Tolerance of Kluyveromyces marxianus Under Acetic Acid-, Isoamyl Alcohol-, Hydrogen Peroxide-, and Ethanol-Induced Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain
2.2. Cytoxicity Assays
2.2.1. MTT Assay
2.2.2. Colony-Forming Unit Counting
2.2.3. Estimating the Inhibitory Concentration 30 (IC30)
2.3. Fermentative Capacity of the Yeast Under Stress Conditions
2.4. Analytical Techniques
2.5. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicity of Stress Agents
3.2. Fermentative Capacity of the Yeast Under Stress Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosaka, T.; Tsuzuno, T.; Nishida, S.; Pattanakittivorakul, S.; Murata, M.; Miyakawa, I.; Lertwattanasakul, N.; Limtong, S.; Yamada, M. Distinct Metabolic Flow in Response to Temperature in Thermotolerant Kluyveromyces marxianus. Appl. Environ. Microbiol. 2022, 88, e02006-21. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.; Cunha, J.T.; Domingues, L. Establishment of Kluyveromyces marxianus as a microbial cell factory for lignocellulosic processes: Production of high value furan derivatives. J. Fungi 2021, 7, 1047. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.C.; Yang, C.Y.; Mathew, D.C.; Huang, C.C. Growth and autolysis of the kefir yeast Kluyveromyces marxianus in lactate culture. Sci. Rep. 2021, 11, 14552. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef]
- Du, C.; Li, Y.; Zong, H.; Yuan, T.; Yuan, W.; Jiang, Y. Production of bioethanol and xylitol from non-detoxified corn cob via a two-stage fermentation strategy. Bioresour. Technol. 2020, 310, 123427. [Google Scholar] [CrossRef]
- Kılmanoğlu, H.; Hoşoğlu, M.İ.; Güneşer, O.; Yüceer, Y.K. Optimization of pretreatment and enzymatic hydrolysis conditions of tomato pomace for production of alcohols and esters by Kluyveromyces marxianus. LWT 2021, 138, 110728. [Google Scholar] [CrossRef]
- Su, M.; Hu, Y.; Cui, Y.; Wang, Y.; Yu, H.; Liu, J.; Dai, W.; Piao, C. Production of β-glucosidase from okara fermentation using Kluyveromyces marxianus. J. Food Sci. Technol. 2021, 58, 366–376. [Google Scholar] [CrossRef]
- González-Orozco, B.D.; Kosmerl, E.; Jiménez-Flores, R.; Alvarez, V.B. Enhanced probiotic potential of Lactobacillus kefiranofaciens OSU-BDGOA1 through co-culture with Kluyveromyces marxianus bdgo-ym6. Front. Microbiol. 2023, 14, 1236634. [Google Scholar] [CrossRef]
- Câmara, A.d.A.; Maréchal, P.A.; Tourdot-Maréchal, R.; Husson, F. Oxidative stress resistance during dehydration of three non-Saccharomyces wine yeast strains. Food Res. Int. 2019, 123, 364–372. [Google Scholar] [CrossRef]
- Fernandes, M.A.; Mota, M.N.; Faria, N.T.; Sá-Correia, I. An Evolved Strain of the Oleaginous Yeast Rhodotorula toruloides, Multi-Tolerant to the Major Inhibitors Present in Lignocellulosic Hydrolysates, Exhibits an Altered Cell Envelope. J. Fungi 2023, 9, 1073. [Google Scholar] [CrossRef]
- Acosta-García, E.D.; Páez-Lerma, J.B.; Moreno-Jiménez, M.R.; Rojas-Contreras, J.A.; Soto-Cruz, N.O. Yeast competition during alcoholic fermentation of agave. Its comprehension as a way to reach sustainable mezcal production. Int. J. Food Sci. Technol. 2023, 58, 6674–6688. [Google Scholar] [CrossRef]
- Fink, J.W.; Manhart, M. How do microbes grow in nature? The role of population dynamics in microbial ecology and evolution. Curr. Opin. Syst. Biol. 2023, 36, 100470. [Google Scholar] [CrossRef]
- Postaru, M.; Tucaliuc, A.; Cascaval, D.; Galaction, A.I. Cellular Stress Impact on Yeast Activity in Biotechnological Processes—A Short Overview. Microorg. Multidiscip. Digit. Publ. Inst. 2023, 11, 2522. [Google Scholar] [CrossRef] [PubMed]
- De Nadal, E.; Posas, F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022, 22, foac013. [Google Scholar] [CrossRef]
- Toyoda, Y.; Saitoh, S. Fission yeast torc2 signaling pathway ensures cell proliferation under glucose-limited, nitrogen-replete conditions. Biomolecules 2021, 11, 1465. [Google Scholar] [CrossRef]
- Cheng, J.S.; Ding, M.Z.; Tian, H.C.; Yuan, Y.J. Inoculation-density-dependent responses and pathway shifts in Saccharomyces cerevisiae. Proteomics 2009, 9, 4704–4713. [Google Scholar] [CrossRef]
- Betlej, G.; Bator, E.; Oklejewicz, B.; Potocki, L.; Górka, A.; Slowik-Borowiec, M. Long-term adaption to high osmotic stress as a tool for improving enological characteristics in industrial wine yeast. Genes 2020, 11, 576. [Google Scholar] [CrossRef]
- Tatebayashi, K.; Yamamoto, K.; Tomida, T.; Nishimura, A.; Takayama, T.; Oyama, M. Osmostress enhances activating phosphorylation of Hog1 MAP kinase by mono-phosphorylated Pbs2 MAP 2K. EMBO J. 2020, 39, e103444. [Google Scholar] [CrossRef]
- Fu, X.; Li, P.; Zhang, L.; Li, S. Understanding the stress responses of Kluyveromyces marxianus after an arrest during high-temperature ethanol fermentation based on integration of RNA-Seq and metabolite data. Appl. Microbiol. Biotechnol. 2019, 103, 2715–2729. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Tachon, S.; Michelon, D.; Chambellon, E.; Cantonnet, M.; Mezange, C.; Henno, L. Experimental conditions affect the site of tetrazolium violet reduction in the electron transport chain of Lactococcus lactis. Microbiology 2009, 155, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Foongladda, S.; Roengsanthia, D.; Arjrattanakool, W.; Chuchottaworn, C.; Chaiprasert, A.; Franzblau, S.G. Rapid and simple MTT method for rifampicin and isoniazid susceptibility testing of Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 2002, 6, 1118–1122. [Google Scholar]
- Montoro, E.; Lemus, D.; Echemendia, M.; Martin, A.; Portaels, F.; Palomino, J.C. Comparative evaluation of the nitrate reduction assay, the MTT test, and the resazurin microtitre assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2005, 55, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Ionescu, A.; Cazzaniga, G.; Edefonti, V.; Gagliani, M. The influence of antibacterial toothpastes on in vitro Streptococcus mutans biofilm formation: A continuous culture study. Am. J. Dent 2014, 27, 160–166. [Google Scholar]
- Wang, F.; Cao, L.; Hu, S. A rapid and accurate 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide colorimetric assay for quantification of bacteriocins with nisin as an example. J. Zhejiang Univ. Sci. B 2007, 8, 549–554. [Google Scholar] [CrossRef]
- Bilal, M.; Ji, L.; Xu, Y.; Xu, S.; Lin, Y.; Iqbal, M.N.; Cheng, H. Bioprospecting Kluyveromyces marxianus as a Robust Host for Industrial Biotechnology. Front. Bioeng. Biotechnol. 2022, 10, 851768. [Google Scholar] [CrossRef]
- Holguín-Loya, A.H.; Salazar-Herrera, A.E.; Soto-Cruz, N.O.; Kirchmayr, M.R.; Lopes, C.A.; Rojas-Contreras, J.A. Enhancing Mezcal Production Efficiency by Adding an Inoculant of Native Saccharomyces cerevisiae to a Standardized Fermentation Must. Foods 2025, 14, 341. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kobori, T.; Ganesh, D.; Aoyagi, H. Fungal Pigment–Assisted Silver Nanoparticle Synthesis and Their Antimicrobial and Cytotoxic Potential. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2022; pp. 65–78. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Xu, H.; Liu, J.; Wang, J.; Zhang, H. A Physiogenomic Study of the Tolerance of Saccharomyces cerevisiae to Isoamyl Alcohol. Fermentation 2024, 10, 4. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Fonseca, G.G.; Gombert, A.K.; Heinzle, E.; Wittmann, C. Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 2007, 7, 422–435. [Google Scholar]
- Kavšček, M.; Stražar, M.; Curk, T.; Natter, K.; Petrovič, U. Yeast as a cell factory: Current state and perspectives. Microb. Cell Factories 2015, 14, 94. [Google Scholar] [CrossRef]
- de Moura Ferreira, M.A.; da Silveira, F.A.; da Silveira, W.B. Ethanol stress responses in Kluyveromyces marxianus: Current knowledge and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Wang, M.; Zhan, R.; Yu, Y.; He, Y.; Lu, H. Kluyveromyces marxianus developing ethanol tolerance during adaptive evolution with significant improvements of multiple pathways. Biotechnol. Biofuels 2019, 12, 63. [Google Scholar] [CrossRef]
- Guaragnella, N.; Bettiga, M. Acetic acid stress in budding yeast: From molecular mechanisms to applications. Yeast 2021, 38, 391–400. [Google Scholar] [CrossRef]
- Sainz-Mellado, D.C.; Mendez-Hernández, J.E.; López-Miranda, J.; Páez-Lerma, J.B.; Aguilar, C.N.; Soto-Cruz, N.O. Gradually supply of isoamyl alcohol increases the isoamyl acetate production in solid-state fermentation. Appl. Microbiol. 2023, 76, ovac061. [Google Scholar] [CrossRef]
- Eleutherio, E.; Brasil, A.d.A.; França, M.B.; de Almeida, D.S.G.; Rona, G.B.; Magalhães, R.S.S. Oxidative stress and aging: Learning from yeast lessons. Fungal Biol. 2018, 122, 514–525. [Google Scholar] [CrossRef]
- Fai, P.B.; Grant, A. A comparative study of Saccharomyces cerevisiae sensitivity against eight yeast species sensitivities to a range of toxicants. Chemosphere 2009, 75, 289–296. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Dmitrenko, V.V. Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 2015, 574, 193–203. [Google Scholar] [CrossRef]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar]
- Bleoanca, I.; Silva, A.R.C.; Pimentel, C.; Rodrigues-Pousada, C.; Menezes, R.d.A. Relationship between ethanol and oxidative stress in laboratory and brewing yeast strains. J. Biosci. Bioeng. 2013, 116, 697–705. [Google Scholar]
- Vilela, L.d.F.; de Araujo, V.P.G.; Paredes, R.d.S.; Bon, E.P.d.S.; Torres, F.A.G.; Neves, B.C. Enhanced xylose fermentation and ethanol production by engineered Saccharomyces cerevisiae strain. AMB Express 2015, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-López, F.N.; Querol, A.; Barrio, E. Application of a substrate inhibition model to estimate the effect of fructose concentration on the growth of diverse Saccharomyces cerevisiae strains. J. Ind. Microbiol. Biotechnol. 2009, 36, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.A.; Vitorino, M.V.; Godinho, C.P.; Bourbon-Melo, N.; Robalo, T.T.; Fernandes, F. Yeast adaptive response to acetic acid stress involves structural alterations and increased stiffness of the cell wall. Sci. Rep. 2021, 11, 12652. [Google Scholar] [CrossRef] [PubMed]
- Picazo, C.; Molin, M. Impact of hydrogen peroxide on protein synthesis in yeast. Antioxidants 2021, 10, 952. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Erten, H.; Cabaroglu, T. Enhanced production of isoamyl acetate from beet molasses with addition of fusel oil by Williopsis saturnus var. saturnus. Food Chem. 2009, 112, 290–294. [Google Scholar] [CrossRef]
- Quilter, M.G.; Hurley, J.C.; Lynch, F.J.; Murphy, M.G. The production of isoamyl acetate from amyl alcohol by Saccharomyces cerevisiae. J. Inst. Brew. 2003, 109, 34–40. [Google Scholar] [CrossRef]
- Rentería-Martínez, O.; Sánchez-Castañeda, A.K.; Hernández-Carbajal, G.; Rutiaga-Quiñones, O.M.; Rojas-Contreras, J.A.; López-Miranda, J.; Páez-Lerma, J.B.; Soto-Cruz, N.O. Isoamyl acetate production by Pichia fermentans isolated from alcoholic fermentation of Agave juice. In Sustainable and Integrated Use of Agave; CIATEJ-CONACYT: Zapopan, Mexico, 2016; pp. 85–88. [Google Scholar]
- Martínez-Anaya, C.; Dickinson, J.R.; Sudbery, P.E. In yeast, the pseudohyphal phenotype induced by isoamyl alcohol results from the operation of the morphogenesis checkpoint. J. Cell Sci. 2003, 116, 3423–3431. [Google Scholar] [CrossRef][Green Version]
- Rentería-Martínez, O.; Páez-Lerma, J.B.; Rojas-Contreras, J.A.; López-Miranda, J.; Martell-Nevárez, M.A.; Soto-Cruz, N.O. Enhancing isoamyl acetate biosynthesis by Pichia fermentans. Rev. Mex. Ing. Quim. 2021, 20, 621–633. [Google Scholar] [CrossRef]
- Nikolaidis, A.; Andreadis, M.; Moschakis, T. Effect of heat, pH, ultrasonication and ethanol on the denaturation of whey protein isolate using a newly developed approach in the analysis of difference-UV spectra. Food Chem. 2017, 232, 425–433. [Google Scholar] [CrossRef]
- Vamvakas, S.S.; Kapolos, J. Factors affecting yeast ethanol tolerance and fermentation efficiency. World J. Microbiol. Biotechnol. 2020, 36, 114. [Google Scholar] [CrossRef]
- Arellano-Plaza, M.; Noriega-Cisneros, R.; Clemente-Guerrero, M.; González-Hernández, J.C.; Robles-Herrera, P.D.; Manzo-Ávalos, S. Fermentative capacity of Kluyveromyces marxianus and Saccharomyces cerevisiae after oxidative stress. J. Inst. Brew. 2017, 123, 519–526. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces Cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 5, 1937–1949. [Google Scholar] [CrossRef]
- Fujii, T.; Kobayashi, O.; Yoshimoto, H.; Furukawa, S.; Tamaim, Y. Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1997, 63, 910–915. [Google Scholar] [CrossRef]
- Kuroda, K.; Hammer, S.K.; Watanabe, Y.; Montaño-López, J.; Fink, G.R.; Stephanopoulos, G. Critical Roles of the Pentose Phosphate Pathway and GLN3 in Isobutanol-Specific Tolerance in Yeast. Cell Syst. 2019, 9, 534–547. [Google Scholar] [CrossRef]
- Yoshida, M.; Kato, S.; Fukuda, S.; Izawa, S. Acquired Resistance to Severe Ethanol Stress in Saccharomyces cerevisiae Protein Quality Control. Appl. Environ. Microbiol. 2021, 87, e02353-20. [Google Scholar] [CrossRef]
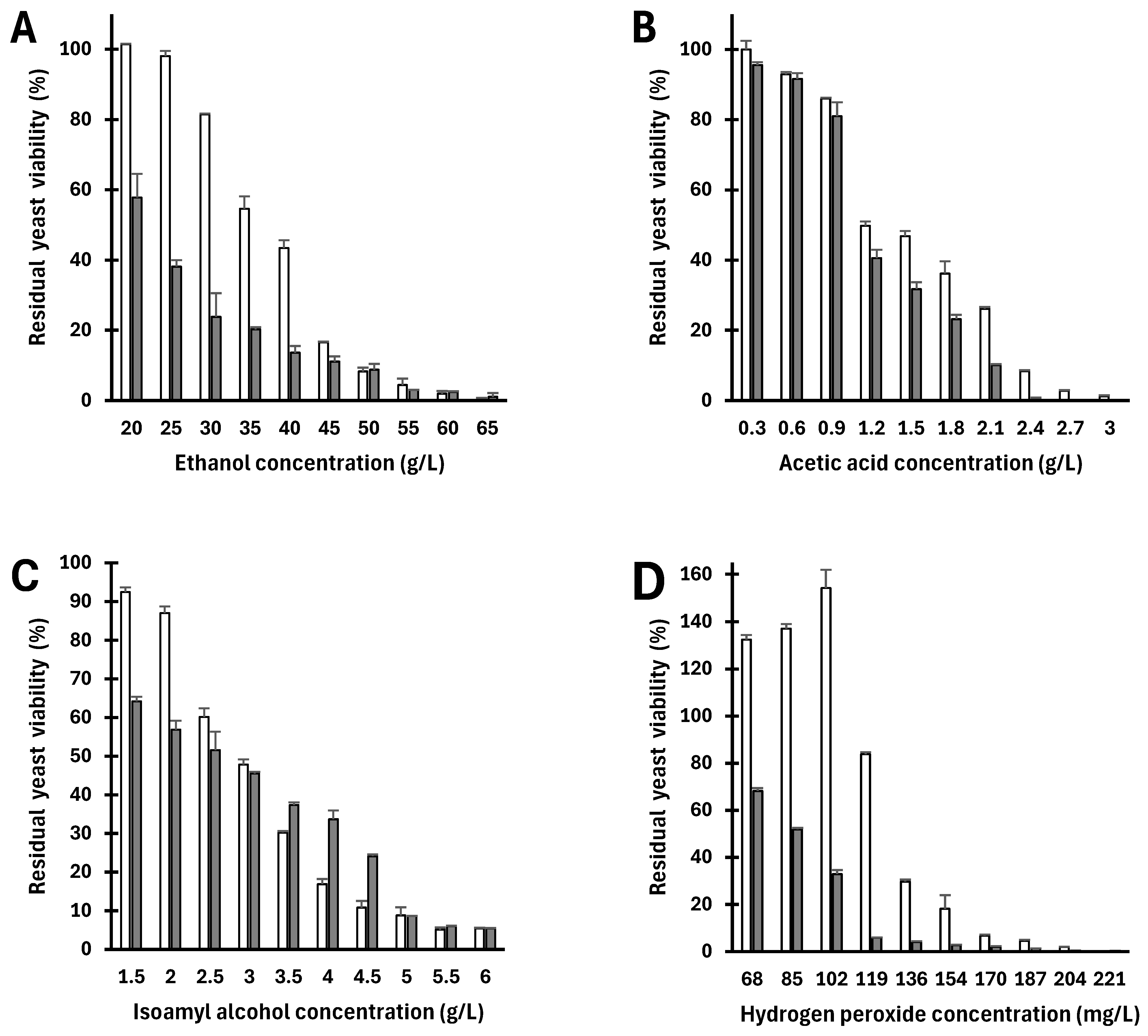
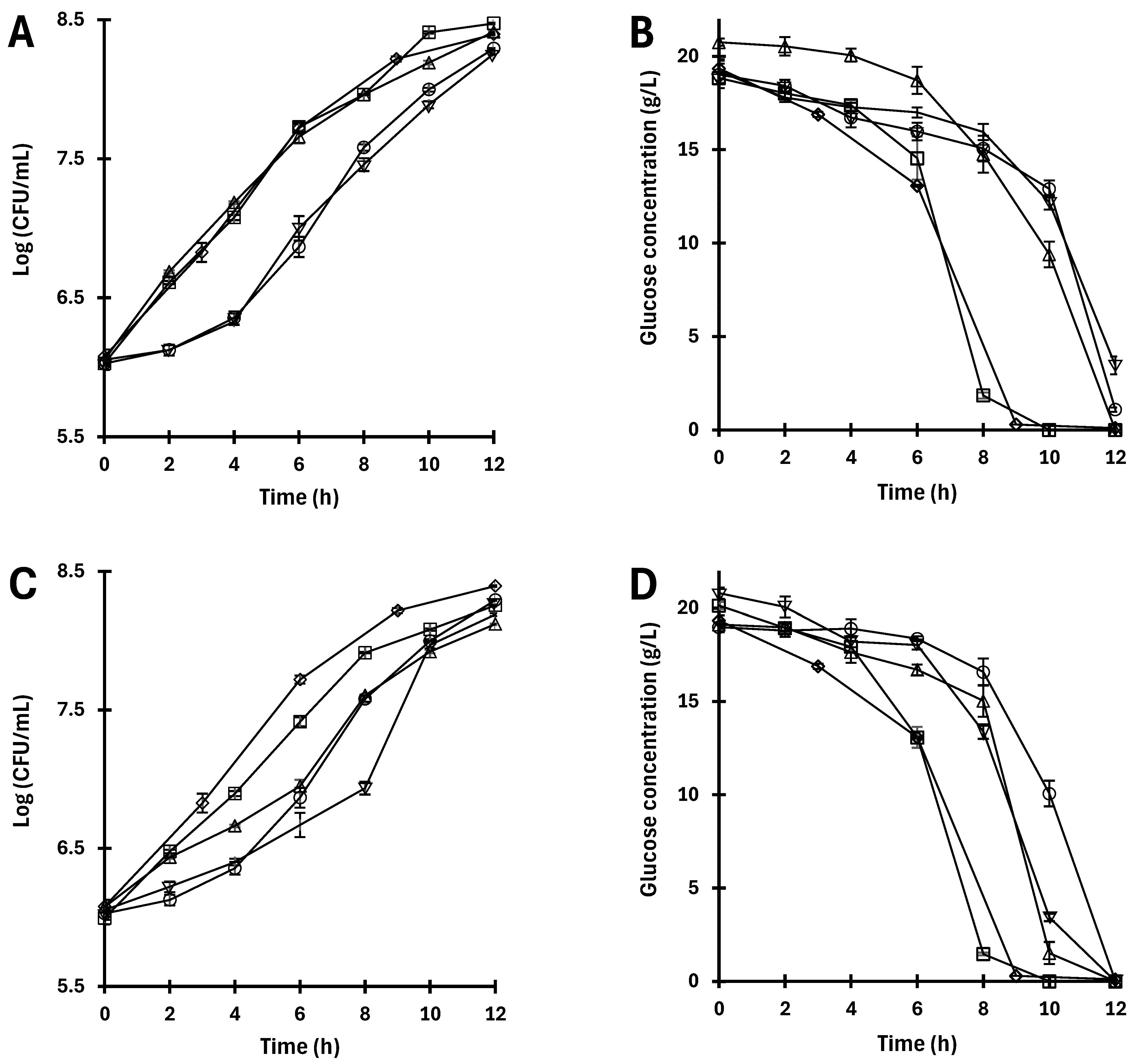
| Stress Agent | Viability by Direct Method (CFU Counting) | Viability by Indirect Method (MTT) | ||||
|---|---|---|---|---|---|---|
| IC50 (g/L) | IC30 (g/L) | R2 | IC50 (g/L) | IC30 (g/L) | R2 | |
| Ethanol | 21.82 | 17.12 | 0.9854 | 36.70 | 32.79 | 0.9961 |
| Acetic acid | 1.19 | 0.97 | 0.9914 | 1.29 | 0.93 | 0.9805 |
| Isoamyl alcohol | 2.74 | 1.07 | 0.9630 | 2.84 | 2.40 | 0.9964 |
| Hydrogen peroxide | 0.09 | 0.06 | 0.9949 | 0.128 | 0.123 | 0.9907 |
| Stress Agent | ||||||
|---|---|---|---|---|---|---|
| Control | Ethanol | Acetic Acid | Isoamyl Alcohol | Hydrogen Peroxide | ||
(h−1) | IC30 | 0.549 ± 0.009 a | 0.422 ± 0.007 c | 0.550 ± 0.010 a | 0.459 ± 0.010 b | 0.437 ± 0.008 bc |
| IC50 | 0.549 ± 0.009 a | 0.413 ± 0.009 bc | 0.442 ± 0.018 b | 0.398 ± 0.016 c | 0.342 ± 0.02 d | |
(h−1) | IC30 | 0.0547 ± 0.0026 a | 0.0286 ± 0.0088 b | 0.0207 ± 0.0018 b | 0.0100 ± 0.0061 b | 0.0308 ± 0.0129 ab |
| IC50 | 0.0547 ± 0.0026 a | 0.0260 ± 0.0032 b | 0.0283 ± 0.0037 b | 0.0236 ± 0.0032 b | 0.0054 ± 0.0019 c | |
| × 10−7 (cells/g S) | IC30 | 1.400 ± 0.081 a | 1.133 ± 0.085 b | 1.197 ± 0.091 ab | 1.305 ± 0.046 ab | 1.141 ± 0.095 b |
| IC50 | 1.400 ± 0.081 a | 0.668 ± 00.018 b | 0.707 ± 0.027 b | 0.626 ± 0.022 b | 0.371 ± 0.016 c | |
| Isoamyl Acetate (mg/L) | IC30 | 40.90 ± 6.08 b | 7.77 ± 0.27 b | 26.52 ± 0.54 b | 596.57 ± 41.60 a | 71.40 ± 7.90 b |
| IC50 | 40.90 ± 6.08 b | 6.77 ± 0.56 c | 23.94 ± 1.40 bc | 238.61 ± 10.00 a | 30.60 ± 0.12 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Cuevas, C.K.; Páez-Lerma, J.B.; Rojas-Contreras, J.A.; Rodríguez-Sifuentes, L.; Sánchez-Castañeda, A.K.; Soto-Cruz, N.O. Tolerance of Kluyveromyces marxianus Under Acetic Acid-, Isoamyl Alcohol-, Hydrogen Peroxide-, and Ethanol-Induced Stress. Fermentation 2025, 11, 171. https://doi.org/10.3390/fermentation11040171
Acosta-Cuevas CK, Páez-Lerma JB, Rojas-Contreras JA, Rodríguez-Sifuentes L, Sánchez-Castañeda AK, Soto-Cruz NO. Tolerance of Kluyveromyces marxianus Under Acetic Acid-, Isoamyl Alcohol-, Hydrogen Peroxide-, and Ethanol-Induced Stress. Fermentation. 2025; 11(4):171. https://doi.org/10.3390/fermentation11040171
Chicago/Turabian StyleAcosta-Cuevas, Cesia K., Jesús B. Páez-Lerma, Juan A. Rojas-Contreras, Lucio Rodríguez-Sifuentes, Ana K. Sánchez-Castañeda, and Nicolas O. Soto-Cruz. 2025. "Tolerance of Kluyveromyces marxianus Under Acetic Acid-, Isoamyl Alcohol-, Hydrogen Peroxide-, and Ethanol-Induced Stress" Fermentation 11, no. 4: 171. https://doi.org/10.3390/fermentation11040171
APA StyleAcosta-Cuevas, C. K., Páez-Lerma, J. B., Rojas-Contreras, J. A., Rodríguez-Sifuentes, L., Sánchez-Castañeda, A. K., & Soto-Cruz, N. O. (2025). Tolerance of Kluyveromyces marxianus Under Acetic Acid-, Isoamyl Alcohol-, Hydrogen Peroxide-, and Ethanol-Induced Stress. Fermentation, 11(4), 171. https://doi.org/10.3390/fermentation11040171







