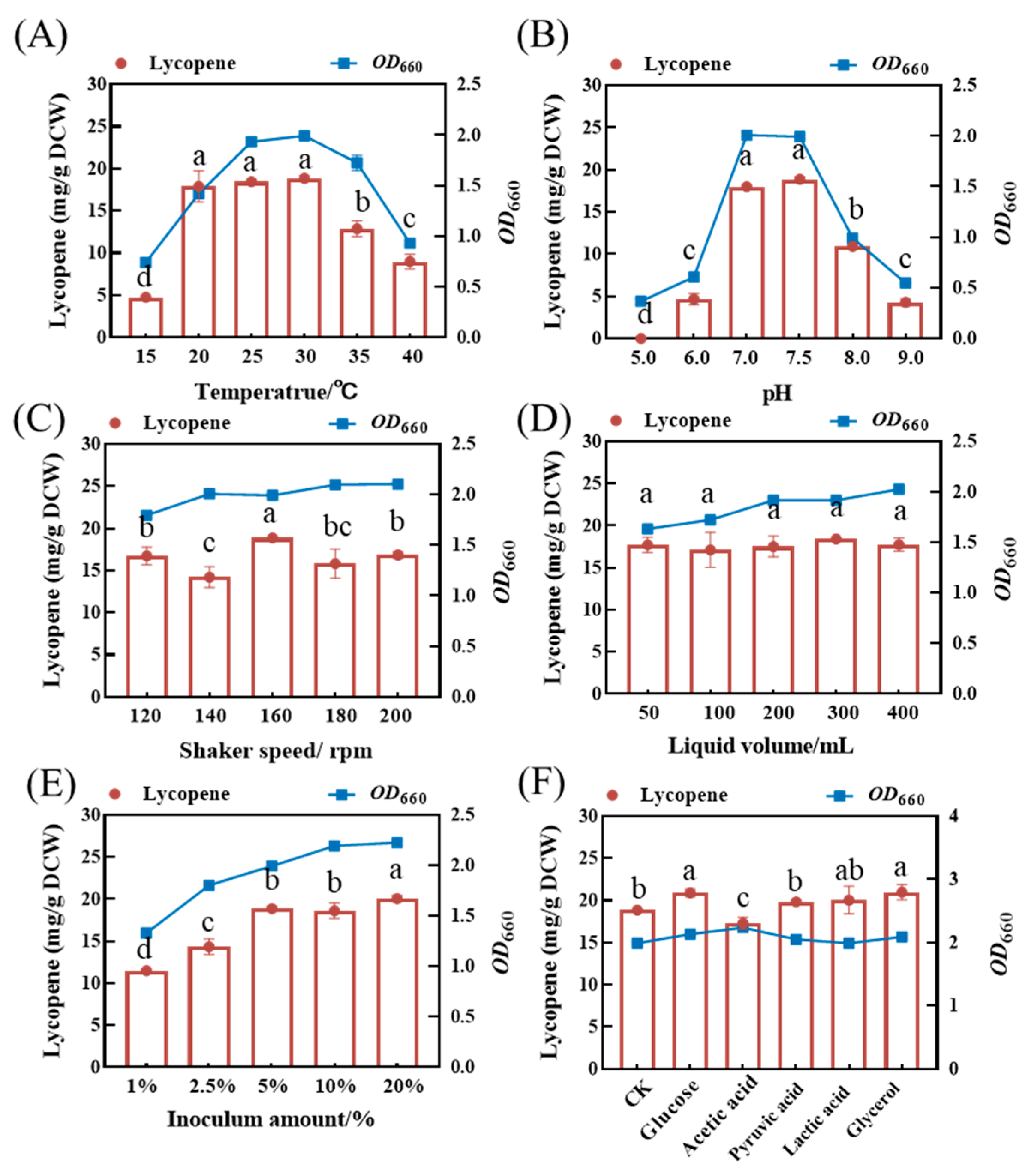
To optimize the fermentative conditions, the effects of different temperatures, pH values, shaker speeds, inoculum amount, liquid volumes, and selected carbon sources on lycopene accumulation in the ΔcrtC knockout mutant strain were investigated in a series of shake flask experiments. The yeast extract medium supplemented with 400 mg/L proline was used as the fermentation medium. The results show that both culture temperature and medium pH significantly affected the growth of and lycopene accumulation in the mutant strain. Optimal growth and lycopene yield were observed at 25–30 °C and at a pH of 7.0–7.5. Beyond the optimal temperature or pH range, both biomass and lycopene accumulation decreased significantly (Figure 7A,B).
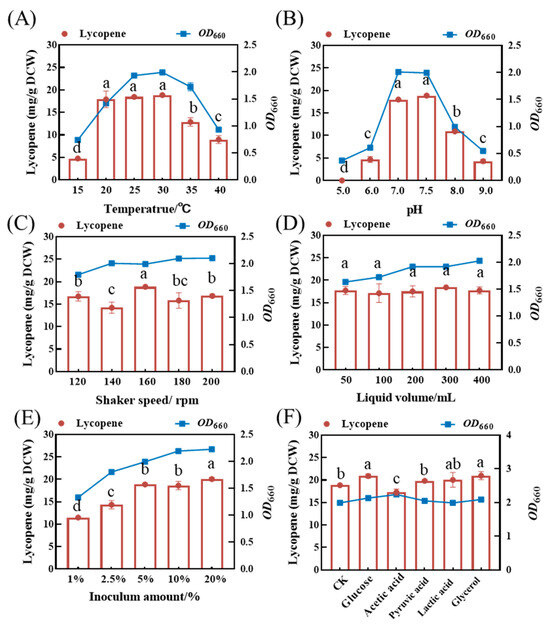
Figure 7.
The impact of different fermentation conditions on OD660 and lycopene accumulation in the strain DT.1. (A) Temperature, (B) pH, (C) shaker speed, (D) liquid volume, (E) inoculum amount, and (F) carbon source. The data with different lower-case letters in the figures are statistically different in terms of the content of lycopene (p < 0.05).
Bacterial biomass, expressed as OD660 of the ΔcrtC mutant strain, was not significantly influenced by rotating speed, although the highest lycopene yield was observed at 160 rpm (Figure 7C). The growth curve of the ΔcrtC mutant strain showed a gradually increasing trend, while the lycopene yield remained almost unchanged (17.11 ± 0.35 mg/L to 18.41 ± 0.28 mg/L) as the liquid volume increased from 50 mL to 400 mL (Figure 7D). This suggests that a relatively lower oxygen supply is sufficient to sustain the growth of the ΔcrtC mutant strain. Thus, the fermentation cost may be significantly reduced as bacterial growth and lycopene accumulation can be accomplished at relatively higher loading volume and microaerobic conditions.
Within the inoculum volume range of 1–20%, both growth and lycopene yield increased with the increases in the inoculum size. The optimal inoculum volumes for bacterial growth and lycopene yield in the ΔcrtC mutant strain are 10 and 5%, respectively. Raising the inoculum volume up to 20% will be restricted by higher cost of seed preparation, although this is beneficial for promoting microbial growth and lycopene accumulation in the mutant strain (Figure 7E). Therefore, the recommended inoculum size is 5%, which is conducive to balance the operational cost and lycopene yield.
Supplementation of several other carbon sources did not impact the growth of the ΔcrtC mutant strain but exerted a significant effect on lycopene accumulation. Among these, glucose, pyruvate, lactic acid, and glycerol promoted lycopene accumulation in the ΔcrtC mutant strain, with lycopene yield increased by 12.21 ± 0.26%, 6.43 ± 0.21%, 7.62 ± 0.14%, and 12.64 ± 0.26%, respectively, as compared with the control. The only exception is acetic acid, the addition of which resulted in a decrease in lycopene yield by 7.04 ± 0.25% (Figure 7F).
3.8. Fed-Batch Fermentation for Lycopene Production
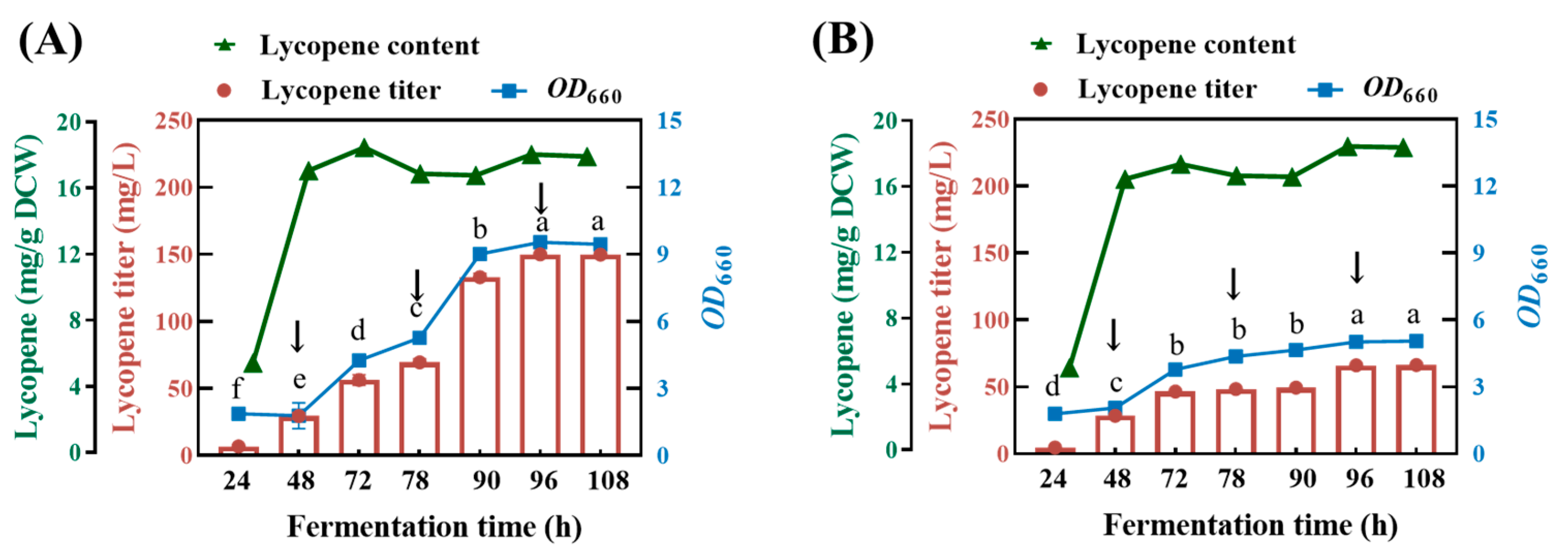
Based on the results above, the fermentation medium was modified as follows: 3 g/L yeast extract, 400 mg/L proline and 2 g/L glycerol (medium A) or 2 g/L glucose (medium B), MgSO4·7H2O 0.5 g/L, NH4Cl 0.5 g/L, NaCl 0.5 g/L, K2HPO4 0.5 g/L, pH 7.0. The lycopene-producing capacity for the ΔcrtC mutant strains was evaluated in a 500 mL fed-batch cultivation under aerobic dark conditions. The results show that, during the initial stage following the inoculation of the ΔcrtC mutant strain to medium A and the subsequent two rounds of fed-batch cultivation, both the growth of the ΔcrtC mutant strain and the production of lycopene continued to increase, while the intracellular lycopene content remained relatively stable. During the two batches of fermentation period, there was an increasing trend toward bacterial proliferation and lycopene accumulation, which confirmed the feasibility of lycopene production using fed-batch cultivation of the ΔcrtC mutant strain. However, further increase in the OD660 and lycopene yield was not observed in the third batch of fermentation. After three rounds of fed-batch cultivation, the yield of lycopene reached 151.10 ± 0.13 mg/L (Figure 8A), which is comparable to the yield 150.15 mg/L reported by Qu et al. [14].
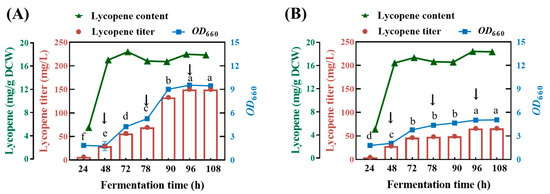
Figure 8.
Lycopene production in fed-batch fermentation. Initial medium: 3 g/L yeast extract, 400 mg/L proline and 2 g/L glycerol (A), 2 g/L glucose (B), MgSO4·7H2O 0.5 g/L, NH4Cl 0.5 g/L, NaCl 0.5 g/L, K2HPO4 0.5 g/L, pH 7.0. The arrowheads indicated that addition of fresh medium at 4 N, 8 N and 16 N concentrations, respectively. The data with different lower-case letters in the figures are statistically different in the content of lycopene (p < 0.05).
In contrast, the fed-batch cultivation of the ΔcrtC mutant strain proved to be less effective in medium B with glucose as a carbon source. In fact, active growth and lycopene accumulation merely occurred in the initial stage, with the second stage receiving the first round of fresh medium addition. The subsequent addition of fresh medium did not lead to a further increase in either bacterial biomass or lycopene production. This was because the fermentation of glucose by this bacterium led to the progressive acidification of the medium B until the lowest value of pH 6.0 was reached, creating an environment condition unfavorable for bacterial growth. At the end of the three rounds of fed-batch cultivation, the maximum lycopene yield harvested was 66.11 ± 0.12 mg/L, which was far below that obtained in the medium A (Figure 8B). Therefore, glycerol can be used as a carbon source that is superior to glucose, in that it is a non-fermentative substrate, precluding the possibility of medium acidification during lycopene production using the ΔcrtC mutant strain. As a by-product during oleochemical and biodiesel fuel production, it can be used as a more cost-effective, sustainable and environmentally friendly substrate for industrial applications in the future [13].
4. Discussion
Like most prokaryotic microorganisms, the newly isolated strain C. sphaeroides DT.1 synthesizes lycopene via the MEP pathway [30]. The critical regions responsible for functional divergence in desaturation activity, key catalytic sites, and micro-evolutionary variations of CrtI enzymes across diverse biological species remain to be elucidated [31,32,33,34]. Currently, the functional characteristics of CrtI can only be inferred from the products of enzymatic reactions [35]. Previous studies have shown that the CrtI in C. sphaeroides ATH 2.4.1 functions as a three-step desaturase, catalyzing the conversion of phytoene to neurosporene as the end product, and is thus incapable of synthesizing lycopene [14]. However, besides lycopene, torulene, a downstream product derived from the five-step desaturation of phytoene was also detected, indicating that CrtI in strain DT.1 possesses four-step or even five-step desaturation capabilities. Zhang et al. showed that CrtI from Rhodobacter azotoformans can catalyze the three-step and four-step desaturation of phytoene to produce neurosporene and lycopene, respectively, and under certain conditions, it can also perform five-step desaturation to form 3,4-didehydrolycopene [35]. In our study, the amino acid sequence similarity of CrtI between strain DT.1 and Rhodobacter azotoformans was calculated to be as high as 92.47%, supporting the aforementioned hypothesis.
Results from the present study show that the addition of glutamate or proline promoted the lycopene accumulation in either wildtype or ΔcrtC mutant strains (Figure 6B) and this was achieved by downregulating the expression of the crtC gene related to lycopene conversion (the first mechanism) and promoting the accumulation of lycopene synthesis precursors (second mechanism). This phenomenon has not been reported in previous literature. The addition of glucose or glycerol also enhanced lycopene accumulation through the second mechanism, as both substrates can be converted via glycolytic pathway to glyceraldehyde-3-phosphate (G-3-P), one of the key precursors for lycopene synthesis [13,36]. Glutamate and proline exhibited similar promoting effects, as shown in Figure 3 and Figure 6. This can be explained by the fact that these two amino acids can be interconverted and share the same precursor (α-ketoglutarate) (Figure 5). According to Zhu et al., glutamate can be reduced to proline through the catalytic action of gamma-glutamyl transferase (γ-GK) and pyrroline-5-carboxylate reductase (P5CR), while proline can be oxidized to glutamate through the catalytic action of proline dehydrogenase (PRODH) and delta-1-pyrroline-5-carboxylate dehydrogenase (P5CDH) [37]. Here PRODH and P5CDH formed a bifunctional enzyme which is encoded by the putA gene [38]. This has been confirmed by the successful annotation of the putA gene in the genome of strain DT.1, as well as expression of this gene as revealed in the transcriptomic results.
Cultivation of PNSB under anaerobic light conditions represents the traditional manner of lycopene production [13]. However, industrial scale-up of this process would be restricted by drawbacks such as long fermentation period and insufficient light intensity caused by high cell density. Theoretically, aerobic fermentation would be preferred to overcome this problem. However, photosynthesis genes, including those of carotenoids, are repressed by PpsR, leading to the inhibition of lycopene synthesis under aerobic conditions [39,40]. Recently Sun et al. obtained lycopene of 50.6 mg/g DCW in E. coli by regulating the expression of exogenously introduced genes such as dxs, idi, crtCDF, sucAB, sdhABCD, and talB [41]. Qu et al. succeeded in promoting the lycopene yield by up to 10.32 mg/g DCW under aerobic conditions by knocking out the crtC gene to reduce lycopene conversion, followed by knocking out ppsR to activate the expression of PS genes in C. sphaeroides RL1 [14]. In our study, simply knocking out the crtC gene in the newly isolated C. sphaeroides DT.1 led to an accumulation of lycopene up to 13.48 ± 0.32 mg/g DCW under aerobic dark conditions. Such a discrepancy in lycopene yield may be due to the different properties of the CrtI-type desaturases in these two strains. The crtI4 gene in strain RL1 was introduced externally from Rhodospirillum rubrum and it may be difficult to maintain the optimal catalytic activity in the new host [14], thus inevitably affecting the intracellular biosynthesis of lycopene. In our study, the strain DT.1 contained a native CrtI4, which is conducive to the synthesis of lycopene [42,43]. In particular, lycopene accumulation in strain DT.1 was slightly affected by the variations in rotating speed and filling volume during shake flask fermentation (Figure 7). This crtC mutant of strain DT.1 is characterized by relatively lower oxygen demand and the lack of a need for illumination, making it a potential platform for the mass production of lycopene under aerobic conditions.
5. Conclusions
In this study, a new strain of PNSB DT.1, capable of synthesizing lycopene under aerobic dark conditions, was isolated and identified as Cereibacter sphaeroides. It contained CrtI4, capable of converting phytoene to lycopene via four-step desaturation. We successfully constructed a crtC knockout mutant, which accumulated intracellular lycopene 8.70 folds higher than that of the original strain. Supplementation with glutamate or proline significantly promoted lycopene accumulation in both wildtype and ΔcrtC mutant strains and this promoting effect was achieved mainly by downregulating the expression of the ΔcrtC gene and enhancing the accumulation of lycopene synthesis precursors. This ΔcrtC mutant strain exhibited a distinct advantage, as its lycopene accumulation was slightly affected by variations in rotating speed and filling volume during shake flask fermentation. The optimized fermentation condition for lycopene production was as follows: 25–30 °C, pH 7.0–7.5, 160 rpm, 400 mL liquid volume in 500 mL flasks and 5% inoculation amount. Feasibility for lycopene production was confirmed in a fed-batch fermentation with the highest yield of 151.10 ± 0.13 mg/L lycopene achieved. Relatively lower oxygen demand and the lack of a need for illumination enable the crtC mutant strain to be a potential platform for lycopene production under aerobic conditions.
Author Contributions
Y.Z.: writing—original draft, visualization, formal analysis, investigation. X.M., Z.X. and X.G. (Xiangyu Gu): investigation, review and editing. X.G. (Xiangyang Gu): writing—review and editing, methodology, funding acquisition, conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China [grant number: 2019YFC1906404]; Jiangsu Province’s Science and Technology Innovation Special Project for Carbon Peak and Carbon Neutrality [grant number: BE2022309].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PNSB | Purple nonsulfur bacteria |
| DCW | Dry cell weight |
| MEP pathway | Methylerythritol phosphate pathway |
| POS | Positive ion mode |
| NEG | Negative ion mode |
| MVA pathway | Mevalonate pathway |
| DMAPP | Dimethylallyl diphosphate |
| IPP | Isopentenyl diphosphate |
| GPP | Geranyl diphosphate |
| FPP | Farnesyl diphosphate |
| BChl a | Bacteriochlorophyll a |
| crtE | FPP/GGPP synthase, CrtE |
| crtB | Phytoene synthase, CrtB |
| crtI | Phytoene desaturase, CrtI |
| crtC | Acyclic carotenoid 1,2-hydratase, CrtC |
| crtD | FAD dependent oxidoreductase, CrtD |
| crtF | O-Methyltransferase family 2, CrtF |
| gltB | Glutamine synthetase, GltB |
| gltA | Citrate synthase, GltA |
| fabB | 3-oxoacyl-[acyl-carrier-protein] synthase I, FabB |
| fabF | 3-oxoacyl-[acyl-carrier-protein] synthase II, FabF |
| fabG | 3-oxoacyl-[acyl-carrier protein] reductase, FabG |
| fabI | Enoyl-[acyl-carrier protein] reductase I, FabI |
| fabZ | 3-hydroxyacyl-[acyl-carrier-protein] dehydratase, FabZ |
| putA | Proline utilization A, PutA |
| acnA | Aconitate hydratase, AcnA |
| dxs | 1-deoxy-D-xylulose-5-phosphate synthase, Dxs |
| ispD | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase, IspD |
| PpsR | Transcriptional regulator PpsR |
| PrrA | Transcription factor PrrA |
| Mvd | Pyrophospomevalonate decarboxylase |
| Pmk | Phosphomevalonate kinase |
| P5CR | Pyrroline-5-carboxylate reductase |
| PRODH | Proline dehydrogenase |
| P5CDH | Delta-1-pyrroline-5-carboxylate dehydrogenase |
References
- Zhu, J.; Hu, Q.; Shen, S. Enhanced antitumor efficacy and attenuated cardiotoxicity of doxorubicin in combination with lycopene liposomes. J. Liposome Res. 2020, 30, 37–44. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Guo, S.; Ren, Y.; Li, M.; Wang, J.; Zhang, H.; Gong, G.; Xu, Y. Decreased protein abundance of lycopene β-cyclase contributes to red flesh in domesticated watermelon. Plant Physiol. 2020, 183, 1171–1183. [Google Scholar] [PubMed]
- Pasupuleti, V.; Kulkarni, S.G. Lycopene fortification on the quality characteristics of beverage formulations developed from pink flesh guava (Psidium guajava L.). J. Food Sci. Technol. 2014, 5, 4126–4131. [Google Scholar]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food sources, biological activities, and human health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar]
- Chernyshova, M.P.; Pristenskiy, D.V.; Lozbiakova, M.V.; Chalyk, N.E.; Bandaletova, T.Y.; Petyaev, I.M. Systemic and skin-targeting beneficial effects of lycopene-enriched ice cream: A pilot study. J. Dairy Sci. 2019, 102, 14–25. [Google Scholar] [PubMed]
- Wang, Y.H.; Zhang, R.R.; Yin, Y.; Tan, G.F.; Wang, G.L.; Liu, H.; Zhuang, J.; Zhang, J.; Zhuang, F.Y.; Xiong, A.S. Advances in engineering the production of the natural red pigment lycopene: A systematic review from a biotechnology perspective. J. Adv. Res. 2023, 46, 31–47. [Google Scholar] [PubMed]
- Li, Y.; Cui, Z.; Hu, L. Recent technological strategies for enhancing the stability of lycopene in processing and production. Food Chem. 2023, 30 Pt A, 134799. [Google Scholar]
- Wang, Q.; Chen, Y.; Yang, Q.; Zhao, J.; Feng, L.; Wang, M. SR5AL serves as a key regulatory gene in lycopene biosynthesis by Blakeslea trispora. Microb. Cell Factories 2022, 21, 126. [Google Scholar]
- Xu, J.; Xu, X.; Xu, Q.; Zhang, Z.; Jiang, L.; Huang, H. Efficient production of lycopene by engineered E. coli strains harboring different types of plasmids. Bioprocess Biosyst. Eng. 2018, 41, 489–499. [Google Scholar]
- Su, A.; Chi, S.; Li, Y.; Tan, S.; Qiang, S.; Chen, Z.; Meng, Y. Metabolic redesign of Rhodobacter sphaeroides for lycopene production. J. Agric. Food Chem. 2018, 66, 5879–5885. [Google Scholar]
- Wang, G.S.; Grammel, H.; Abou-Aisha, K.; Sägesser, R.; Ghosh, R. High-level production of the industrial product lycopene by the photosynthetic bacterium Rhodospirillum rubrum. Appl. Environ. Microbiol. 2012, 78, 7205–7215. [Google Scholar] [CrossRef] [PubMed]
- Tuly, J.A.; Zabed, H.M.; Nizami, A.S.; Mehedi Hassan, M.; Roknul Azam, S.M.; Kumar Awasthi, M.; Janet, Q.; Chen, G.; Dzidzorgbe Kwaku Akpabli-Tsigbe, N.; Ma, H. Bioconversion of agro-food industrial wastes into value-added peptides by a Bacillus sp. Mutant through solid-state fermentation. Bioresour. Technol. 2022, 346, 126513. [Google Scholar] [CrossRef]
- Li, M.; Xia, Q.; Lv, S.; Tong, J.; Wang, Z.; Nie, Q.; Yang, J. Enhanced CO2 capture for photosynthetic lycopene production in engineered Rhodopseudomonas palustris, a purple non-sulfur bacterium. Green Chem. 2022, 24, 7500–7518. [Google Scholar] [CrossRef]
- Qu, Y.; Su, A.; Li, Y.; Meng, Y.; Chen, Z. Manipulation of the regulatory genes ppsR and prrA in Rhodobacter sphaeroides enhances lycopene production. J. Agric. Food Chem. 2021, 69, 4134–4143. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, M.R.; Avalos, J.; Luisa Bonet, M.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Swofford, C.A.; Sinskey, A.J. Modular engineering for microbial production of carotenoids. Metab. Eng. Commun. 2019, 15, e00118. [Google Scholar] [CrossRef]
- Ye, V.M.; Bhatia, S.K. Pathway engineering strategies for production of beneficial carotenoids in microbial hosts. Biotechnol. Lett. 2012, 34, 1405–1414. [Google Scholar] [CrossRef]
- Iwasaka, H.; Koyanagi, R.; Satoh, R.; Nagano, A.; Watanabe, K.; Hisata, K.; Satoh, N.; Aki, T. A possible trifunctional β-carotene synthase gene identified in the draft genome of Aurantiochytrium sp. Strain KH105. Genes 2018, 9, 200. [Google Scholar] [CrossRef]
- Shimizu, T.; Teramoto, H.; Inui, M. Introduction of glyoxylate bypass increases hydrogen gas yield from acetate and l-glutamate in Rhodobacter sphaeroides. Appl. Environ. Microbiol. 2019, 85, e01873-18. [Google Scholar] [CrossRef]
- Koga, A.; Yamasaki, T.; Hayashi, S.; Yamamoto, S.; Miyasaka, H. Isolation of purple nonsulfur bacteria from the digestive tract of ayu (Plecoglossus altivelis). Biosci. Biotechnol. Biochem. 2022, 24, 407–412. [Google Scholar] [CrossRef]
- Caetano-Lopes, J.; Rodrigues, A.; Lopes, A.; Vale, A.C.; Pitts-Kiefer, M.A.; Vidal, B.; Perpétuo, I.P.; Monteiro, J.; Konttinen, Y.T.; Vaz, M.F.; et al. Rheumatoid arthritis bone fragility is associated with upregulation of il17 and dkk1 gene expression. Clin. Rev. Allergy Immunol. 2014, 47, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A. Enhanced lycopene extraction from tomato peels by optimized mixed-polarity solvent mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, J.; Jiang, W.; Li, Y.; Zhang, L.; Ding, Z.; Gu, Z.; Shi, G.; Xu, S. Construction of a highly efficient synthetic lycopene engineered Saccharomyces cerevisiae. Sheng Wu Gong Cheng Xue Bao 2020, 36, 1334–1345. [Google Scholar]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Garrity, G.M. List of new names and new combinations previously effectively, but not validly, published. Int. J. Syst. Evol. Microbiol. 2020, 70, 2960–2966. [Google Scholar]
- Shi, B.; Ma, T.; Ye, Z.; Li, X.; Huang, Y.; Zhou, Z.; Ding, Y.; Deng, Z.; Liu, T. Systematic metabolic engineering of Saccharomyces cerevisiae for lycopene overproduction. J. Agric. Food Chem. 2019, 67, 11148–11157. [Google Scholar]
- Hunter, C.N.; Hundle, B.S.; Hearst, J.E.; Lang, H.P.; Gardiner, A.T.; Takaichi, S.; Cogdell, R.J. Introduction of new carotenoids into the bacterial photosynthetic apparatus by combining the carotenoid biosynthetic pathways of Erwinia herbicola and Rhodobacter sphaeroides. J. Bacteriol. 1994, 176, 3692–3697. [Google Scholar]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumor cells with defective mitochondria. Nature 2011, 481, 385–388. [Google Scholar]
- Fournié, M.; Truan, G. Multiplicity of carotene patterns derives from competition between phytoene desaturase diversification and biological environments. Sci. Rep. 2020, 10, 21106. [Google Scholar]
- Steiger, S.; Astier, C.; Sandmann, G. Substrate specificity of the expressed carotenoid 3,4-desaturase from Rubrivivax gelatinosus reveals the detailed reaction sequence to spheroidene and spirilloxanthin. Biochem. J. 2000, 349, 635–640. [Google Scholar] [PubMed]
- Choi, B.H.; Kim, S.H.; Lee, P.C. Hot spots of phytoene desaturase from Rhodobacter sphaeroides influencing the desaturation of phytoene. Catalysts 2021, 11, 1248. [Google Scholar] [CrossRef]
- Choi, S.; Seo, B.; Lim, K.; Nam, S.W.; Kim, G. Molecular cloning and overexpression of phytoene desaturase (CrtI) from Paracoccus haeundaensis. Microbiol. Biotechnol. Lett. 2018. 46, 145–153.
- Schaub, P.; Yu, Q.; Gemmecker, S.; Poussin-Courmontagne, P.; Mailliot, J.; McEwen, A.G.; Ghisla, S.; Al-Babili, S.; Cavarelli, J.; Beyer, P. On the structure and function of the phytoene desaturase CrtI from Pantoea ananatis, a membrane-peripheral and FAD-dependent oxidase/isomerase. PLoS ONE 2012, 7, e39550. [Google Scholar]
- Zhang, J.; Lu, L.; Yin, L.; Xie, S.; Xiao, M. Carotenogenesis gene cluster and phytoene desaturase catalyzing both three- and four-step desaturations from Rhodobacter azotoformans. FEMS Microbiol. Lett. 2012, 333, 138–145. [Google Scholar]
- Wang, L.; Song, L.; He, X.; Teng, F.; Hu, M.; Tao, Y. Production of isofloridoside from galactose and glycerol using α-galactosidase from Alicyclobacillus hesperidum. Enzym. Microb. Technol. 2020, 134, 109480. [Google Scholar]
- Zhu, J.; Schwörer, S.; Berisa, M.; Kyung, Y.J.; Ryu, K.W.; Yi, J.; Jiang, X.; Cross, J.R.; Thompson, C.B. Mitochondrial NADP(H) generation is essential for proline biosynthesis. Science 2021, 372, 968–972. [Google Scholar] [PubMed]
- Ye, P.; Li, X.; Cui, B.; Song, S.; Shen, F.; Chen, X.; Wang, G.; Zhou, X.; Deng, Y. Proline utilization A controls bacterial pathogenicity by sensing its substrate and cofactors. Commun. Biol. 2022, 5, 496. [Google Scholar]
- Imam, S.; Noguera, D.R.; Donohue, T.J. Global analysis of photosynthesis transcriptional regulatory networks. PLoS Genet. 2014, 10, e1004837. [Google Scholar]
- Imam, S.; Noguera, D.R.; Donohue, T.J. An integrated approach to reconstructing genome-scale transcriptional regulatory networks. PLoS Comput. Biol. 2015, 11, e1004103. [Google Scholar]
- Sun, T.; Miao, L.; Li, Q.; Dai, G.; Lu, F.; Liu, T. Production of lycopene by metabolically-engineered Escherichia coli. Biotechnol. Lett. 2014, 36, 1515–1522. [Google Scholar] [PubMed]
- Xu, Y.; Liu, K.; Han, Y.; Xing, Y.; Zhang, Y.; Yang, Q.; Zhou, M. Codon usage bias regulates gene expression and protein conformation in yeast expression system P. pastoris. Microb. Cell Factories 2021, 20, 91. [Google Scholar]
- Keasling, J.D. Manufacturing molecules through metabolic engineering. Science 2010, 330, 1355–1358. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).