Abstract
Electro-fermentation (EF) is an emerging bioprocess with the ability to regulate the metabolism of electrochemically active microorganisms. In various fermentation processes, electrodes perform either as an electron acceptor or donor, facilitating the formation and movement of electrons and protons. The bioelectric activity created by external electrodes enhances the metabolic reactions, resulting in a higher yield of value-added chemicals. The conventional fermentation process has a number of limitations in terms of usability and economic feasibility, whereas electro-fermentation presents a hybrid technology, minimizing redox instabilities and enhancing the metabolic process in general to achieve increased product production and a higher biomass yield. Electrochemically active microorganisms such as Geobacter and Shewanella species can carry out the exchange of electrons with electrodes directly or indirectly by using electron mediators. Furthermore, the integration of microbial fuel cells (MFCs) with microbial electrolysis cells (MECs) precludes the need for external manipulation of the fermentation system as the required change in electrochemical gradient is provided by the MFC counterpart. The major beneficial aspects of electro-fermentation include its role as a potential tool for enhancing the production of value-added compounds. The mixed-culture system clearly had a favorable impact on the synthesis of butyric acid from rice straw. Furthermore, cathodic electro-fermentation (CEF) exhibited benefits over anaerobic fermentation, influencing NADH/NAD+, enabling a higher product titer, and reducing the accumulation of byproducts. Hence, in this review, we emphasize the importance of electro-fermentation over conventional fermentation for biofuel and biochemical production, covering its fundamentals, interactions, types, future challenges, and ability to provide several benefits to boost the fermentation process, such as the process efficiency and product yield, on an industrial scale.
1. Introduction
Climate change and the energy crisis are the two most pressing issues facing the world today. Consequently, the scientific community is dedicated to finding alternative biofuels to replace conventional fossil fuels and advancing sustainable processes to create a circular economy faster. Bioelectrochemical processes have proven to be effective in generating bioenergy and valuable products from various waste materials. The promotion of renewable resources is essential for transitioning from a fossil fuel-based economy to a sustainable one. The European Bioeconomy Strategy emphasizes the need for a shift towards resource efficient production that simultaneously reduces and valorizes waste streams. Biofuels such as biomethanol, bioethanol, biogas, and biodiesel, along with energy carriers such as hydrogen, are crucial for societal decarbonization. Biofuels can serve as fuel additives or be utilized in their pure form, whereas hydrogen can be converted into electricity through fuel cell technology. These fossil fuel alternatives have the potential to reduce greenhouse gas emissions, mitigate global warming, and advance sustainable development [1,2].
Recently, electro-fermentation has gained significant attention for its potential to enhance the production of biofuels such as biohydrogen, biochemicals such as biomethane, and biopolymers. However, conventional fermentation processes face several practical and economic challenges. Integrating two electrodes into a bioreactor addresses the redox instabilities observed in traditional fermentation, thereby increasing the biomass yield and enhancing product formation. Electro-fermentation is an innovative bioprocess that modulates the metabolism of electrochemically active microorganisms. The incorporation of electrodes into the fermentation process, which serve as electron acceptors, facilitates the transfer of electrons and protons. This not only generates bioelectricity but also produces valuable chemicals [3].
In various fermentation processes, electrodes act either as an electron acceptor or donor, facilitating the formation and movement of electrons and protons. Subsequently, the bioelectric activity created by the external electrodes enhances the metabolic reactions, resulting in a higher yield of value-added chemicals [4,5]. The traditional fermentation method faces a number of limitations in usability and economic feasibility, whereas electro-fermentation presents a hybrid technology that reduces redox instabilities and improves the metabolic process as a whole to produce more biomass and better product formation. Electrochemically active microorganisms such as Geobacter and Shewanella species have the ability to carry out either the direct or indirect exchange of electrons with electrodes [6].
2. Electro-Fermentation over Conventional Fermentation
Fermentation, an anaerobic process used by various microorganisms and cells to degrade glucose, relies solely on glycolysis and a few subsequent reactions to produce energy. Fermentation promotes the accumulation of volatile fatty acids (VFAs) and alcohols by inhibiting anaerobic digestion and methanogenesis [7,8]. Fermentation has recently become a key biotechnological platform for producing a variety of products, including fermented foods, flavor, enzymes, solvents, biofuels, biopolymers, and probiotics. Despite its advantages, the process remains labor-intensive and economically challenging for upstream activities, such as providing pure substances or creating controlled environments, whether for inoculum preparation or fermenter operation [9]. In certain cases, microbial culture growth media must be highly selective and require carefully optimized strains. It is important to remember that the biochemical activities of these microorganisms are crucial aspects of this process.
Conventional fermentation is significantly limited by feedback inhibition, which is a major limitation. The accumulation of byproducts like organic acids can impact the physiological functions of microbial cells, disrupting their metabolism and growth [4]. To overcome these challenges, innovative approaches have been explored, including integrating conventional anaerobic digestion with photo-fermentation or leveraging bioelectrochemical systems (BESs) to enhance stability and optimize chemical production [10]. The ability of microorganisms to establish electrical connections with devices has been recognized since 1911 when Potter demonstrated that certain bacteria could generate an electrical current by converting chemical energy from organic substrates into electricity [11]. However, over the past two decades, microbial electrochemistry has emerged as a revolutionary technological approach, driven by intensive research. Bioelectrochemical systems (BESs) encompass various systems where microorganisms and other biocatalysts are combined with electrochemical techniques to enhance and regulate oxidative and/or reductive metabolic pathways. Over the past decade, electro-fermentation (EF) has gained significant attention from the scientific community as a promising alternative to conventional anaerobic digestion processes. Similarly to other bioelectrochemical systems (BESs), electrodes play a crucial role in optimizing microbial fermentation pathways in EF. These electrodes function as both electron donors and acceptors, thereby modifying the medium by altering the redox balance [4,6,12]. In BESs, electrodes are essential for optimizing the microbial fermentation pathways. They act as both electron donors and acceptors, thereby modifying the medium by adjusting the redox balance [12]. In recent years, Rabaey and coworkers have solidified the concept of EF, demonstrating its potential to offer superior process control and yield higher-purity products compared to conventional fermentation. This technique enhances the stability and density of microbial cell growth and can facilitate chain elongation, such as the production of medium-chain fatty acids from volatile fatty acids [13]. Similarly to conventional processes, EF involves the fermentation of energy-rich substrates such as alcohols and carbohydrates. However, it differs by applying an external potential that activates oxidative and reductive pathways [14].
Thermodynamics pose a limitation in fermentative processes because many reactions occurring during fermentation are spontaneous. The primary challenge in achieving high yields is cellular regulation, which helps maintain the metabolic redox balance. Introducing electrodes into the fermentation medium disrupts this balance, theoretically enabling the stoichiometric conversion of substrates into final products. Consequently, EF technology has the potential to achieve higher yields than theoretically possible under balanced fermentation conditions [15]. Table 1 summarizes the key merits and demerits of electro-fermentation and conventional fermentation.

Table 1.
Merits and demerits of fermentation and electro-fermentation.
3. Electro-Fermentation Fundamentals and Its Principle
The bidirectional electron flow between microbes or biomolecules facilitates transition mechanisms within bioelectrochemical systems (BESs). Recently, BESs have gained attention as a promising technology for producing biofuels and value-added chemicals. The prioritization and advancement of existing BES technology have been significantly influenced by microbial fuel cells (MFCs), which are widely used for bioelectrogenesis through various microbial metabolic reactions [16,17,18].
Although MFCs are the most extensively studied type of BES, the potential of BESs to produce value-added chemicals has captivated the industrial sector. The ability to synthesize essential building-block chemicals, such as acetate from CO/syngas or carbon dioxide (CO2), through electro-fermentation has already been demonstrated. Additionally, supplying external power to BESs, known as microbial electrolysis cells (MECs), can generate fuels such as H2 and CH4. Under specific conditions, the electrical wire between the anode and cathode facilitates electron flow. Upon reaching the cathode, electrons are reduced in the presence of a reducing agent [5]. Numerous researchers have studied microbe–electrode interactions to elucidate the electron transfer mechanism (ETM), discovering that outer-membrane cytochromes, enzymes such as hydrogenases, and cytoplasmic diffusion mediators play critical roles in electron and proton transfer [17]. MFCs, which generate electricity through substrate oxidation in the anode compartment and subsequent electron reduction at the cathode in the presence of oxygen, are one of the most extensively studied models [19]. Additionally, certain microbes have been shown to reduce electron acceptors such as nitrate and metal ions in the cathode chamber [14]. By integrating biological, electrochemical, and material sciences with an external power source, BESs can synthesize value-added chemicals. The first widely accepted BES model, developed in the late 2000s, is an enzymatic fuel cell designed for amperometric biosensors [20]. In the absence of redox mediators, electron tunneling occurs over only a few nanometers. The immobilization of enzymes on electrodes is essential for facilitating direct electron transfer in practical applications. The main mechanism by which microbes interact with electron donors or acceptors is extracellular electron transport (EET) [21,22,23].
Electro-Fermentation Principle
A key parameter in this process is the oxidoreduction potential (ORP), which reflects the oxidation–reduction capacity of the fermentation medium and acts as an indicator of bacterial metabolic activity [24]. Extracellular and intracellular ORPs differ due to the cytomembrane barrier and cellular redox homeostasis. Extracellular ORP is influenced by the composition of the fermentation medium and other factors such as temperature. This is crucial because it directly impacts the intracellular potential by altering the ratio of nicotinamide adenine dinucleotide to its reduced (NADH) and oxidized (NAD+) forms. Maintaining cellular redox homeostasis at the cellular level is crucial for metabolic function. Therefore, alterations in the extracellular potential necessitate adjustments in metabolic electron flow by modifying the NADH+ ratio [25,26].
Metabolic pathways can be adjusted through various strategies to direct them towards the production of desired products. These include controlling extracellular ORP via enzyme synthesis, genetic engineering [27], and chemical methods [28]. Consequently, electro-fermentation (EF) offers an alternative approach to regulating extracellular potential and metabolic pathways by integrating electrodes into the fermentation medium to facilitate electron transfer. In electro-fermentation (EF), fermentation pathways can be enhanced through electrochemical means, which involve boosting specific electron transport routes and improving energy conservation mechanisms, such as ATP formation. Additionally, the inclusion of both inorganic and organic molecules during fermentation acts as a mediator to enhance electron transfers and can serve as a final or intermediate acceptor. These mediators are particularly useful when the microorganisms are not fully electrochemically active.
One option for electro-fermentation (EF) is to use pure cultures of microorganisms or mixed cultures. Certain electroactive bacteria families, such as Geobacteraceae and Shewanellaceae, are particularly valuable for these systems because they can directly engage with both anodic and cathodic electrodes, facilitating electron transfer through the formation of biofilms on electrode surfaces [29]. However, it is important to note that the choice of electrode material can significantly affect the EFefficiency.
In summary, optimizing electrodes is crucial for facilitating efficient biofilm development and electron exchange in electro-fermentation (EF). Ideally, electrodes should possess high conductivity, biocompatibility, corrosion resistance, and a large specific surface area with porosity. Additionally, using catalysts such as noble and non-noble metals on the electrodes can help reduce the overpotential of redox reactions [3]. As a result, Moscoviz et al. [12] introduced the concept of EFefficiency to evaluate the energy performance of an EF process. Here, Qe− is the charge transferred through the electric circuit, and Qproduct is the charge produced by the total oxidation of the desired product.
EFefficiency = Qe−/Qproduct
4. Interaction Between Electrode and Microorganisms
The impact of different metabolites was predicted by analyzing a fundamental electro-fermentation model for glucose and glycerol. Redox cofactors like NADH and NADPH, generated at the cathode and involved in specific reduction reactions, can effectively contribute to carbon chain elongation in organic metabolites [30]. Conversely, as opposed to standard anaerobic catabolism, the anode, acting as an electron sink, can enhance the biomass yield [31,32].
Recent research has extensively studied and investigated the electron transfer mechanisms (ETMs) of the anodic compartment by exo-electrogenic bacteria. Direct electron transfer involves cytochrome dehydrogenase interactions, hydrogenase electron transmission through the periplasm, and direct electron transfer via cytochrome c-type proteins. This has been demonstrated in various exo-electrogenic bacteria, including Geobacter and Shewanella. Researchers have examined electron transmission systems such as cytochromes, quinones, and ferredoxins, which are key components in electrode–microbe interactions, to understand their potential catalytic functions [32,33].
Electron Transfer Mechanism
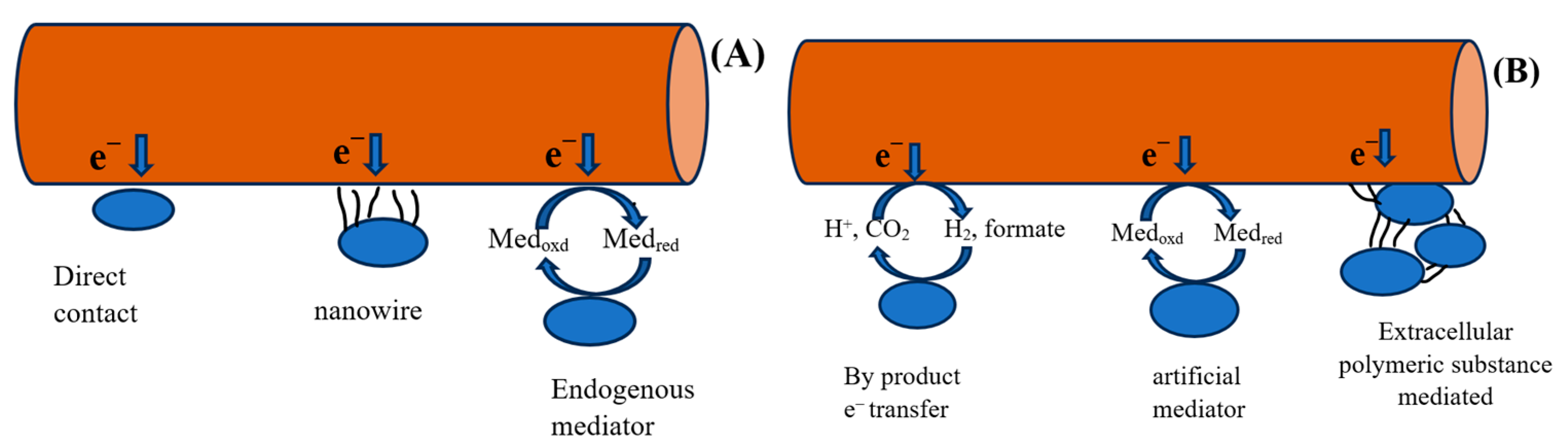
Electron movement occurs in three stages: oxidation, electron conversion, and final transport of extracellular electrons [34]. The transfer of electrons to associated proteins occurs in three stages [33]. Key proteins that facilitate electron transfer include hydrogenase, formate dehydrogenase, cytochrome, ferredoxin, and rubredoxin. Concurrently, numerous biological and electrochemical reactions are catalyzed throughout the electron transfer (ET) cycle. In the initial stages, electrons are transferred by diffusible intracellular electron carriers, such as NAD+/NADH, to proteins located in the external membrane [35]. Three pathways of electron transfer have been identified: direct electron transfer (ET), indirect ET, and transfer via biological projections, as shown in Figure 1. These electrons can move to the cathode terminal, and vice versa. Direct ET is considered one of the most efficient methods for electron transfer, moving electrons from the cathode to the respective microorganisms. This process is well documented in microbial communities, including Betaproteobacteria in biocathodes [36]. Proteins such as ferredoxin and other redox proteins are critical candidates for extracellular electron transfer (ET). Among microorganisms, Geobacter and Shewanella species are highly regarded for their effectiveness in extracellular electron transmission [36].
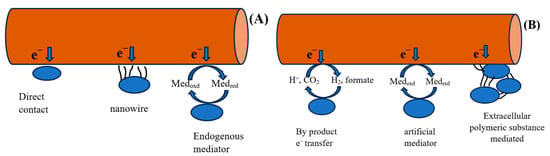
Figure 1.
(A) Direct and (B) indirect electron transfer mechanism.
Recent research suggests two alternative systems owing to certain limitations, such as inadequate surface area and diffusion issues in mediators, which prevent high-current-density production. However, previous studies have demonstrated that extracellular electron transfer (EET) can occur through direct interactions or mediators [5]. Alternatively, microorganisms utilize conduction-based EET to enhance transport efficiency and achieve high current densities by employing biological structures such as pili and nanowires. Additional proposed mechanisms include redox conduction, also known as electron hopping, and conduction through the biofilm matrix.
5. Anode-Mediated Electron Transfer Mechanism
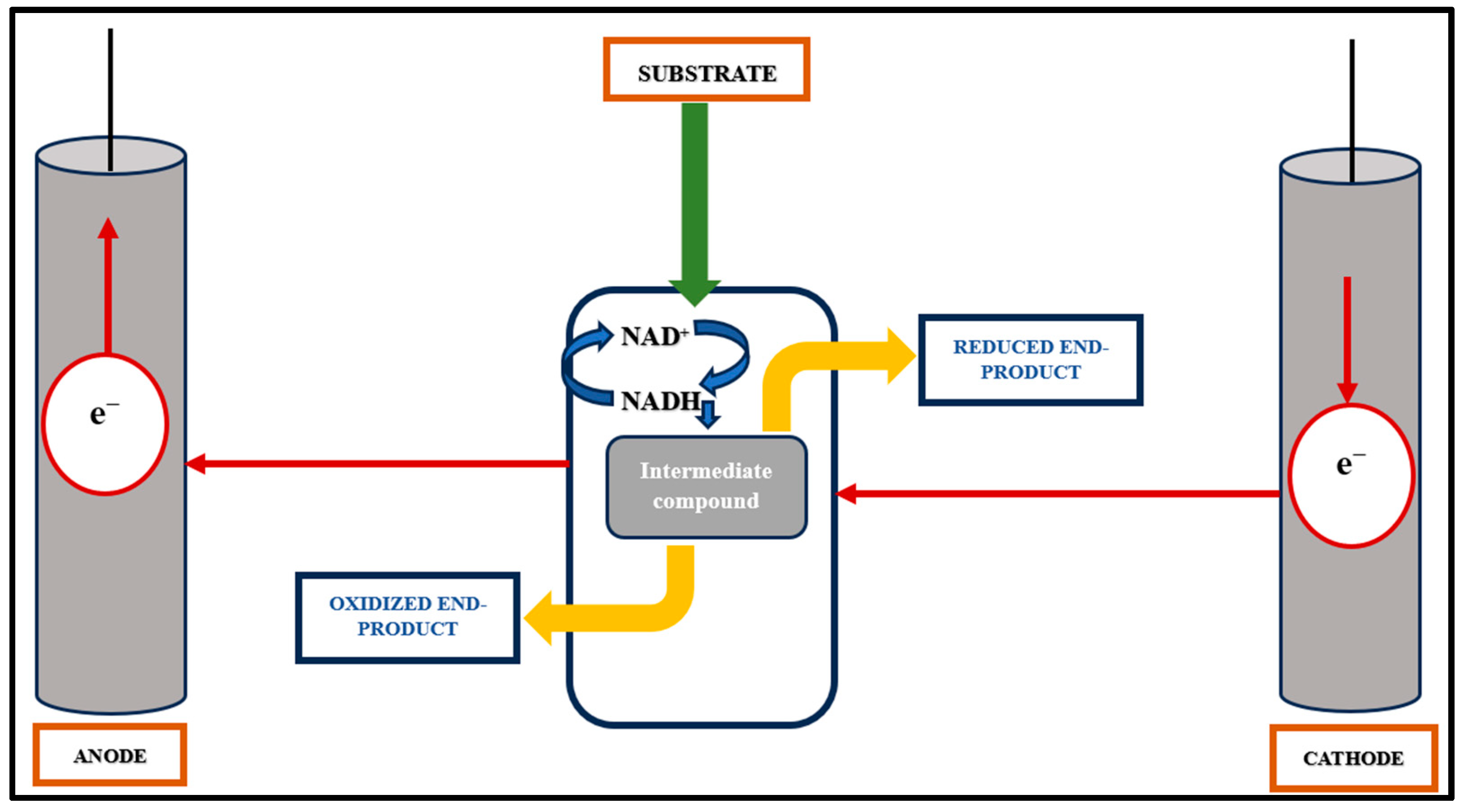
The primary application of anodic microbial processes has traditionally focused on generating electric current in fuel cells, as reviewed in [17]. The primary objective of this process is electron transfer from the oxidation of a complex or defined substrate rather than the production of value-added compounds, as illustrated in Figure 2. Microorganisms proficient in anodic electron discharge, such as Shewanella oneidensis and Geobacter sulfurreducens, are not known to synthesize biotechnologically significant products. Microorganisms with well-known biotechnological potential, such as Pseudomonas sp., are not efficient for anodic electron discharge. Significant efforts have been made to heterologously integrate and re-engineer efficient cytochrome-based electron transfer pathways from S. oneidensis into the biotechnological host Escherichia coli [37,38,39].
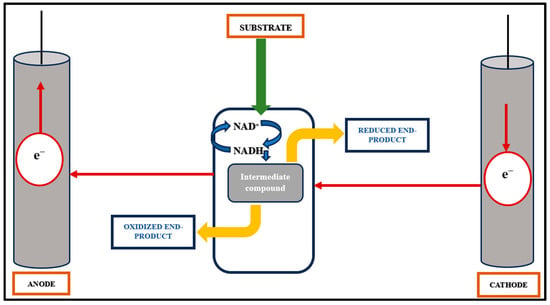
Figure 2.
Anode- and cathode-mediated electron transfer mechanism. Red arrows representing the direction of flow of electrons.
Designing a more efficient anodic chamber involves ensuring high conductivity, non-corrosiveness, a large surface area, high porosity, and low construction costs. These features are crucial for processes such as electron adhesion and transfer by exo-electrogenic bacteria. Carbon materials are widely used for anode construction because of their consistently high electrical conductivity and adaptable morphology [5]. Carbon-based materials, such as graphite granules and fiber brushes, are excellent candidates for anode development because of their high surface areas and conductivities [40,41,42].
6. Cathode-Mediated Electron Transfer Mechanism
Anodic reactions generate current, while reactions in the cathodic chamber utilize this current to synthesize value-added chemicals. The mixed bioelectrochemical reaction is catalyzed by extracellular electron transfer (EET) from the biocatalyst to the cathode chamber, as shown in Figure 2. Methane (CH4) production in the cathode chamber follows a direct pathway, starting with electron transfer to a proton and then the reduction of CO2 [35,43,44]. Another approach involves incorporating porosity into carbon materials using multiwalled carbon nanotubes (MWCNTs) doped with catalytic materials such as cobalt-tetramethylphenyl porphyrine or manganese oxide for cathode electrode preparation. This method has been recently explored [5,45]. Efforts are underway to enhance electron flow from the biocatalyst to the cathode, including increasing the interfacial area and interactions. Various materials such as nano-nickel, carbon nanotubes, and reticulated vitreous carbon have been tested to immobilize nanoparticles on cathodes [46]. Furthermore, studies have shown that the production of bioelectrochemical H2 in a microbial electrolysis cell (MEC) system can be assisted and improved by graphene-modified biocathodes [47].
7. Electro-Fermenter Design
The electro-fermenter’s design, including material selection and operating conditions, directly influences both the system’s cost and efficiency. Various configurations of anaerobic fermenters powered by an external power supply have been utilized for electro-fermentation. Among these, the electrode material and reactor configuration are critical factors in enhancing device performance. The industry employs a vast array of fermenter configurations, ranging from anaerobic fermenters for producing various bioproducts to reactors that utilize electric currents to facilitate fermentation, known as electrochemical reactors [48]. To optimize their electrochemical requirements, these reactors should be integrated into conventional fermenters.
Economic feasibility, performance, and stability are crucial factors in the design of instruments for industrial and large-scale applications. Carbon and graphene are extensively used in electro-fermentation because of their high conductivity, low cost, and chemical stability. Enhancing the properties of electrodes increases their electronic conductivity and electron transfer capabilities, which are highly desirable [49]. The ability of microorganisms to form biofilms and adhere to electrodes is influenced by the porosity, surface area, and biocompatibility of materials. Surface functional groups such as oxygen and nitrogen can further enhance electrochemical reactions. Typically, counter-electrode functions and their respective reactions can occur either within the reactor or in separate chambers divided by ion-exchange membranes (IEMs).
8. Types of Electro-Fermentation Systems
8.1. Single-Chamber Reactor
In various studies, membrane-less, single-chamber electro-fermenters have been identified as effective configurations for converting various substrates into methane, hydrogen, bioethanol, and other products. Recent studies on electro-fermentation processes have primarily focused on single-chamber reactors. In 2015, Zhao et al. [48] conducted an experiment in which they applied an external voltage of 0.6 V across a graphite-rod cathode and a graphite-brush anode submerged in a cylindrical reactor inoculated with activated sludge to produce methane. After 33 days, the electro-fermenter accumulated 2998.4 mL of methane, significantly surpassing the 904.5 mL produced through conventional fermentation. These results underscore the increased methane yield achieved by utilizing polarized electrodes during fermentation. Similarly, in 2016, Zhen et al. [50] investigated the impact of different applied voltages on methane production using a single-chamber reactor with a submerged cathode. Their study revealed that the synergistic interaction between fermenting and electroactive bacteria contributed to process stability and improved energy recovery.
In addition to producing various types of biogases, single-chamber electro-fermenters have also been utilized to simultaneously generate fatty acids, such as acetic acid, lactic acid, and butyric acid. Notably, Sravan et al. [51] designed an air-cathode single-chamber electro-fermenter powered by food waste. Paiano et al. [52] employed a similar electro-fermenter setup to produce butyric acid. They utilized a membrane-less reactor inoculated with anaerobic sludge and supplied it with a mixture of glucose, acetate, and ethanol. Two graphite rods functioned as electrodes, with an applied voltage ranging from 0.6 V to 1.5 V. The highest butyric acid production occurred at 1.3 V, yielding a 2.7-fold increase compared to the open-circuit potential. However, the application of a voltage greater than 1.4 V resulted in decreased butyric acid production and increased energy consumption. Moreover, the type of inoculum may not significantly affect the performance of the electro-fermentation process.
8.2. Double-Chamber Reactor
An alternative to single-chamber reactors is the use of double-chamber systems, which incorporate a proton exchange membrane as a separator. Mostafazadeh et al. (2016) [53] employed a pure culture of Clostridium pasteurianum cultivated in standard minimum media supplemented with glucose to produce biobutanol in an electro-fermenter. Their findings were compared with those obtained using stainless-steel electrodes. When the applied voltage exceeded 1.5 V, a decline in butanol production was observed for both electrode materials. Similarly, Villano et al. (2017) [54] utilized an H-type reactor equipped with a commercial proton exchange membrane, two graphite rod electrodes, and a Ag/AgCl reference electrode (+199 mV vs. SHE) to explore the bioelectrochemical synthesis of isobutyrate. The reactors, inoculated with anaerobic sludge, were supplied with various carbon sources—either individually or in combination (glucose, ethanol, and acetate)—under a set voltage of −0.7 V (vs. SHE) to polarize the electrodes. The results indicate that metabolite distribution can be precisely controlled by adjusting the applied potential and pH of the media, enabling greater regulation of the overall process.
9. Biofuel and Biochemical Production via Electro-Fermentation
In EF technology, electricity is used to synthesize chemicals with the assistance of microorganisms attached to the cathode/anode surface. These microorganisms act as biocatalysts capable of performing oxidation–reduction reactions as efficiently as traditional chemical catalysts [12,55,56]. The effectiveness of the synthesis process is influenced by factors such as the biocatalyst, reduction potential, electrochemical redox mediators, and type of bioelectrode used. Yu et al. [57] present an innovative approach to enhancing extracellular electron uptake by developing an electro-controlled dynamic regulation system in engineered S. oneidensis. Their study effectively integrates a dual-stage microbial electro-fermentation (MEF) process, an NADH/NAD⁺ ratio-responsive biosensor, and an eCRISPRi-based transcription inhibition system to optimize the supply and targeted distribution of reducing equivalents for isobutanol synthesis. Notably, their strategy leads to a 10.4-fold increase in isobutanol yield. A recent study [58] showed that the introduction of a biocathode enhanced medium-chain and longer-chain fatty acid production.
9.1. Bioalcohol Production
Electrons released in the anodic compartment can reduce the production of volatile fatty acid (VFA) intermediates, thereby facilitating the production of alcohols during the electro-fermentation process. The kinetics of these reactions determine the functionality of the biocathode, which in turn regulates the entire electro-fermentation process [59]. Kim et al. [59] investigated the impact of cathode-mediated bioconversion on the transformation of glycerol to 1,3-Propanediol (1,3-PDO) using Klebsiella pneumoniae L17 as a pure culture. By applying an externally poised potential to the cathode (−900 mV vs. Ag/AgCl), they observed an increase in 1,3-PDO production (35.5 mm) compared with fermentation without the bioelectrochemical system (23.7 mm).
In another study, Engel et al. [60] explored the impact of mediator-less electro-fermentation with Clostridium acetobutylicum for the first time. They found that during bioelectrosynthesis, the highest butanol production (13.31 g/L) was achieved at an applied potential of 1.32 V. The batch fermentation of C. acetobutylicum demonstrated an increase in solvent yield when influenced by a poised electrode. Specifically, the cumulative acetone–butanol–ethanol yield increased from 0.141 g/L to 0.202 g/L.
9.2. Biohydrogen Production
Harnessing biological pathways for H2 production from renewable substrates is not only ingenious but also renders the process environmentally sustainable and economically beneficial [23,61]. Electron donors, often derived from substances such as glucose, stimulate the growth of microbes involved in biological H2 processing. As biological H2 is produced, the electrons generated during the process need to be discharged to maintain an electrical balance. Microbial electrochemical cells (MECs) are a significant platform for biological H2 production. Within MECs, exo-electrogenic bacteria oxidize carbon-rich organic substrates to generate electrons and protons because of the potential provided by the in situ electrode. These electrons and protons are then transferred to the cathodic compartment by bacteria. Protons are also transported to the cathode, where they undergo reduction to facilitate biological H2 production. Such reactions can occur within both single- and double-chamber MEC setups.
MECs utilize the anodic terminal as an electron sink to oxidize organic substrates, which is facilitated by electrochemically active microorganisms that release protons and electrons. These protons are then transported to the cathodic terminal through a proton exchange membrane (PEM), where they combine with electrons transferred through an external electrical circuit to generate H2 [62]. Several researchers have explored various types of low-value wastewater as feedstock for H2 production using electro-fermentation technology [63]. In a recent study, increased hydrogen production was evaluated via electro-fermentation, and it was demonstrated that applying negative potentials suppresses biomass generation while promoting hydrogen production. In contrast, positive potentials are observed to enhance ethanol production by using mixed microbial cultures [64].
10. Design Considerations and Challenges in Reactor Upscaling
Single-chamber reactors have been utilized because of their relatively simple construction and operation [52]. However, their main limitation is the formation of toxic compounds at the counter-electrode, which can hinder the process [65].
One major challenge is optimizing the electrode design and materials to enhance the conductivity, stability, and biocompatibility, ensuring efficient electron transfer and microbial activity. Additionally, selecting and engineering microorganisms with high electroactivity and metabolic efficiency are essential for maximizing product yield and selectivity. Anodic glucose fermentation, facilitated by engineered Corynebacterium glutamicum for lysine and organic acid production, was successfully scaled up from 0.35 L to 2.40 L without any significant reduction in process efficiency [66]. Previously, researchers designed specialized lids for conventional bioreactors that incorporated ports to accommodate electrode placement. Large-scale electro-fermenters require durable and straightforward designs that accommodate variations in the feedstock (gas/liquid streams) and the operational durations necessary for the growth of electroactive populations. Furthermore, the selection of suitable electrode materials and fermenter architectures optimized for large-scale applications has been identified as a critical factor [67]. Increasing the volume of the electrode chambers may introduce challenges in ensuring the uniform distribution of substrates [68,69]. Additionally, the existing literature on the design parameters of bioelectrochemical reactors remains limited and lacks standardized data representation [70]. Some studies have applied scaled-up criteria for the transition from the laboratory to the pilot scale while maintaining similar geometries and operational parameters [71]. However, significant variations were observed in the electrode distance and abiotic current density, resulting in performance discrepancies owing to the increased electrode resistance. Advanced process modeling techniques, such as computational fluid dynamics (CFD) analysis, can aid in optimizing mixing conditions [72,73,74]. Utilizing electrode materials with enhanced electronic properties or employing stacked configurations can help mitigate electrical resistance at larger scales. A recent study shows the validity of anodic electro-fermentation as a strategy for the generation of simultaneous electricity and butanol production by using engineered electroactive E. coli cultivated on organic byproducts. Notably, increasing the electrode surface area led to an enhancement in electron output [75]. A transcriptome analysis conducted in another study revealed distinct gene expression patterns under anodic electro-fermentation conditions, whereas minimal differences were observed under cathodic electro-fermentation [76]. Additionally, certain electro-fermentation setups may require sacrificial counter-electrodes, which degrade over time and necessitate periodic replacements. Modular designs are preferred when the replacement of electrodes and membranes is required, as they avoid disturbing the biological community. Scaling up electro-fermentation processes from the laboratory to the industrial scale, while maintaining product quality and process efficiency, presents significant challenges.
11. Conclusions
Electro-fermentation enables the efficient conversion of renewable feedstocks into a diverse range of valuable products, including biofuels such as hydrogen, methane, ethanol, and butanol, as well as high-value biochemicals with various industrial applications. For example, a synergistic microbial consortium can enhance the H2 production rate. In addition, the microbial mixed culture increased acid production along with an increase in the relative abundance of butyric acid-fermenting bacteria under the influence of a voltage [77]. While electro-fermentation presents several challenges, such as optimizing the electrode design, selecting suitable microorganisms, and scaling up processes, ongoing research and technological advancements continue to overcome these barriers and unlock their full potential. One of the major limitations in scaling up processes is the reactor size, which affects the process performance and increases the energy demand. Maintaining efficient electron transfer from the exo-electrogen to the anode is essential, as efficiency declines with increasing anode size. This, in turn, increases the production cost of hydrogen or other value-added compounds. To minimize energy losses, controlling the pH gradient is crucial, because it influences both the cathode potential and exo-electrogen metabolism on the anode surface. In principle, electro-fermentation does not face the same scalability challenges as microbial electrochemical technologies do. Therefore, it is possible that conventional anaerobic bioreactors with relatively small electrode surface areas could be repurposed for electro-fermentation applications, meaning that upscaling electro-fermentation reactors could begin by retrofitting existing fermenters. However, it is important to note that no studies have yet explored the impact of the electrode material and surface area on electro-fermentation efficiency. Addressing these factors requires a deeper understanding of microbe–electrode interactions, which are crucial for the successful advancement and deployment of this promising technology.
Author Contributions
P.P. and U.G.P. wrote the original draft and facilitated literature collection. K.B. provided resources, conceptualization, supervision, writing, reviewing, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This review did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| EF | Electro-fermentation |
| BES | Bioelectrochemical system |
| VFAs | Volatile fatty acids |
| MFC | Microbial fuel cell |
| MEC | Microbial electrolysis cell |
| ETM | Electron transfer mechanism |
| EET | Extracellular electron transfer |
| ORP | Oxidoreduction potential |
| NADH | Nicotinamide adenine dinucleotide |
| ET | Electron transfer |
| MWCNTs | Multiwalled carbon nanotubes |
| IEM | Ion exchange membrane |
| PEM | Proton exchange membrane |
| CFD | Computational fluid dynamics |
References
- Su, W.; Li, D.; Tan, H.; Yu, D.; Liu, Z.; He, Z.; Liu, W. Boosting the biosynthesis of medium-chain fatty acids from waste activated sludge electro-fermentation: Roles of syngas and ethanol serving as co-electron donors. J. Clean. Prod. 2025, 502, 145383. [Google Scholar] [CrossRef]
- Tharak, A.; Suresh, G.; Kaveti, S.; Jain, N.; Mohan, S.V. Heterologous Expression of the carbon monoxide dehydrogenase Gene from Clostridium sp. to Enhance Acetic Acid and Alcohol Production from CO2. bioRxiv 2024. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, A.N.; Kumar, G.; Kim, D.H.; Song, Y.C.; Kim, S.H. Electro-fermentation for biofuels and biochemicals production: Current status and future directions. Bioresour. Technol. 2021, 323, 124598. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.; Wong, J.W.C.; Pradhan, N. Recent advances in electro-fermentation technology: A novel approach towards balanced fermentation. Bioresour. Technol. 2022, 360, 127637. [Google Scholar] [CrossRef]
- Salar-García, M.J.; Ortiz-Martínez, V.M.; Sánchez-Segado, S.; Valero Sánchez, R.; Sáez López, A.; Lozano Blanco, L.J.; Godínez-Seoane, C. Sustainable Production of Biofuels and Biochemicals via Electro-Fermentation Technology. Molecules 2024, 29, 834. [Google Scholar] [CrossRef]
- Virdis, B.; Hoelzle, R.D.; Marchetti, A.; Boto, S.T.; Rosenbaum, M.A.; Blasco-Gómez, R.; Puig, S.; Freguia, S.; Villano, M. Electro-fermentation: Sustainable bioproductions steered by electricity. Biotechnol. Adv. 2022, 59, 107950. [Google Scholar] [CrossRef]
- Fernandez-Domínguez, D.; Astals, S.; Peces, M.; Frison, N.; Bolzonella, D.; MataAlvarez, J.; Dosta, J. Volatile fatty acids production from biowaste at mechanical-biological treatment plants: Focusing on fermentation temperature. Bioresour. Technol. 2020, 314, 123729. [Google Scholar] [CrossRef]
- Kumar, P.; Ray, S.; Kalia, V.C. Production of co-polymers of polyhydroxyalkanoates by regulating the hydrolysis of biowastes. Bioresour. Technol. 2016, 200, 413–419. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Laurinavichene, T.; Tekucheva, D.; Laurinavichius, K.; Tsygankov, A. Utilization of distillery wastewater for hydrogen production in one-stage and two-stage processes involving photofermentation. Enzym. Microb. Technol. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical Effects Accompanying the Decomposition of Organic Compounds. Proc. R. Soc. B Biol. Sci. 1911, 84, 260–276. [Google Scholar]
- Moscoviz, R.; Toledo-Alarcón, J.; Trably, E.; Bernet, N. Electro-Fermentation: How to drive fermentation using electrochemical systems. Trends Biotechnol. 2016, 34, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Ragauskas, A.J. Editorial overview: Energy Biotechnology. Curr. Opin. Biotechnol. 2014, 27, v–vi. [Google Scholar] [CrossRef] [PubMed]
- Bhagchandanii, D.D.; Babu, R.P.; Sonawane, J.M. A Comprehensive Understanding of Electro-Fermentation. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Kracke, F.; Krömer, J.O. Identifying target processes for microbial electrosynthesis by elementary mode analysis. BMC Bioinform. 2014, 15, 410. [Google Scholar] [CrossRef]
- Yeruva, D.K.; Jukuri, S.; Velvizhi, G.; Kumar, A.N.; Swamy, Y.V.; Venkata Mohan, S. Integrating sequencing batch reactor with bio-electrochemical treatment for augmenting remediation efficiency of complex petrochemical wastewater. Bioresour. Technol. 2015, 188, 33–42. [Google Scholar] [CrossRef]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Saratale, R.G.; Kuppam, C.; Mudhoo, A.; Saratale, G.D.; Periyasamy, S.; Zhen, G.; Kook, L.; Bakonyi, P.; Nemestothy, N.; Kumar, G. Bioelectrochemical systems using microalgae—A concise research update. Chemosphere 2017, 177, 35–43. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef]
- Habermüller, K.; Mosbach, M.; Schuhmann, W. Electron-transfer mechanisms in amperometric biosensors. Fresenius’ J. Anal. Chem. 2000, 366, 560–568. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrochemical remediation of real field petroleum sludge as an electron donor with simultaneous power generation facilitates biotransformation of PAH: Effect of substrate concentration. Bioresour. Technol. 2012, 110, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Velvizhi, G.; Venkata Mohan, S. Bioelectrogenic role of anoxic microbial anode in the treatment of chemical wastewater: Microbial dynamics with bioelectro-characterization. Water Res. 2015, 70, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Qin, J.C.; Lin, Y.H.; Liu, C.G.; Qin, J.C.; Lin, Y.H. Fermentation and Redox Potential. In Fermentation Processes; IntechOpen: London, UK, 2017. [Google Scholar]
- Liu, X.; Shi, L.; Gu, J.D. Microbial electrocatalysis: Redox mediators responsible for extracellular electron transfer. Biotechnol. Adv. 2018, 36, 1815–1827. [Google Scholar] [CrossRef]
- Hoelzle, R.D.; Virdis, B.; Batstone, D.J. Regulation mechanisms in mixed and pure culture microbial fermentation. Biotechnol. Bioeng. 2014, 111, 2139–2154. [Google Scholar] [CrossRef]
- Liu, L.; Bilal, M.; Luo, H.; Zhao, Y.; Iqbal, H.M.N. Metabolic Engineering and Fermentation Process Strategies for L-Tryptophan Production by Escherichia coli. Processes 2019, 7, 213. [Google Scholar] [CrossRef]
- Liu, C.G.; Xue, C.; Lin, Y.H.; Bai, F.W. Redox potential control and applications in microaerobic and anaerobic fermentations. Biotechnol. Adv. 2013, 31, 257–265. [Google Scholar] [CrossRef]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Sharma, M.; Aryal, N.; Sarma, P.M.; Vanbroekhoven, K.; Lal, B.; Benetton, X.D.; Pant, D. Bioelectrocatalyzed reduction of acetic and butyric acids via direct electron transfer using a mixed culture of sulfate-reducers drives electrosynthesis of alcohols and acetone. Chem. Commun. 2013, 49, 6495. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Amulya, K.; Venkata Mohan, S. Solid phase bioelectrofermentation of food waste to harvest value-added products associated with waste remediation. Waste Manag. 2015, 45, 57–65. [Google Scholar] [CrossRef]
- Choi, O.; Sang, B.I. Extracellular electron transfer from cathode to microbes: Application for biofuel production. Biotechnol. Biofuels 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Kokko, M.; Epple, S.; Gescher, J.; Kerzenmacher, S. Effects of wastewater constituents and operational conditions on the composition and dynamics of anodic microbial communities in bioelectrochemical systems. Bioresour. Technol. 2018, 58, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.H.; Cui, D.; Gao, L.; Cheng, H.Y.; Wang, A.J. Analysis of electrode microbial communities in an up-flow bioelectrochemical system treating azo dye wastewater. Electrochim. Acta 2016, 220, 252–257. [Google Scholar] [CrossRef]
- Shamsuddin, R.A.; Abu Bakar, M.H.; Wan Daud, W.R.; Hong, K.B.; Jahim, J.M. Can electrochemically active biofilm protect stainless steel used as electrodes in bioelectrochemical systems in a similar way as galvanic corrosion protection? Int. J. Hydrogen Energy 2019, 44, 30512–30523. [Google Scholar] [CrossRef]
- Barbosa, S.G.; Peixoto, L.; Alves, J.I.; Alves, M.M. Bioelectrochemical systems (BESs) towards conversion of carbon monoxide/syngas: A mini-review. Renew. Sustain. Energy Rev. 2021, 135, 110358. [Google Scholar] [CrossRef]
- Goldbeck, C.P.; Jensen, H.M.; Teravest, M.A.; Beedle, N.; Appling, Y.; Hepler, M.; Cambray, G.; Mutalik, V.; Angenent, L.T.; Ajo-Franklin, C.M. Tuning promoter strengths for improved synthesis and function of electron conduits in Escherichia coli. ACS Synth. Biol. 2013, 2, 150–159. [Google Scholar] [CrossRef]
- Sturm-Richter, K.; Golitsch, F.; Sturm, G.; Kipf, E.; Dittrich, A.; Beblawy, S.; Kerzenmacher, S.; Gescher, J. Unbalanced fermentation of glycerol in Escherichia coli via heterologous production of an electron transport chain and electrode interaction in microbial electrochemical cells. Bioresour. Technol. 2015, 186, 89–96. [Google Scholar] [CrossRef]
- Teravest, M.A.; Zajdel, T.J.; Ajo-Franklin, C.M. The Mtr pathway of Shewanella oneidensis MR-1 couples substrate utilization to current production in Escherichia coli. ChemElectroChem 2014, 1, 1874–1879. [Google Scholar] [CrossRef]
- Ayanda, O.S.; Mmuoegbulam, A.O.; Okezie, O.; Durumin Iya, N.I.; Mohammed, S.A.E.; James, P.H.; Muhammad, A.B.; Unimke, A.A.; Alim, S.A.; Yahaya, S.M.; et al. Recent progress in carbon-based nanomaterials: Critical review. J. Nanopart. Res. 2024, 26, 106. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Kumar, G.; Venkata Mohan, S.; Pandey, A.; Jeon, B.H.; Jang, M.; Kim, S.H. Microbial Electro-Remediation (MER) of hazardous waste in aid of sustainable energy generation and resource recovery. Environ. Technol. Innov. 2020, 19, 100997. [Google Scholar] [CrossRef]
- Pandit, S.; Chandrasekhar, K.; Kakarla, R.; Kadier, A.; Jeevitha, V. Basic principles of microbial fuel cell: Technical challenges and economic feasibility. In Microbial Applications; Springer Nature: London, UK, 2017; pp. 165–188. [Google Scholar]
- Modestra, J.A.; Chiranjeevi, P.; Mohan, S.V. Cathodic material effect on electron acceptance towards bioelectricity generation and wastewater treatment. Renew. Energy 2016, 98, 178–187. [Google Scholar] [CrossRef]
- Shang, G.; Xu, G.; Ren, J.; Yu, J.P.; Cai, W.; Cui, K.; Guo, K. A cathodic electro-fermentation system for enhanced methane production from high-concentration potato starch industrial wastewater. J. Water Process Eng. 2024, 59, 105006. [Google Scholar] [CrossRef]
- Chandrasekhar, K. Effective and Nonprecious Cathode Catalysts for Oxygen Reduction Reaction in Microbial Fuel Cells. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 485–501. [Google Scholar]
- Kumar, A.; Hsu, L.H.; Kavanagh, P.; Barriere, F.; Lens, P.N.L.; Lapinsonniere, L.; Lienhard, J.H.; Schroder, U.; Jiang, X.; Leech, D. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 2017, 1, 24. [Google Scholar] [CrossRef]
- Jourdin, L.; Grieger, T.; Monetti, J.; Flexer, V.; Freguia, S.; Lu, Y.; Chen, J.; Romano, M.; Wallace, G.G.; Keller, J. High Acetic Acid Production Rate Obtained by Microbial Electrosynthesis from Carbon Dioxide. Environ. Sci. Technol. 2015, 49, 13566–13574. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Wang, L.; Quan, X. Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 11094. [Google Scholar] [CrossRef]
- Hafez, H.M. Method and System for Electro-Assisted Hydrogen Production from Organic Material. U.S. Patent 9,458,474, 4 October 2016. [Google Scholar]
- Zhen, G.; Kobayashi, T.; Lu, X.; Kumar, G.; Xu, K. Biomethane recovery from Egeria densa in a microbial electrolysis cell-assisted anaerobic system: Performance and stability assessment. Chemosphere 2016, 149, 121–129. [Google Scholar] [CrossRef]
- Sravan, J.S.; Butti, S.K.; Sarkar, O.; Mohan, S.V. Electrofermentation: Chemicals and Fuels. In Biomass, Biofuels, Biochemicals: Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 723–737. [Google Scholar]
- Paiano, P.; Premier, G.; Guwy, A.; Kaur, A.; Michie, I.; Majone, M.; Villano, M. Simplified reactor design for mixed culture-based electrofermentation toward butyric acid production. Processes 2021, 9, 417. [Google Scholar] [CrossRef]
- Mostafazadeh, A.K.; Drogui, P.; Brar, S.K.; Tyagi, R.D.; Le Bihan, Y.; Buelna, G.; Rasolomanana, S.D. Enhancement of biobutanol production by electromicrobial glucose conversion in a dual chamber fermentation cell using C. pasteurianum. Energy Convers. Manag. 2016, 130, 165–175. [Google Scholar] [CrossRef]
- Villano, M.; Paiano, P.; Palma, E.; Miccheli, A.; Majone, M. Electrochemically Driven Fermentation of Organic Substrates with Undefined Mixed Microbial Cultures. ChemSusChem 2017, 10, 3091–3097. [Google Scholar] [CrossRef]
- Borole, A.P.; Tsouris, C.; Pavlostathis, S.G.; Yiacoumi, S.; Lewis, A.J.; Zeng, X.; Park, L. Efficient conversion of aqueous-wastecarbon compounds into electrons, hydrogen, and chemicals via separations and microbial electrocatalysis. Front. Energy Res. 2018, 6, 94. [Google Scholar] [CrossRef]
- Mogollón, C.A.G.; Díaz, J.C.Q.; Posada, J.O.G. Production of acetone, butanol, and ethanol by electro-fermentation with Clostridium saccharoperbutylacetonicum N1-4. Bioelectrochemistry 2023, 152, 108414. [Google Scholar]
- Yu, H.; Li, F.; Wang, Y.; Hu, C.; Zhang, B.; Qiao, C.; Song, H. Electro-controlled distribution of reducing equivalents to boost isobutanol biosynthesis in microbial electro-fermentation of S. oneidensis. Joule 2025, 9, 101773. [Google Scholar] [CrossRef]
- Sun, X.; Yin, Y.; Chen, H.; Zhao, L.; Wang, C.; Wang, J. Enhanced Medium-Chain Fatty Acids Production by Electro-fermentation: Insights into the Effect of Biocathode and Ethanol Supply. ACS ES&T Eng. 2024, 5, 179–190. [Google Scholar]
- Kim, C.; Lee, J.H.; Baek, J.; Kong, D.S.; Na, J.; Lee, J.; Sundstrom, E.; Park, S.; Kim, J.R. Small Current but Highly Productive Synthesis of 1,3-Propanediol from Glycerol by an Electrode-Driven Metabolic Shift in Klebsiella pneumoniae L17. ChemSusChem 2020, 13, 564–573. [Google Scholar] [CrossRef]
- Engel, M.; Holtmann, D.; Ulber, R.; Tippkotter, N. Increased Biobutanol Production by Mediator-Less Electro-Fermentation. Biotechnol. J. 2019, 14, 1800514. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Chang, J.S. Biohydrogen from Renewable Resources; Pandey, A., Chang, J.S., Hallenbecka, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 185–221. [Google Scholar]
- Rathinam, N.K.; Bibra, M.; Salem, D.R.; Sani, R.K. Thermophiles for biohydrogen production in microbial electrolytic cells. Bioresour. Technol. 2019, 277, 171–178. [Google Scholar] [CrossRef]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 2015, 68, 601–615. [Google Scholar] [CrossRef]
- Cardeña, R.; Valencia-Ojeda, C.; Chazaro-Ruiz, L.F.; Razo-Flores, E. Regulation of the dark fermentation products by electro-fermentation in reactors without membrane. Int. J. Hydrogen Energy 2024, 49, 107–116. [Google Scholar] [CrossRef]
- Chen, H.; Dong, F.; Minteer, S.D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nat. Catal. 2020, 3, 225–244. [Google Scholar] [CrossRef]
- Krieg, T.; Phan, L.M.P.; Wood, J.A.; Sydow, A.; Vassilev, I.; Krömer, J.O.; Mangold, K.-M.; Holtmann, D. Characterization of a membrane-separated and a membrane- less electrobioreactor for bioelectrochemical syntheses. Biotechnol. Bioeng. 2018, 115, 1705–1716. [Google Scholar] [CrossRef]
- Hiebl, C.; Fuchs, W. Electro-Enhanced Gas Fermentation for Bioproduction of Volatile Fatty Acids and Alcohols. Microorganisms 2025, 13, 249. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Deutzmann, J.S.; Jayathilake, B.S.; Pang, S.H.; Chandrasekaran, S.; Baker, S.E.; Spormann, A.M. Efficient hydrogen delivery for microbial electrosynthesis via 3D-printed cathodes. Front. Microbiol. 2021, 12, 696473. [Google Scholar] [CrossRef] [PubMed]
- Krige, A.; Rova, U.; Christakopoulos, P. 3D bioprinting on cathodes in microbial electrosynthesis for increased acetate production rate using Sporomusa ovata. J. Environ. Chem. Eng. 2021, 9, 106189. [Google Scholar] [CrossRef]
- Santoro, C.; Babanova, S.; Cristiani, P.; Artyushkova, K.; Atanassov, P.; Bergel, A.; Bretschger, O.; Brown, R.K.; Carpenter, K.; Colombo, A.; et al. How comparable are microbial electrochemical systems around the globe? An electrochemical and microbiological cross-laboratory study. ChemSusChem 2021, 14, 2313–2330. [Google Scholar] [CrossRef]
- Enzmann, F.; Holtmann, D. Rational Scale-Up of a methane producing bio-electrochemical reactor to 50 L pilot scale. Chem. Eng. Sci. 2019, 207, 1148–1158. [Google Scholar] [CrossRef]
- Korth, B.; Harnisch, F. Modeling microbial electrosynthesis. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 127–141. [Google Scholar]
- Sharma, M.; Bajracharya, S.; Gildemyn, S.; Patil, S.A.; Alvarez-Gallego, Y.; Pant, D.; Rabaey, K.; Dominguez-Benetton, X. A critical revisit of the key parameters used to describe microbial electrochemical systems. Electrochim. Acta 2014, 140, 191–208. [Google Scholar] [CrossRef]
- Vilà-Rovira, A.; Puig, S.; Balaguer, M.D.; Colprim, J. Anode hydrodynamics in bioelectrochemical systems. RSC Adv. 2015, 5, 78994–79000. [Google Scholar] [CrossRef]
- Jong, B.; Haritos, V.S. Co-production of bioelectricity and butanol by engineered Escherichia coli fed organic wastes in anodic fermentation. Green Chem. 2025, 27, 1356–1364. [Google Scholar] [CrossRef]
- Liang, Y.C.; Li, K.; Zhao, X.Q.; Bai, F.W.; Liu, C.G. Enhanced glucose utilization and succinic acid production in Corynebacterium glutamicum by integration of metabolic engineering and electro-fermentation. Chem. Eng. J. 2025, 505, 159522. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Meng, J.; Sun, K.; Yan, H. A neutral red mediated electro- fermentation system of Clostridium beijerinckii for effective co-production of butanol and hydrogen. Bioresour. Technol. 2021, 332, 125097. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).