Effects of Fermentation Temperature on the Physicochemical Properties, Bioactive Compounds, and In Vitro Digestive Profile of Cacao (Theobroma cacao) Seeds
Abstract
1. Introduction
2. Materials and Methods
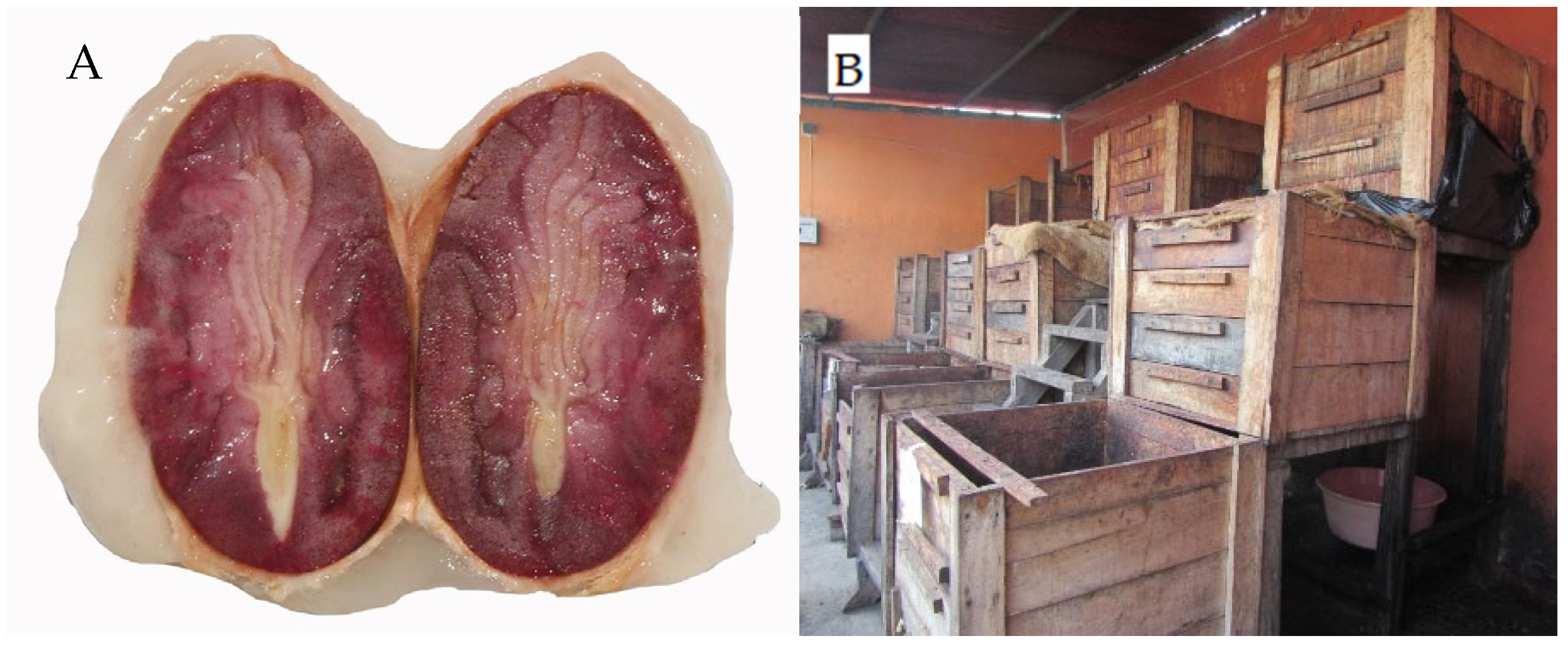
2.1. Sample Collection and Inoculation Process
2.2. Fermentation Profiles
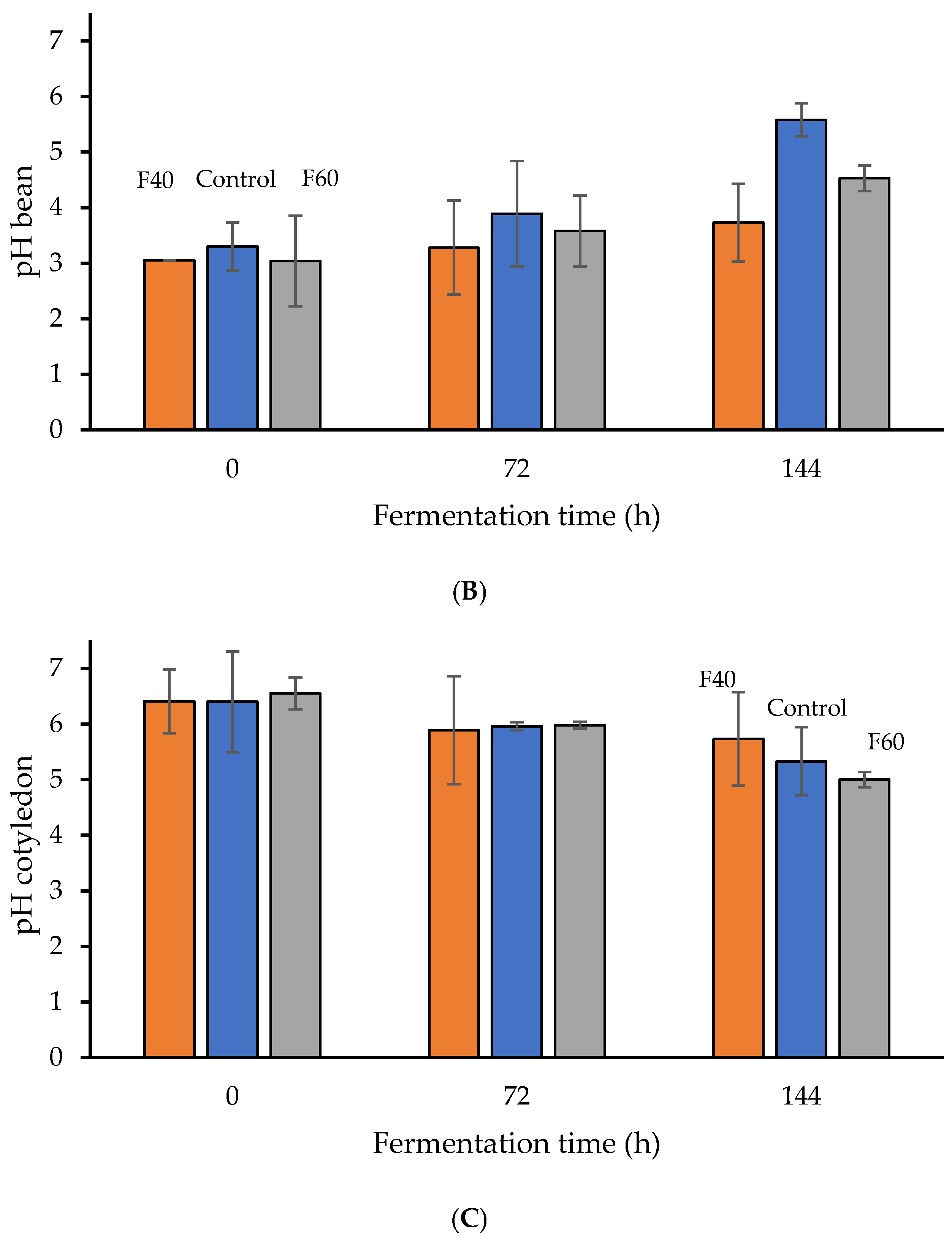
2.3. pH of Pulp and Cotyledons
2.4. Chemical Analysis
2.5. Free Sugars
2.6. Fatty Acid Determination
2.7. Free Amino Nitrogen (FAN)
2.8. Total Phenolic Compounds
2.9. Antioxidant Activity
2.10. Enzyme Activity
2.11. Seed Structural Analysis
2.12. In Vitro Available Carbohydrate Digestibility
2.13. In Vitro Protein Digestibility
2.14. Statistical Analysis
3. Results and Discussion
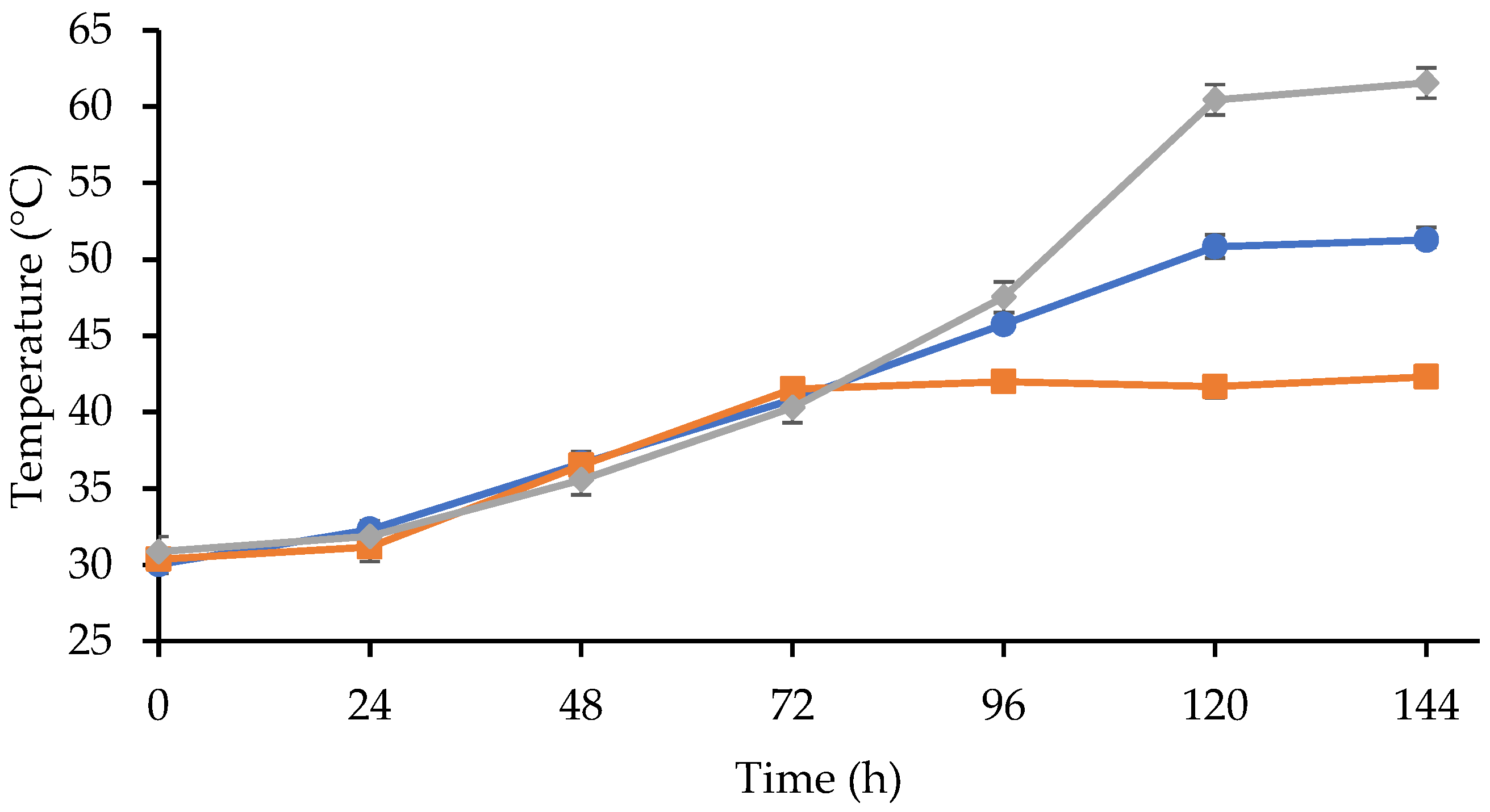
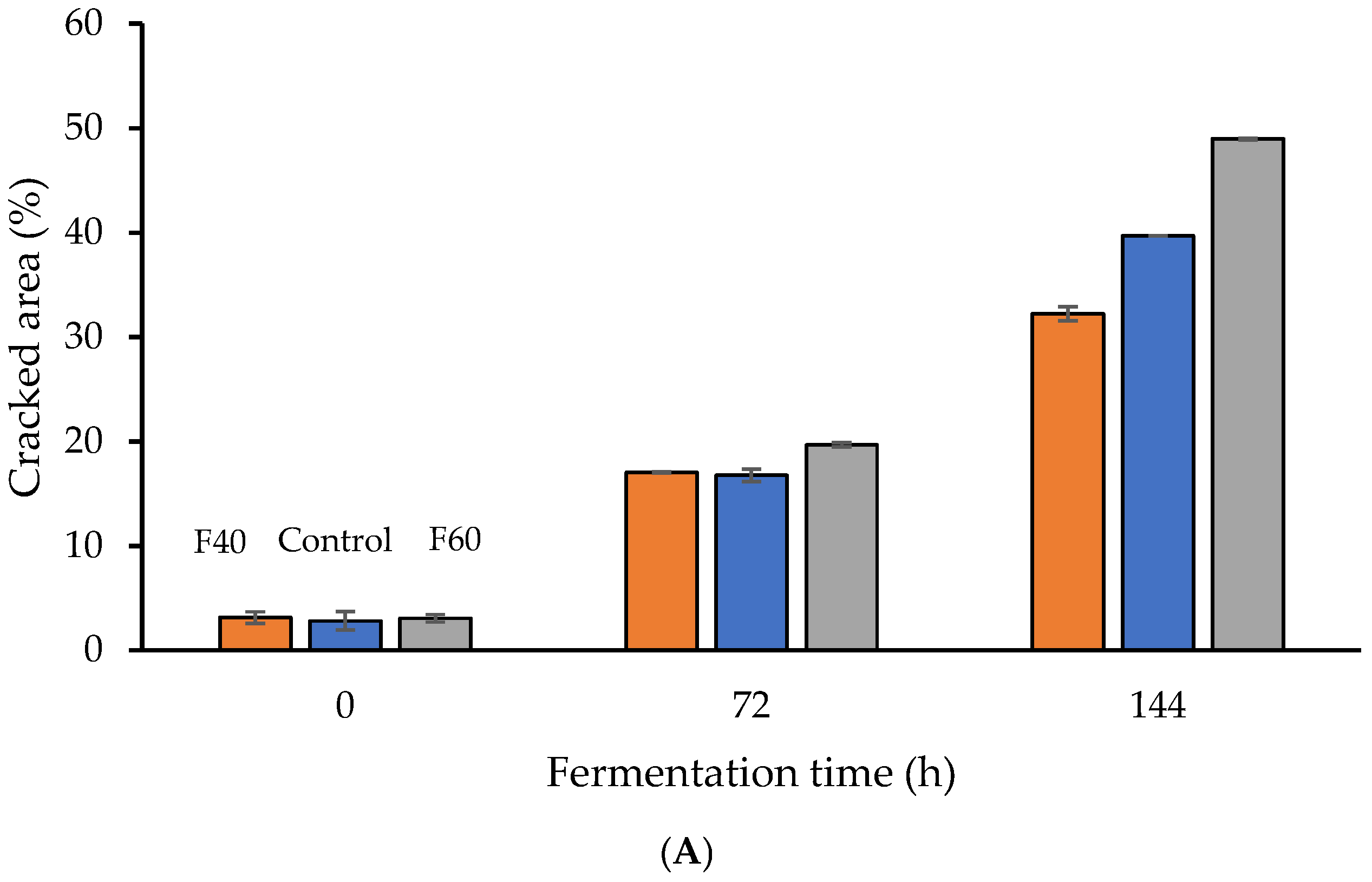
3.1. Effect of Fermentation Dynamics on Seed Characteristics
3.2. Cacao Seed Proximal Analysis
3.3. Fatty Acid Determination
3.4. Free Amino Nitrogen (FAN), Phenolics, and Antioxidants
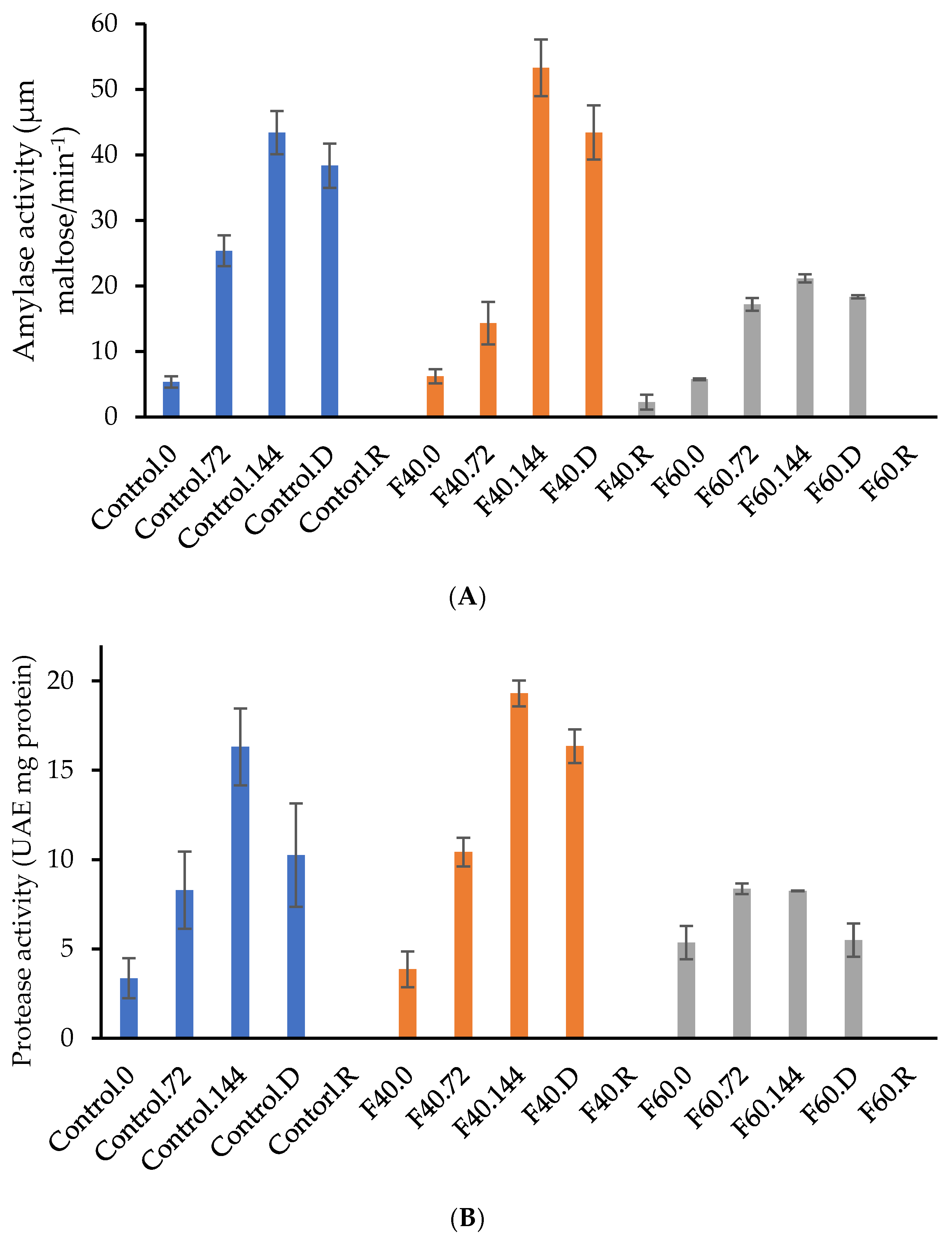
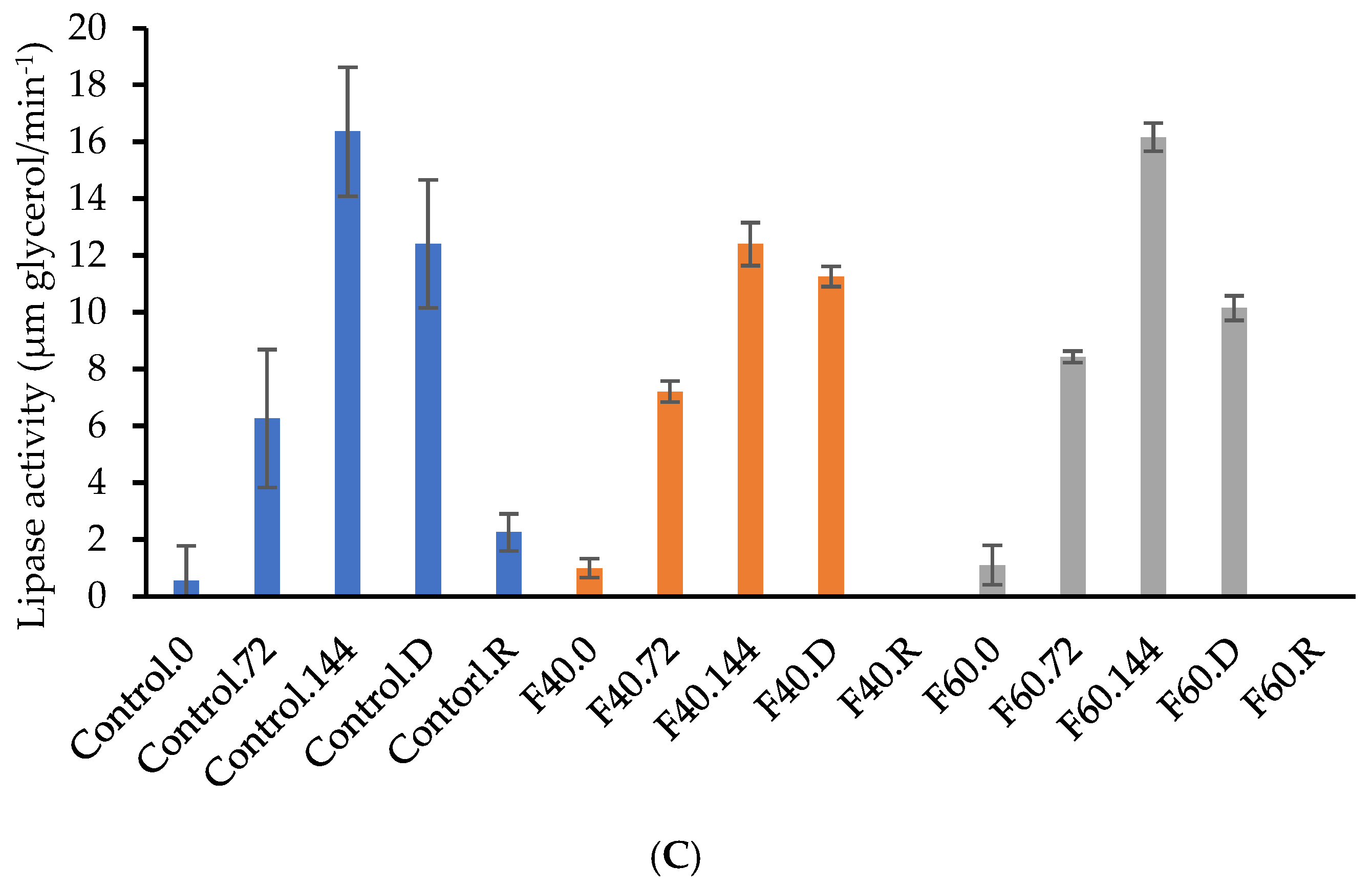
3.5. Enzymatic Activity
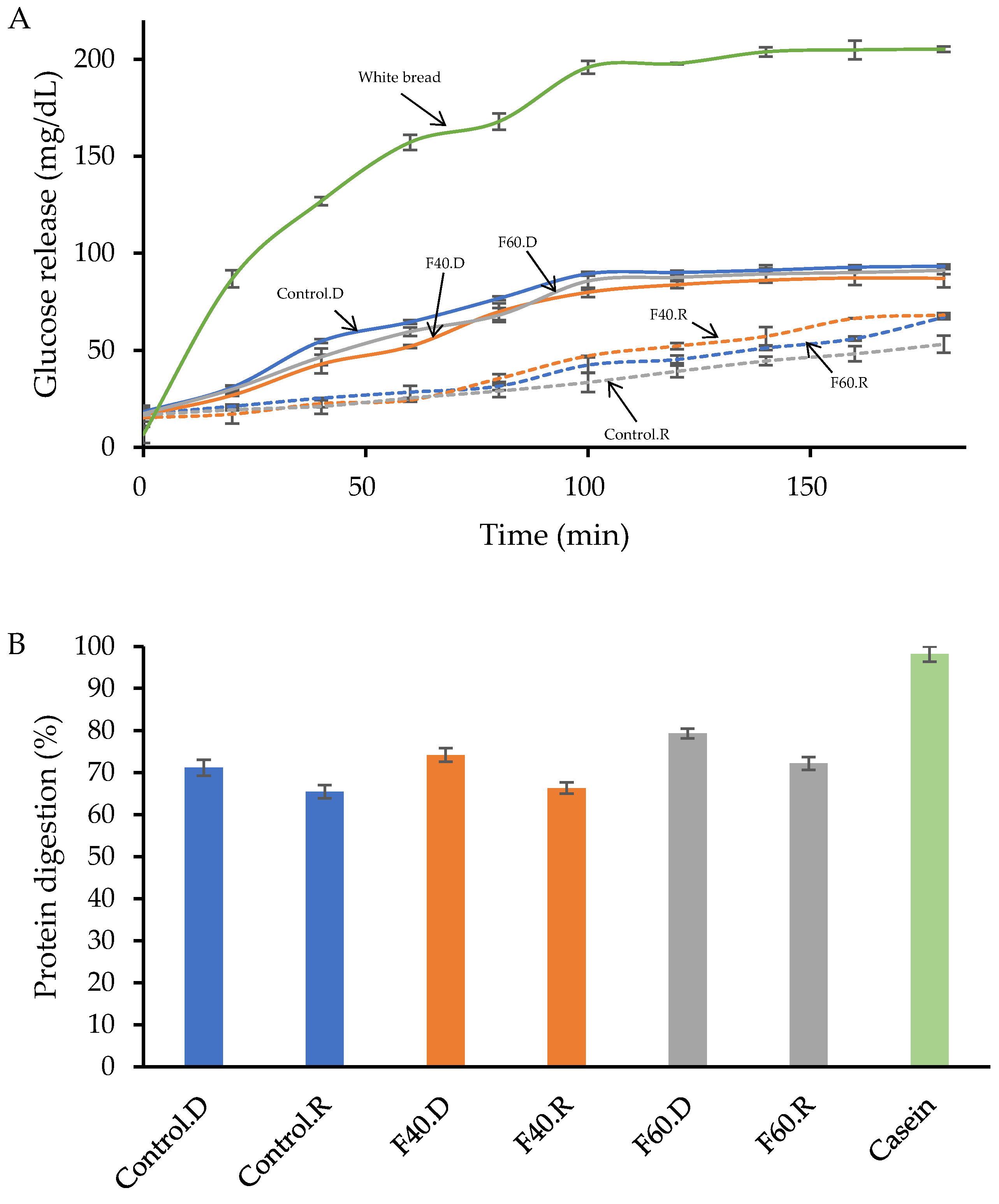
3.6. In Vitro Carbohydrate and Protein Digestion
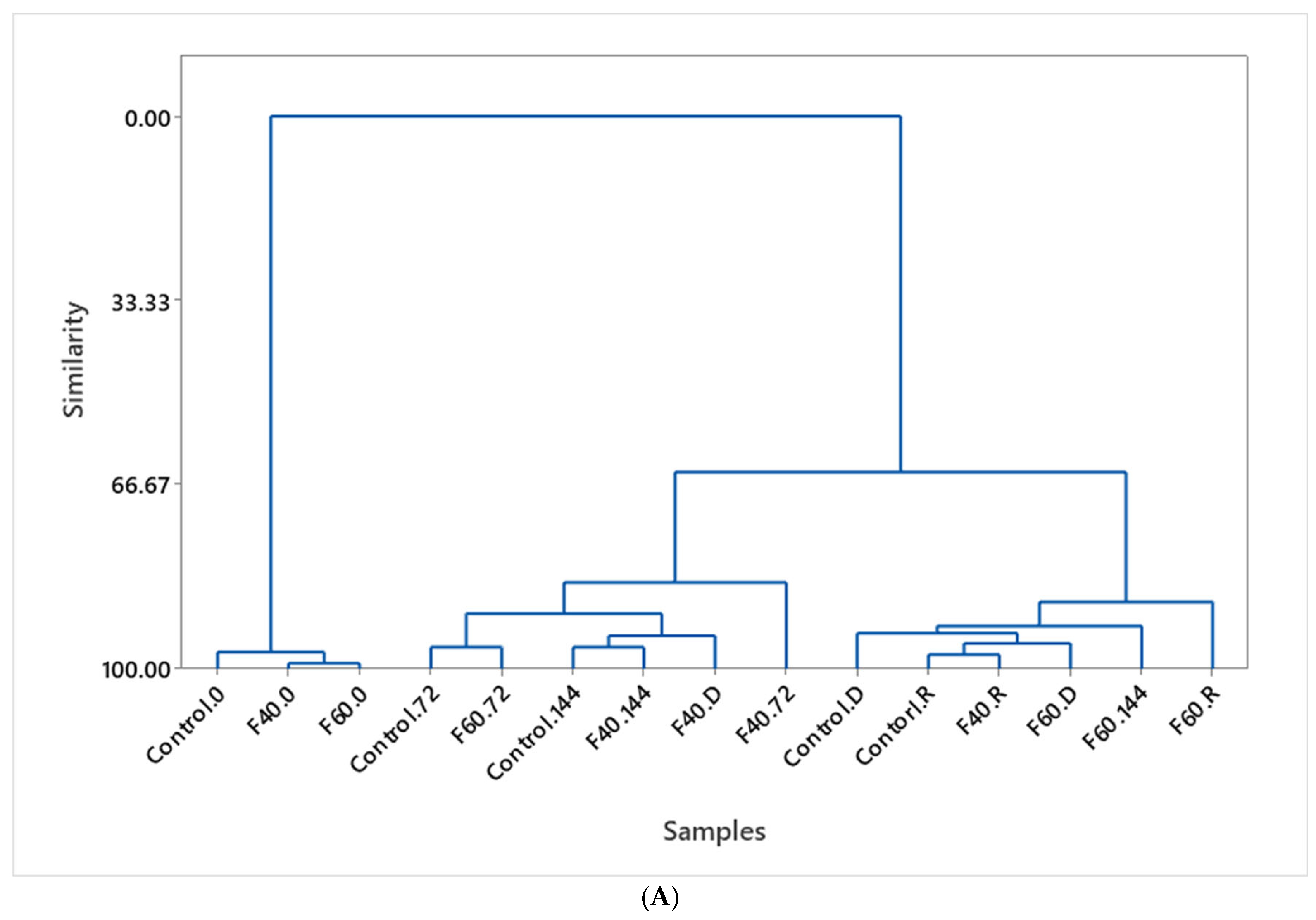
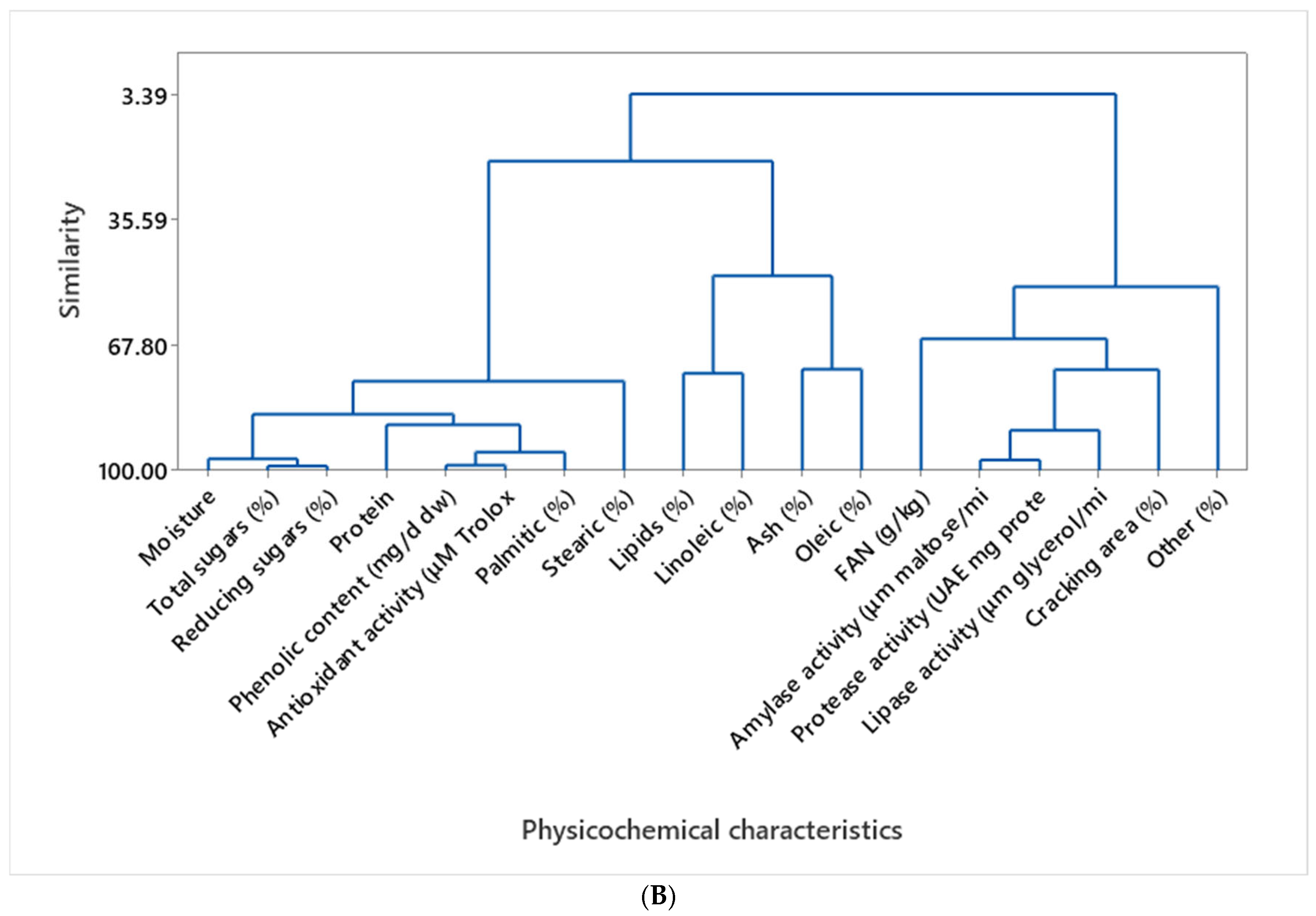
3.7. Data Processing by Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cruz, J.F.M.; Leite, P.B.; Soares, S.E.; Bispo, E.S. Bioactive compounds in different cocoa (Theobroma cacao, L) cultivars during fermentation. Food Sci. Technol. 2015, 35, 279–284. [Google Scholar] [CrossRef]
- Kumari, N.; Grimbs, A.; D’Souza, R.N.; Verma, S.K.; Corno, M.; Kuhnert, N.; Ullrich, M.S. Origin and varietal based proteomic and peptidomic fingerprinting of Theobroma cacao in non-fermented and fermented cocoa beans. Food Res. Int. 2018, 111, 137–147. [Google Scholar] [CrossRef] [PubMed]
- John, W.A.; Böttcher, N.L.; Aßkamp, M.; Bergounhou, A.; Kumari, N.; Ho, P.-W.; D’Souza, R.N.; Nevoigt, E.; Ullrich, M.S. Forcing fermentation: Profiling proteins, peptides and polyphenols in lab-scale cocoa bean fermentation. Food Chem. 2019, 278, 786–794. [Google Scholar] [CrossRef]
- Kumari, N.; Kofi, K.J.; Grimbs, S.; D’Souza, R.N.; Kuhnert, N.; Vrancken, G.; Ullrich, M.S. Biochemical fate of vicilin storage protein during fermentation and drying of cocoa beans. Food Res. Int. 2016, 90, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kotey, R.N.; Odoom, D.A.; Kumah, P.; Akowuah, J.O.; Donkor, E.F.; Quartey, E.K.; Sam, E.K.; Owusu-Kwarteng, J.; Santo, K.G.; Kwami-Adala, F.; et al. Effects of Fermentation Periods and Drying Methods on Postharvest Quality of Cocoa (Theobroma Cacao) Beans in Ghana. J. Food Qual. 2022, 2022, 7871543. [Google Scholar] [CrossRef]
- Meersman, E.; Struyf, N.; Kyomugasho, C.; Kermani, Z.J.; Santiago, J.S.; Baert, E.; Hemdane, S.; Vrancken, G.; Verstrepen, K.J.; Courtin, C.M.; et al. Characterization and Degradation of Pectic Polysaccharides in Cocoa Pulp. J. Agric. Food Chem. 2017, 65, 9726–9734. [Google Scholar] [CrossRef]
- Wahyuni, S.; Susilowati, P.; Tamrin; Holilah; Asranudin; Utomo, W.; Dueris, Y.; Samarindu, L. Characterization and application of polygalacturonase from trinitario (Theobroma cacao L.) pulp. Asian J. Chem. 2016, 28, 2261–2266. [Google Scholar] [CrossRef]
- Moretti, L.K.; Ramos, K.K.; Ávila, P.F.; Goldbeck, R.; Vieira, J.B.; Efraim, P. Influence of cocoa varieties on carbohydrate composition and enzymatic activity of cocoa pulp. Food Res. Int. 2023, 173, 113393. [Google Scholar] [CrossRef]
- García-Ríos, E.; Lairón-Peris, M.; Muñiz-Calvo, S.; Heras, J.M.; Ortiz-Julien, A.; Poirot, P.; Rozès, N.; Querol, A.; Guillamón, J.M. Thermo-adaptive evolution to generate improved Saccharomyces cerevisiae strains for cocoa pulp fermentations. Int. J. Food Microbiol. 2021, 342, 109077. [Google Scholar] [CrossRef]
- Chetschik, I.; Kneubühl, M.; Chatelain, K.; Schlüter, A.; Bernath, K.; Hühn, T. Investigations on the Aroma of Cocoa Pulp (Theobroma cacao L.) and Its Influence on the Odor of Fermented Cocoa Beans. J. Agric. Food Chem. 2018, 66, 2467–2472. [Google Scholar] [CrossRef]
- Besançon, L.; Poirot, P.; Lebrun, M.; Ortiz-Julien, A.; Boulanger, R. Investigating Key Volatile Compound Diffusion in Cocoa Beans during Yeast Fermentation-like Incubation. J. Agric. Food Chem. 2024, 72, 15788–15800. [Google Scholar] [CrossRef] [PubMed]
- Niemenak, N.; Evina, V.J.E.; Mouafi, A.S.D.; Ngouambe, A.G.T.; Bernhardt, C.; Lieberei, R.; Bisping, B. Assessment of the profile of free amino acids and reducing sugars of cacao beans from local Cameroonian Trinitario (SNK varieties) and Forastero (TIKO varieties) using fermentation-like incubation. J. Appl. Bot. Food Qual. 2020, 93, 321–329. [Google Scholar] [CrossRef]
- Melo, T.S.; Pires, T.C.; Engelmann, J.V.P.; Monteiro, A.L.O.; Maciel, L.F.; Bispo, E.S. Evaluation of the content of bioactive compounds in cocoa beans during the fermentation process. J. Food Sci. Technol. 2021, 58, 1947–1957. [Google Scholar] [CrossRef]
- de Nazaré Melo Ramos, S.; Glezer, J.W.; de Oliveira Garcia, A.; Behrens, J.H.; Efraim, P. Cupuassu from bean to bar: Sensory and hedonic characterization of a chocolate-like product. Food Res. Int. 2022, 155, 111039. [Google Scholar] [CrossRef] [PubMed]
- Mougang, N.N.; Tene, S.T.; Zokou, R.; Kohole, H.A.F.; Solefack, E.N.; Mboukap, A.N.; Abaoabo, A.A.F.; Womeni, H.M. Influence of fermentation time, drying time and temperature on cocoa pods (Theobroma cacao L.) marketability. Appl. Food Res. 2024, 4, 100460. [Google Scholar] [CrossRef]
- Mohamed, R.; Abdullah, A.; Yap, K.C.; Mustapha, W.A.W. Comparative study of flavor precursors, volatile compounds and sensory between Malaysian and Ghanaian cocoa beans. Sains Malays. 2019, 48, 589–598. [Google Scholar] [CrossRef]
- Kadow, D.; Niemenak, N.; Rohn, S.; Lieberei, R. Fermentation-like incubation of cocoa seeds (Theobroma cacao L.)—Reconstruction and guidance of the fermentation process. LWT 2015, 62, 357–361. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) Official Methods of Analysis of AOAC International, 22nd ed.; AOAC Publications: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Schmidt, Š. Lupin composition and possible use in bakery—A review. Czech J. Food Sci. 2011, 29, 203–211. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Moreira, A.V.B.; Mancini Filho, J. Influência dos compostos fenólicos de especiarias sobre a lipoperoxidação eo perfil lipídico de tecidos de ratos. Rev. Nutr. 2004, 17, 411. [Google Scholar] [CrossRef]
- Rodríguez, Y.B.; Aguilar, I.G.; de Almeida e Silva, J. Estudio del desarrollo del nitrógeno amino libre durante el malteado del sorgo. Cent. Azúcar 1974, 44, 39–47. [Google Scholar]
- Granfeldt, Y.; Björck, I.; Drews, A.; Tovar, J. An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Eur. J. Clin. Nutr. 1992, 46, 649–660. [Google Scholar] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A. A Multienzyme Technique for Estimating Protein Digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Ouattara, H.G.; Reverchon, S.; Niamke, S.L.; Nasser, W. Regulation of the synthesis of pulp degrading enzymes in Bacillus isolated from cocoa fermentation. Food Microbiol. 2017, 63, 255–262. [Google Scholar] [CrossRef]
- Da Silva Oliveira Nunes, C.; De Carvalho, G.B.M.; Da Silva, M.L.C.; Da Silva, G.P.; Machado, B.A.S.; Uetanabaro, A.P.T. Cocoa pulp in beer production: Applicability and fermentative process performance. PLoS ONE 2017, 12, 0175677. [Google Scholar] [CrossRef]
- Balcázar-Zumaeta, C.R.; Fernández-Romero, E.; Lopes, A.S.; Ferreira, N.R.; Chagas-Júnior, G.C.A.; Yoplac, I.; López-Trigoso, H.A.; Tuesta-Occ, M.L.; Maldonado-Ramirez, I.; Maicelo-Quintana, J.L.; et al. Amino acid profile behavior during the fermentation of Criollo cocoa beans. Food Chem. X 2024, 22, 101486. [Google Scholar] [CrossRef]
- Tchouatcheu, G.A.N.; Noah, A.M.; Lieberei, R.; Niemenak, N. Effect of cacao bean quality grade on cacao quality evaluation by cut test and correlations with free amino acids and polyphenols profiles. J. Food Sci. Technol. 2019, 56, 2621–2627. [Google Scholar] [CrossRef]
- Hinneh, M.; Semanhyia, E.; Van de Walle, D.; De Winne, A.; Tzompa-Sosa, D.A.; Scalone, G.L.L.; De Meulenaer, B.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; et al. Assessing the influence of pod storage on sugar and free amino acid profiles and the implications on some Maillard reaction related flavor volatiles in Forastero cocoa beans. Food Res. Int. 2018, 111, 607–620. [Google Scholar] [CrossRef]
- Chang, H.; Gu, C.; Wang, M.; Chen, J.; Yue, M.; Zhou, J.; Chang, Z.; Zhang, C.; Liu, F.; Feng, Z. Screening and characterizing indigenous yeasts, lactic acid bacteria, and acetic acid bacteria from cocoa fermentation in Hainan for aroma Development. J. Food Sci. 2025, 90, e17612. [Google Scholar] [CrossRef]
- Mota-Gutierrez, J.; Botta, C.; Ferrocino, I.; Giordano, M.; Bertolino, M.; Dolci, P.; Cannoni, M.; Cocolin, L. Dynamics and biodiversity of bacterial and yeast communities during fermentation of cocoa beans. Appl. Environ. Microbiol. 2018, 84, e01164-18. [Google Scholar] [CrossRef]
- Sofeo, N.; Toi, M.G.; Ee, E.Q.G.; Ng, J.Y.; Busran, C.T.; Lukito, B.R.; Thong, A.; Hermansen, C.; Peterson, E.C.; Glitsos, R.; et al. Sustainable production of lipids from cocoa fatty acid distillate fermentation driven by adaptive evolution in Yarrowia lipolytica. Bioresour. Technol. 2024, 394, 130302. [Google Scholar] [CrossRef] [PubMed]
- Arbter, P.; Sinha, A.; Troesch, J.; Utesch, T.; Zeng, A.-P. Redox governed electro-fermentation improves lipid production by the oleaginous yeast Rhodosporidium toruloides. Bioresour. Technol. 2019, 294, 122122. [Google Scholar] [CrossRef] [PubMed]
- Brückel, K.; Stark, T.D.; Dawid, C.; Hofmann, T. Molecular Changes during Germination of Cocoa Beans, Part 1. J. Agric. Food Chem. 2024, 72, 18606–18618. [Google Scholar] [CrossRef] [PubMed]
- Megías-Pérez, R.; Ruiz-Matute, A.I.; Corno, M.; Kuhnert, N. Analysis of minor low molecular weight carbohydrates in cocoa beans by chromatographic techniques coupled to mass spectrometry. J. Chromatogr. A 2019, 1584, 135–143. [Google Scholar] [CrossRef]
- Afifah, E.N.; Sari, I.A.; Susilo, A.W.; Malik, A.; Fukusaki, E.; Putri, S.P. Characterization of fine-flavor cocoa in parent-hybrid combinations using metabolomics approach. Food Chem. X 2024, 24, 101832. [Google Scholar] [CrossRef]
- Meersman, E.; Steensels, J.; Struyf, N.; Paulus, T.; Saels, V.; Mathawan, M.; Allegaert, L.; Vrancken, G.; Verstrepen, K.J. Tuning chocolate flavor through development of thermotolerant Saccharomyces cerevisiae starter cultures with increased acetate ester production. Appl. Environ. Microbiol. 2016, 82, 732–746. [Google Scholar] [CrossRef]
- Lessa, O.A.; Reis, N.d.S.; Leite, S.G.F.; Gutarra, M.L.E.; Souza, A.O.; Gualberto, S.A.; de Oliveira, J.R.; Aguiar-Oliveira, E.; Franco, M. Effect of the solid state fermentation of cocoa shell on the secondary metabolites, antioxidant activity, and fatty acids. Food Sci. Biotechnol. 2018, 27, 107–113. [Google Scholar] [CrossRef]
- Cevallos-Cevallos, J.M.; Gysel, L.; Maridueña-Zavala, M.G.; Molina-Miranda, M.J. Time-Related Changes in Volatile Compounds during Fermentation of Bulk and Fine-Flavor Cocoa (Theobroma cacao) Beans. J. Food Qual. 2018, 2018, 1758381. [Google Scholar] [CrossRef]
- Caporaso, N.; Whitworth, M.B.; Fowler, M.S.; Fisk, I.D. Hyperspectral imaging for non-destructive prediction of fermentation index, polyphenol content and antioxidant activity in single cocoa beans. Food Chem. 2018, 258, 343–351. [Google Scholar] [CrossRef]
- Cañas, S.; Tosi, N.; Núñez-Gómez, V.; Del Rio, D.; Mena, P.; Aguilera, Y.; Martín-Cabrejas, M.A. Transformations of phenolic compounds in cocoa shell during in vitro colonic fermentation. Curr. Res. Food Sci. 2024, 9, 100930. [Google Scholar] [CrossRef]
- Brito, B.D.N.D.C.; Chisté, R.C.; da Silva Pena, R.; Gloria, M.B.A.; Lopes, A.S. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.K.S.; Barros, R.G.C.; Pereira, U.C.; Gualberto, N.C.; de Oliveira, C.S.; Shanmugam, S.; Narain, N. α-Amylase inhibition, cytotoxicity and influence of the in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds in the peel and seed of Theobroma grandiflorum. Food Chem. 2022, 373, 131494. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Zhou, W.; Shen, H.; Yang, Z.; Wang, G.; Zuo, Z.; Hou, Y.; Zhao, Z.K. Co-fermentation of acetate and sugars facilitating microbial lipid production on acetate-rich biomass hydrolysates. Bioresour. Technol. 2016, 207, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.M.; Khoo, W.; Ye, L.; Lambert, J.D.; Okeefe, S.F.; Neilson, A.P. Loss of Native Flavanols during Fermentation and Roasting Does Not Necessarily Reduce Digestive Enzyme-Inhibiting Bioactivities of Cocoa. J. Agric. Food Chem. 2016, 64, 3616–3625. [Google Scholar] [CrossRef]

| Sample | Moisture (%) | Protein (%) | Lipids (%) | Carbohydrates (%) | Free Sugars (%) | Ash (%) |
|---|---|---|---|---|---|---|
| Control.0 | 65.23 ± 0.69 a | 17.23 ± 0.36 a | 53.23 ± 0.59 a | 24.21 ± 0.64 a | 20.11 ± 0.62 a | 5.33 ± 0.56 a |
| Control.72 | 63.45 ± 0.05 a | 16.52 ± 0.96 a | 54.21 ± 0.07 a | 23.81 ± 0.85 a | 19.82 ± 0.58 a | 5.46 ± 0.06 a |
| Control.144 | 62.73 ± 0.78 a | 16.56 ± 0.48 a | 53.98 ± 0.43 a | 24.25 ± 0.00 a | 15.33 ± 0.23 c | 5.21 ± 0.32 a |
| Control.D | 10.51 ± 0.30 b | 15.36 ± 0.16 a | 53.41 ± 0.85 a | 25.57 ± 0.32 a | 11.43 ± 0.70 dd | 5.66 ± 0.90 a |
| Control.R | 6.23 ± 0.84 c | 15.55 ± 0.93 a | 54.26 ± 0.67 a | 24.41 ± 0.47 a | 9.86 ± 1.00 d | 5.78 ± 0.42 a |
| F40.0 | 65.57 ± 0.76 a | 16.54 ± 0.01 a | 50.57 ± 0.72 c | 23.24 ± 0.66 a | 18.10 ± 0.32 b | 5.12 ± 0.40 a |
| F40.72 | 64.61 ± 0.36 a | 15.52 ± 0.30 a | 50.96 ± 0.18 c | 23.91 ± 0.28 a | 18.23 ± 0.13 b | 5.24 ± 0.43 a |
| F40.144 | 63.97 ± 0.47 a | 15.56 ± 0.42 a | 48.04 ± 0.41 d | 23.04 ± 0.07 a | 14.72 ± 0.64 c | 5.00 ± 0.81 a |
| F40.D | 9.18 ± 0.67 b | 14.28 ± 0.32 b | 50.74 ± 0.31 c | 23.27 ± 0.66 a | 10.52 ± 0.31 | 5.43 ± 0.86 a |
| F40.R | 5.68 ± 0.28 c | 14.13 ± 0.28 b | 51.55 ± 0.03 b | 22.72 ± 0.47 a | 9.27 ± 0.31 d | 5.49 ± 0.14 a |
| F60.0 | 64.27 ± 0.54 a | 17.23 ± 0.08 a | 54.23 ± 0.37 ba | 23.00 ± 0.17 a | 19.51 ± 0.04 a | 5.43 ± 0.09 a |
| F60.72 | 62.71 ± 0.74 a | 15.03 ± 0.34 a | 54.31 ± 0.31 a | 23.10 ± 0.23 a | 19.23 ± 0.71 a | 4.31 ± 0.11 a |
| F60.144 | 62.21 ± 0.55 a | 14.57 ± 0.12 b | 54.21 ± 0.89 a | 23.52 ± 0.92 a | 14.56 ± 0.17 c | 3.39 ± 0.20 b |
| F60.D | 10.09 ± 0.32 b | 14.28 ± 0.72 b | 52.14 ± 0.22 b | 22.80 ± 0.73 a | 11.09 ± 0.89 d | 3.55 ± 0.34 b |
| F60.R | 6.04 ± 0.38 c | 14.15 ± 0.49 b | 51.12 ± 0.77 b | 22.68 ± 0.04 a | 9.47 ± 0.81 d | 2.97 ± 0.00 b |
| Sample | Phenolics (mg/g) | Antioxidant Activity (μM Trolox eq) | Cracking Area (%) | FAN (mg/g) |
|---|---|---|---|---|
| Control.0 | 180.00 ± 4.41 a | 795.00 ± 5.00 a | 2.83 ± 0.96 f | 3.36 ± 0.39 c |
| Control.72 | 86.00 ± 3.08 c | 243.00 ± 6.10 c | 16.76 ± 0.40 e | 8.33 ± 0.91 b |
| Control.144 | 42.00 ± 8.38 e | 196.00 ± 1.41 f | 39.7 ± 1.50 b | 11.41 ± 0.38 a |
| Control.D | 33.14 ± 5.33 f | 112.00 ± 4.51 h | ND | 11.26 ± 0.69 a |
| Control.R | 22.32 ± 4.69 g | 85.00 ± 10.26 j | ND | 9.31 ± 0.75 b |
| F40.0 | 181.3 ± 5.35 a | 790.00 ± 10.03 a | 2.74 ± 0.16 f | 3.29 ± 0.64 c |
| F40.72 | 101.12 ± 12.02 b | 291.00 ± 2.68 b | 17.05 ± 0.03 e | 7.58 ± 0.54 b |
| F40.144 | 66.43 ± 7.50 d | 204.00 ± 1.65 e | 32.24 ± 1.14 c | 10.38 ± 0.23 b |
| F40.D | 54.23 ± 6.40 e | 188.00 ± 5.22 g | ND | 10.02 ± 0.05 b |
| F40.R | 33.14 ± 8.76 f | 101.00 ± 4.36 i | ND | 8.38 ± 0.89 b |
| F60.0 | 179.14 ± 7.96 a | 797.00 ± 2.16 a | 2.81 ± 0.33 f | 3.36 ± 0.16 c |
| F60.72 | 84.33 ± 11.20 c | 214.00 ± 9.38 d | 19.67 ± 0.12 d | 10.75 ± 0.40 b |
| F60.144 | 22.41 ± 6.03 g | 91.00 ± 4.85 j | 48.96 ± 0.31 a | 12.50 ± 0.65 a |
| F60.D | 15.21 ± 4.17 g | 77.13 ± 2.60 j | ND | 11.21 ± 0.31 a |
| F60.R | 8.25 ± 1.36 h | 31.14 ± 7.13 k | ND | 11.57 ± 0.85 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillen-Guerrero, K.M.; de la Rosa-Millan, J. Effects of Fermentation Temperature on the Physicochemical Properties, Bioactive Compounds, and In Vitro Digestive Profile of Cacao (Theobroma cacao) Seeds. Fermentation 2025, 11, 167. https://doi.org/10.3390/fermentation11040167
Guillen-Guerrero KM, de la Rosa-Millan J. Effects of Fermentation Temperature on the Physicochemical Properties, Bioactive Compounds, and In Vitro Digestive Profile of Cacao (Theobroma cacao) Seeds. Fermentation. 2025; 11(4):167. https://doi.org/10.3390/fermentation11040167
Chicago/Turabian StyleGuillen-Guerrero, Karla Maria, and Julian de la Rosa-Millan. 2025. "Effects of Fermentation Temperature on the Physicochemical Properties, Bioactive Compounds, and In Vitro Digestive Profile of Cacao (Theobroma cacao) Seeds" Fermentation 11, no. 4: 167. https://doi.org/10.3390/fermentation11040167
APA StyleGuillen-Guerrero, K. M., & de la Rosa-Millan, J. (2025). Effects of Fermentation Temperature on the Physicochemical Properties, Bioactive Compounds, and In Vitro Digestive Profile of Cacao (Theobroma cacao) Seeds. Fermentation, 11(4), 167. https://doi.org/10.3390/fermentation11040167







