Abstract
In this study, we established a mutagenesis and high-throughput screening system to select a high-yielding glutathione (GSH)-producing strain of Saccharomyces cerevisiae. The parent strain was mutated by atmospheric and room temperature plasma (ARTP) technology and cultivated using ethionine plate cultivation. Subsequently, high-throughput screening was performed using liquid deep microtiter plates (MTPs) for cultivation and a microplate reader for rapid GSH detection. The results demonstrated the successful selection of a stable mutant strain, S-272, which exhibited significantly enhanced GSH production. Fermentation validation in 5 L bioreactors revealed that S-272 achieved a 14.7% higher final GSH concentration and a 19.5% higher intracellular GSH content compared to the parent strain. The improved performance of S-272 was attributed to enhanced ethanol utilization, elevated activity of γ-glutamylcysteine synthetase (γ-GCS), and increased intracellular trehalose content. This study presents an effective strategy for developing high GSH-yield strains using ARTP complex mutagenesis technology combined with high-throughput screening.
1. Introduction
Glutathione (GSH) is a water-soluble tripeptide composed of L-glutamate, L-cysteine, and glycine, with its antioxidant properties primarily attributed to the thiol group of cysteine [1,2]. The significant ability of GSH to scavenge free radicals, protect against oxidative stress, and enhance drug stability and efficacy makes it highly valuable in various industries, including dietary supplements, cosmetics, and pharmaceuticals [3].
Currently, GSH can be produced industrially through chemical, enzymatic, and microbial synthesis methods [4,5]. However, chemical and enzymatic processes are expensive, unstable, and time-consuming, limiting their feasibility for large-scale production. In contrast, the microbial synthesis of GSH by Saccharomyces cerevisiae offers distinct advantages, such as rapid growth in inexpensive media, high intracellular GSH accumulation, and a Generally Recognized as Safe (GRAS) certification [6,7]. The primary challenge to the widespread application of GSH remains its relatively low yield, which can be addressed through the selection and screening of high-yield strains.
Conventional methods for achieving high GSH yields have been hindered by low mutation rates, labor-intensive screening procedures, and the requirement for large fermentation spaces [8]. To overcome these limitations, fully automatic high-throughput screening systems have been developed to facilitate the selection of target strains [9,10]. These systems integrate automated mutagenesis, medium perfusion, downscaled fermentation, high-throughput detection, and colony selection, significantly improving the efficiency of screening for high-yield strains. Over the last decades, atmospheric pressure and room temperature plasma (ARTP) has emerged as a powerful tool to enhance mutation rates and create mutant libraries [11]. The mutation mechanism of ARTP involves enhancing cell permeability and damaging DNA and proteins [12,13]. Additionally, deep microtiter plates (MTPs) enable the miniaturization of culture flasks, conserving space and reducing medium consumption [8,14,15]. Computational fluid dynamics (CFD) simulations are typically employed to predict the hydrodynamic properties of bioreactors and optimize process design [8]. Furthermore, a microplate reader can replace high-performance liquid chromatography (HPLC) for quantifying the majority of compounds, offering a more efficient and cost-effective alternative [14]. Thus, these high-throughput mutagenesis and screening devices are essential for accurately and reproducibly evaluating a large number of mutants.
In this study, we employed ARTP mutagenesis and a high-throughput screening system to enhance GSH production in S. cerevisiae. The ARTP mutagenesis generated a diverse pool of mutants. We conducted an evaluation of the culture conditions for high-throughput screening utilizing deep microtiter plates (MTPs) through CFD analysis. Additionally, a high-throughput detection method using a microplate reader was established for the rapid and accurate quantification of GSH. Subsequently, we validated the performance of the high-yield mutants in a shake flask. At last, we compared the fermentation performance and underlying mechanism of the selected mutant strain against the parent strain in 5 L bioreactors.
2. Materials and Methods
2.1. Strain and Media
The parent strain, Saccharomyces cerevisiae AY-014, was kindly provided by Angel Yeast Co., Ltd. (Yichang, China) and stored at −80 °C in a 20% glycerol solution. The plate medium contained 20 g/L glucose, 10 g/L yeast extract, 20 g/L peptone, and 20 g/L agar. Screening plates were supplemented with 0.2 g/L ethionine. The seed medium consisted of 30 g/L glucose, 6 g/L yeast extract, 3 g/L (NH4)2SO4, 1 g/L KH2PO4, 1 g/L K2HPO4, and 0.8 g/L MgSO4. The fermentation medium contained 25 g/L glucose, 20 g/L yeast extract, 8 g/L (NH4)2SO4, 3 g/L KH2PO4, and 1 g/L MgSO4. All media were adjusted to pH 5.5 with 6 M HCl and sterilized at 115 °C for 20 min. Yeast extract and peptone were purchased from Angel Yeast Co., Ltd. (Yichang, China), and other chemicals were purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Integrated Procedure of the Stain Mutagenesis and Screening
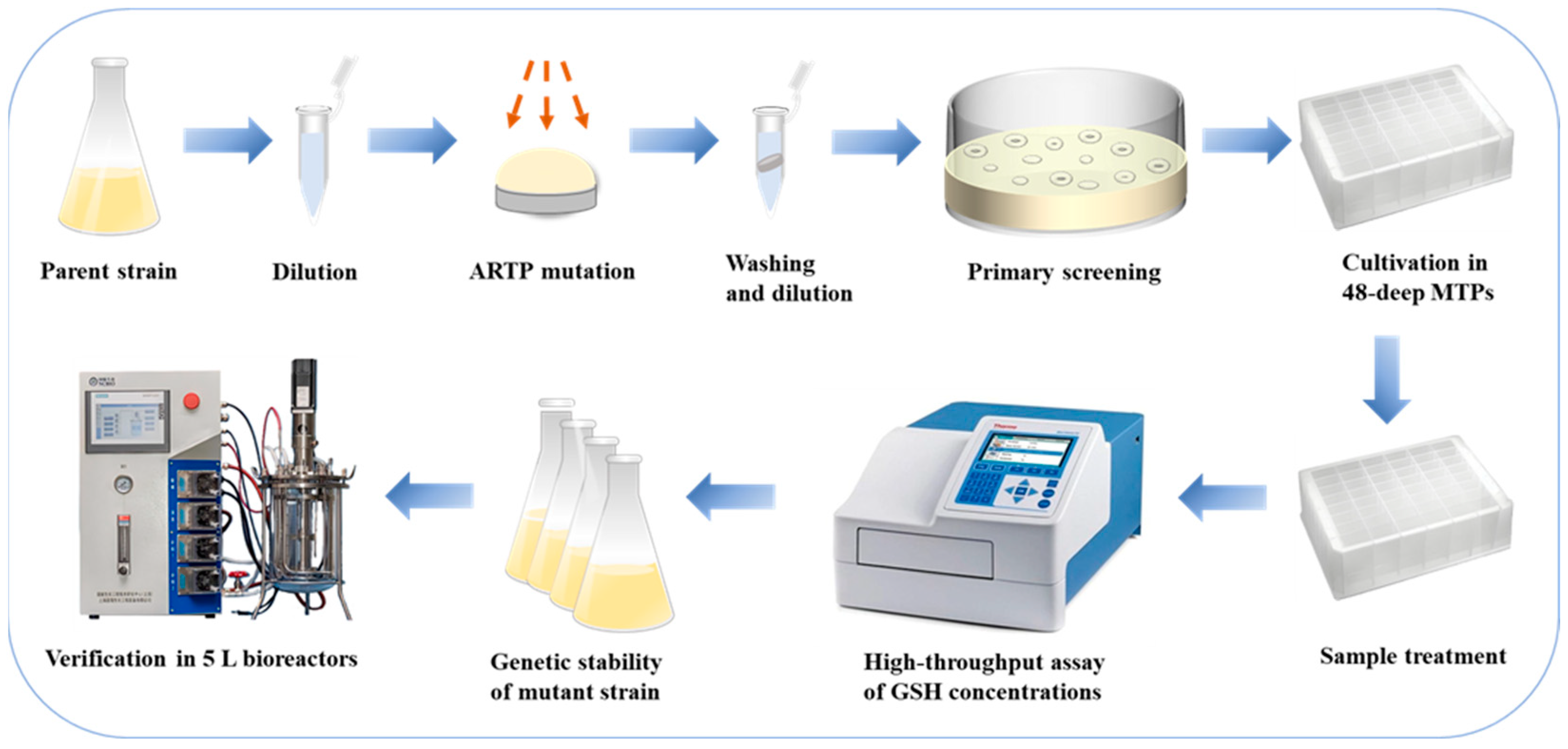
The integrated procedure of the strain mutagenesis and screening is illustrated in Figure 1. The parent strain was cultured in a 500 mL shake flask, and the fermentation broth was harvested during the exponential growth phase of the yeast cells. The cells were collected and subjected to ARTP mutagenesis. The mutagenized cell suspension was then diluted to an appropriate concentration and spread on ethionine-containing plates for cultivation. Ethionine is a structural analog of glutathione. The mutant strain resistant to ethionine effectively relieves the feedback inhibition of GSH. Based on the calculated lethality rate, mutant strains were selected and cultured in deep MTPs (Shanghai Canvic Biotechnology Co., Ltd., Shanghai, China) for high-throughput cultivation. Intracellular GSH concentrations were determined using the alloxan method in conjunction with a microplate reader (Multiskan GO, Thermo Fisher Scientific, Solon, OH, USA). Several strains exhibiting high GSH yields were chosen for genetic stability testing. Ultimately, strains with high and stable GSH production were selected and compared with the parent strain in 5 L bioreactors (NCBIO, Shanghai, China) to validate their fermentation performance.
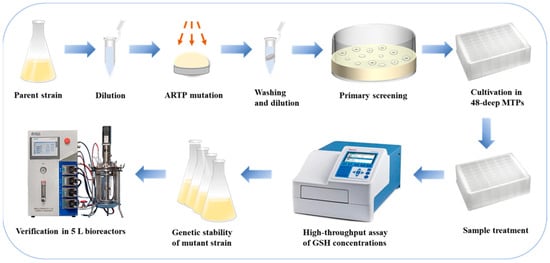
Figure 1.
Integrated procedure of the strain mutagenesis and screening.
2.2.1. Cultivation in Shake Flasks
S. cerevisiae was precultured in a 500 mL shake flask containing 50 mL of seed medium at 30 °C and 200 rpm for 24 h.
2.2.2. ARTP Mutagenesis Procedure
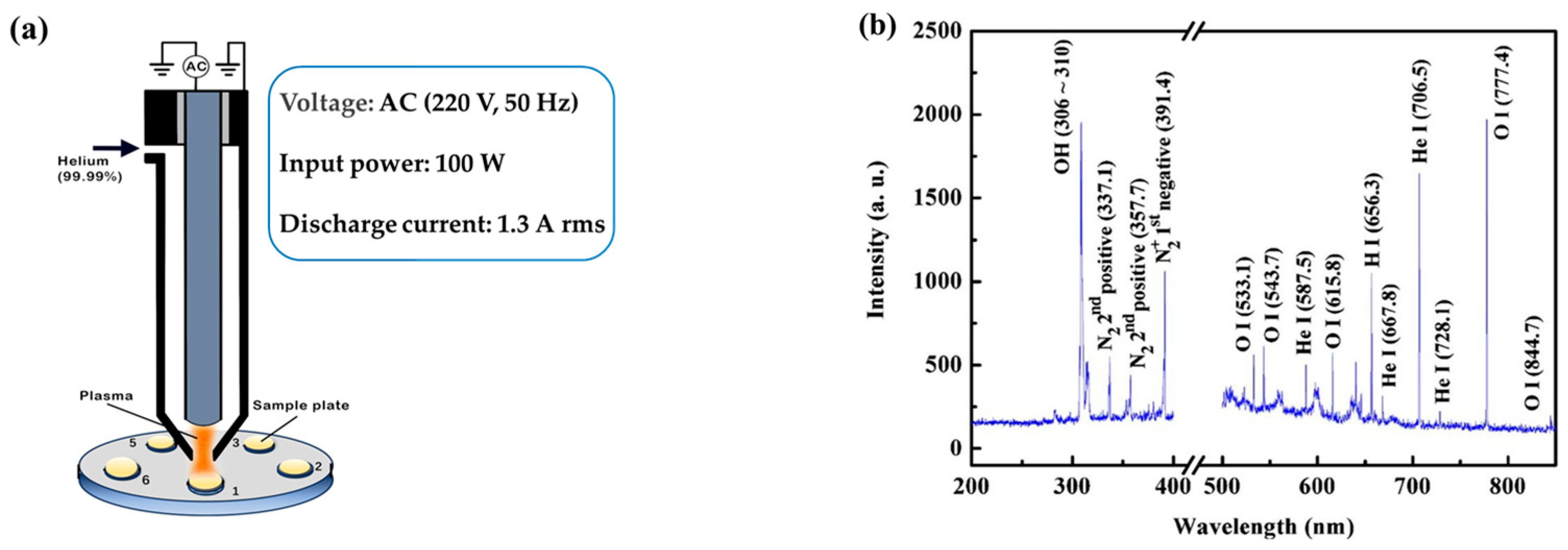
ARTP mutagenesis was performed using an ARTP-IIS mutagenesis system (Wuxi TMAXTREE Biotechnology Co., Ltd., Wuxi, China) [12,16]. This device is driven by an alternating current (AC) source with a voltage of 220 V and a frequency of 50 Hz (Figure 2a). The discharge current is measured to be 1.3 A rms (root mean square). In this system, helium (99.99%) is ionized by a radio-frequency electric field and then blown through a nozzle onto a microbial sample placed on a metal plate attached to a movable platform. This device is capable of generating plasma emitter streams that contain a high concentration of active particles under atmospheric pressure and at room temperature (25–40 °C). These active particles consist of hydroxyl (OH) free radicals, helium atoms, oxygen atoms, and nitrogen atoms (Figure 2b). These particles can induce DNA damage and activate the SOS repair mechanisms within living cells [17,18].
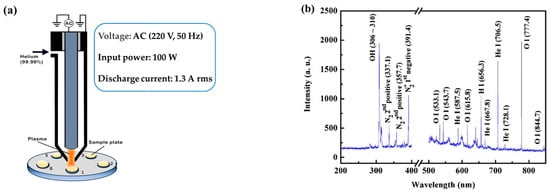
Figure 2.
The plasma jet generator functions as the core component of the ARTP system: (a) the schematic diagram of plasma jet generator; (b) plasma emission spectrum. (These figures were provided by Wuxi TMAXTREE Biotechnology Co., Ltd.).
The S. cerevisiae cells in the logarithmic phase were collected by centrifuging the fermentation broth and washed twice with sterilized saline solution. Then, 10 μL cell suspensions of approximately 2 × 105 CFU/mL were obtained by proper diluting and evenly spread on a sterile steel plate. Pure He2 was used as the plasma working gas in the ARTP mutation, and the operating parameters were as follows: (1) mutagenic input power was 100 W (approximately equal to the consumed power); (2) irradiation distance was 2 mm; and (3) the temperature of the plasma jet was below 30 °C. Under these operating conditions, the ARTP mutagenesis dosage depended solely on the treatment time. Yeast cells were exposed to the ARTP jet for 20, 40, 60, 80, 100, and 120 s, with three parallel slides for each treatment time.
After the mutagenic treatment, cell suspensions were diluted appropriately and spread onto ethionine screening plates for 48 h at 30 °C. The lethality rate of yeast cells was calculated using colony counts:
There were 420 colonies with a specific lethality rate to be selected and inoculated in deep MTPs for high-throughput cultivation.
2.2.3. High-Throughput Cultivation in Both 24-Deep MTPs and 48-Deep MTPs
High-throughput cultivation was performed in both 24-deep MTPs and 48-deep MTPs (Shanghai Canvic Biotechnology Co., Ltd.). The 24-deep MTPs contained 2 mL per well of fermentation medium with 10% (v/v) precultures, while the 48-deep MTPs contained 1 mL per well. A customized sandwich cover plate was designed to optimize mass transfer and mitigate evaporation [15]. It consisted of a microporous filter membrane, a porous silicone spacer plate, and a stainless-steel perforated cover plate. This configuration was particularly effective in preventing the evaporation of ethanol, acetic acid, and culture medium. Other culture conditions were identical to those used in shake flasks. After incubation, 0.5 mL of culture suspension from each well was centrifuged, and the precipitate was used to measure the GSH concentration. Strains with high GSH production were further cultured in shake flasks to validate yields and propagation stability. The target strain was ultimately identified based on these results.
2.2.4. High-Throughput Assay of GSH Concentrations
GSH concentration was measured using the alloxan method [19]. GSH reacts with alloxan to produce an ultraviolet absorbance peak at 305 nm, allowing quantification via a microplate reader (Thermo Fisher Scientific). A solution of 1 g/L alloxan was prepared in 0.1 M HCl, along with 0.1 M glycine solution and 0.24 M Na2HPO4-NaH2PO4 buffer (pH = 7.6). A standard curve was generated using a series of GSH concentrations (0, 40, 80, 120, 160, and 200 μM) prepared by dilution. The reaction mixture contained 0.2 mL of standard solution, 0.7 mL of NaHPO4-NaH2PO4 buffer (pH = 7.6), and 0.1 mL of glycine solution, with 0.2 mL of alloxan solution added to initiate the reaction. After 20 min, the solution was transferred to a 96-well quartz microplate for absorbance measurement at 305 nm. These chemicals were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China).
For sample analysis, 0.5 mL of the culture were loaded into 48-deep MTPs and centrifuged using a deep MTPs centrifuge (HTC-R002, Shanghai Canvic Biotechnology Co., Ltd., Shanghai, China) at 2810× g, 4 °C for 10 min. Intracellular GSH was extracted with 40% ethanol at 30 °C for 2 h and cells were removed using the deep MTPs centrifuge (Shanghai Canvic Biotechnology Co., Ltd.). GSH concentration was determined using the standard curve.
2.2.5. L Bioreactor Cultures
A total of 300 mL of seed culture was precultured in shake flasks and then transferred to a 5 L fermenter with a 3 L working volume of fermentation medium for 24 h. Agitation and aeration were adjusted to maintain adequate dissolved oxygen (DO ≥ 30%). The oxygen uptake rate (OUR) was monitored using a process mass spectrometer (Thermo Fisher Scientific, USA) equipped with Biostar software 5.5 (NCBIO, Shanghai, China). Samples were taken every 2 h until the end of fermentation.
2.3. Analysis of Computational Fluid Dynamics (CFD)
CFD simulations were conducted to analyze the unsteady turbulent flow field in 24-deep MTPs and 48-deep MTPs (Shanghai Canvic Biotechnology Co., Ltd.). The methodology for CFD modeling was adapted from Li’ s research [20]. The flow field of these MTPs was simulated at a rotational speed of 200 rpm and an amplitude of 25 mm. Approximately one million computational grids were generated using the ANSYS ICEM CFD 12.0 (ANSYS Inc., Canonsburg, PA, USA) technique. A free surface model was employed to simulate the two-phase flow, and the CFD model equations were solved using ANSYS CFX 12.0 (ANSYS Inc., Canonsburg, PA, USA). All domain boundaries were defined as no-slip wall boundary conditions. The physical simulation duration was set at 5 s (equivalent to 15 cycles at 200 rpm) to achieve a quasi-steady liquid shape. The results were considered convergent when all root mean squares were below 1 × 10−4 on a 16-process Dell server.
2.4. Other Analytical Methods
2.4.1. DCW Determination
A total of 2 mL of the fermentation broth was centrifuged at 9450× g at 4 °C for 5 min. The cell pellet was washed twice with deionized water and dried at 80 °C for 24 h until a constant weight was reached [21].
2.4.2. Sugar Determination
The residual glucose concentration was measured using an SBA-40E biosensor (Biology Institute of Shandong Academy of Sciences, Jinan, China) [22].
2.4.3. HPLC Assay of GSH Concentrations
Approximately 0.4 g of wet cells were added to 30 mL of 0.01 M HCl and extracted in boiling water for 5 min. After cooling, the mixture was brought to a constant volume of 50 mL, filtered through a 0.45 μm microporous filter, and transferred to HPLC vials.
The GSH concentration was analyzed using HPLC (Agilent 1290, Agilent technologies, CA, Santa Clara, CA, USA), with a C18 column at a flow rate of 1.0 mL/min and column temperature of 35 °C [23]. The injection volume was 20 μL, with a detection wavelength of 210 nm. The mobile phase consisted of A:B = 90:10 (A: phosphate buffer containing 3.4 g potassium dihydrogen phosphate and 1.1 g sodium heptane sulfonate in 500 mL water, adjusted to pH 3.05 with phosphoric acid; B: pure methanol). Methanol was purchased from Sigma Aldrich (Sigma-Aldrich, St. Louis, MO, USA). The GSH concentration could be calculated by the standard curve as follows: GSH concentration (g/L) = (A − 12.7)/23553 (A was the area of the peak, R2 = 0.9997). GSH content was calculated as follows:
2.4.4. Determination of Ethanol Concentration in Fermentation Broth
Ethanol concentrations in the fermentation broth were determined by gas chromatography (Agilent 7820 A; Agilent technologies, Santa Clara, CA, USA) [24]. The supernatant of the fermentation broth was filtered through a 0.22 µm membrane and appropriately diluted with deionized water.
Standard ethanol solutions were prepared by adding 0.1 mL, 0.25 mL, 0.75 mL, 1.0 mL, 1.25 mL, 1.5 mL, 1.75 mL, 2.5 mL, 3 mL, 4 mL, and 5 mL of ethanol (chromatography pure, Sigma-Aldrich, St. Louis, MO, USA) to a 100 mL volumetric flask. After adding the ethanol, the flask was filled to the 100 mL mark with deionized water.
The chromatographic conditions were as follows: initial temperature of 40 °C for 7 min, increased to 200 °C at a rate of 20 °C/min, and maintained for 5 min. Injection port temperature: 200 °C; FID detector: 230 °C; vaporization chamber temperature: 180 °C; tail blowing N2 flow: 0.03 L/min; H2 flow rate: 0.03 L/min; air flow rate: 0.4 L/min. The carrier gas was adjusted by constant tail blowing and gas flow. Injection volume: 0.5 μL; split ratio: 5:1.
2.4.5. Analysis of Intracellular Metabolites and Enzyme Activity Assays
Intracellular precursor amino acids (L-glutamate, L-cysteine, and glycine) (Sigma-Aldrich, St. Louis, MO, USA) and trehalose (Sigma-Aldrich, St. Louis, MO, USA) were quantified using gas chromatography/mass spectrometry (GC/MS) analysis (7890 A GC coupled to 5975 C MSD; Agilent Technologies, Santa Clara, CA, USA) [25]. The sample preparation for GC/MS analysis was performed with meticulous attention to detail. Initially, 1 mL of the culture broth was quickly quenched in cold methanol and then centrifuged at low temperature (4 °C, 6000× g for 5 min). The supernatant was carefully removed, and the pelleted cells were stored at −80 °C. Prior to analysis, the cells were treated with 75% boiling ethanol, and 13C internal standards were added simultaneously. After a 5 min boiling period, the solution was evaporated and concentrated to 600 µL using a rotary evaporator (RapidVap, Labconco, KS, USA) for intracellular metabolite analysis. A volume of 100 µL from the concentrated solution was subjected to derivatization. The derivatized samples were then lyophilized at −70 °C before being analyzed by GC/MS.
For the GC/MS measurement, 100 µL of the concentrated extract was freeze-dried for 12 h. The solid residue was then dissolved and derivatized using a mixture of 75 µL acetonitrile (Sigma-Aldrich, St. Louis, MO, USA) and 75 µL N-methyl-N-(tert-butyldimethylsilyl) trifluoroacetamide (Sigma-Aldrich, St. Louis, MO, USA) at 70 °C for 1 h. The derivatized solution was centrifuged at 13,600× g for 2 min, and 100 µL of the supernatant was transferred to GC/MS analysis. Standard curves for the IDMS IS were generated by injecting a mixture of 13C-labeled cell extract and a series of 12C metabolites from Sigma (Sigma-Aldrich, St. Louis, MO, USA), prepared in a 1:5 volume ratio, into the MS/MS system. The peak height ratios (PH) of 13C to 12C metabolites (PH13C/PH12C) were regressed against the concentration of 12C metabolites to establish the standard curves.
γ-GCS enzyme activity was measured using commercial kits (A091-1-1, Jiancheng Bioengineering Institute, Nanjing, China) [26]. The assay mixture included ATP (50 mM), glutamate (100 mM), bovine serum albumin (50 mg), and post-nuclear supernatant (70−100 mg protein) in a 125 μL volume with 0.1 M Tris-HCl (pH 8.2). The reaction was initiated by adding l-amino butyrate (100 mM) and then incubated at 37 °C for 30 min. It was terminated by adding 125 μL of 10% trichloroacetic acid. After adding 1.0 mL 1.6% ammonium molybdate and 0.4 mL 1% ferrous sulfate, the mixture was centrifuged at 5000× g for 5 min. The absorbance of the supernatant was measured at a wavelength of 660 nm. One unit of γ-GCS activity was defined as 1 μmol Pi released per hour per mg protein.
3. Results and Discussion
3.1. Establishment of a High-Throughput Culture Method for Saccharomyces Cerevisiae
To determine the optimal size of MTPs for high-throughput culture, the fermentation performance of S. cerevisiae was compared across three different bioreactor scales: shake flasks, 24-deep MTPs, and 48-deep MTPs.
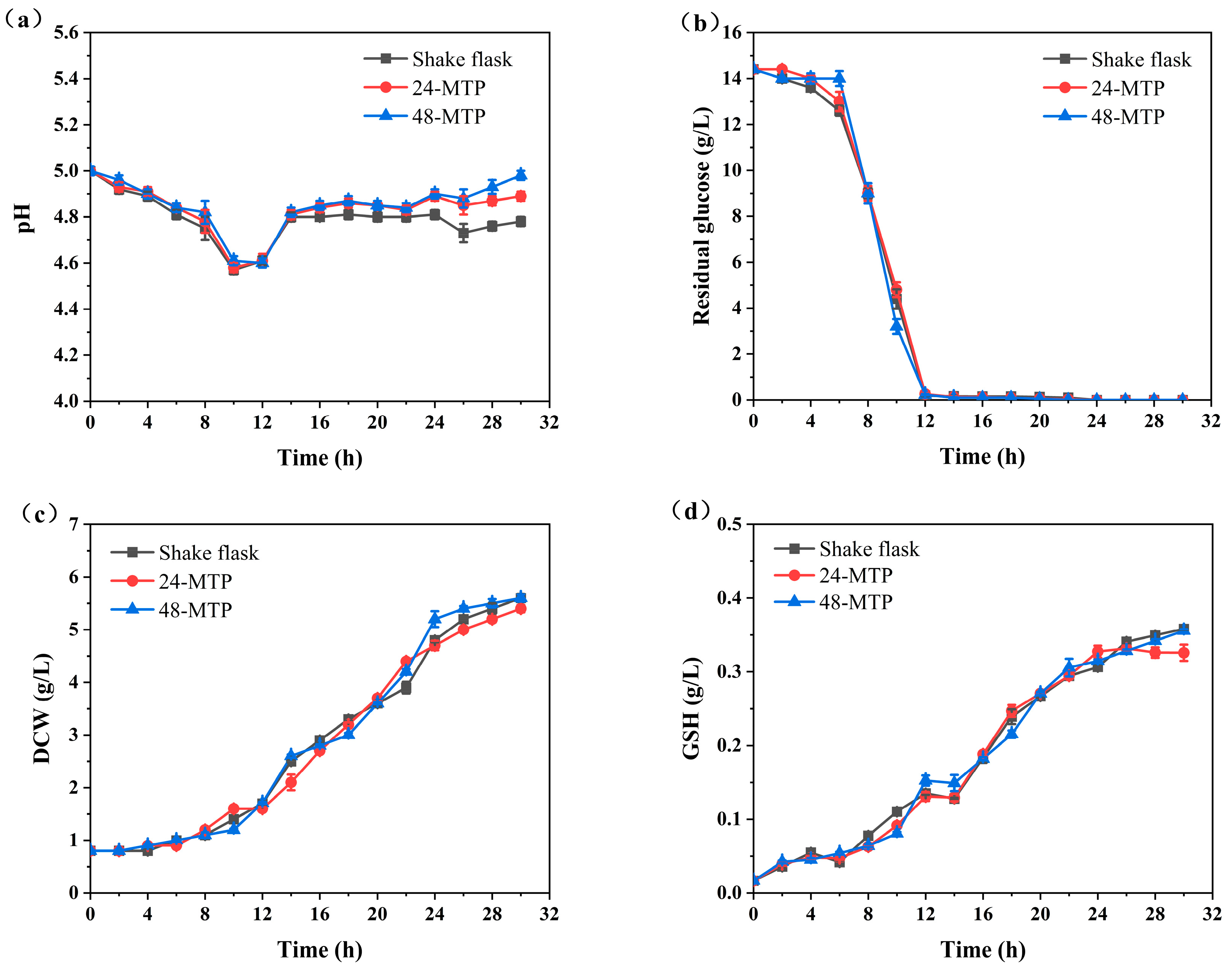
Figure 3 shows a consistent trend in the evolution of DCW and GSH concentrations across all three bioreactor types during 0–30 h. Initially, glucose was consumed, with a portion converting to ethanol, causing the pH to drop from 5.0 to 4.6. After 12 h, when glucose was exhausted, S. cerevisiae began to metabolize ethanol. Notably, during the transition phase from glucose to ethanol utilization (12–14 h), there was no significant increase in GSH concentration (Figure 3d). After 24 h, both DCW and GSH concentrations plateaued, indicating that cell growth had entered a stable phase (Figure 3c,d). Therefore, fermentation broth samples were collected at 24 h in subsequent high-throughput screening experiments to better reflect yeast cell metabolism.
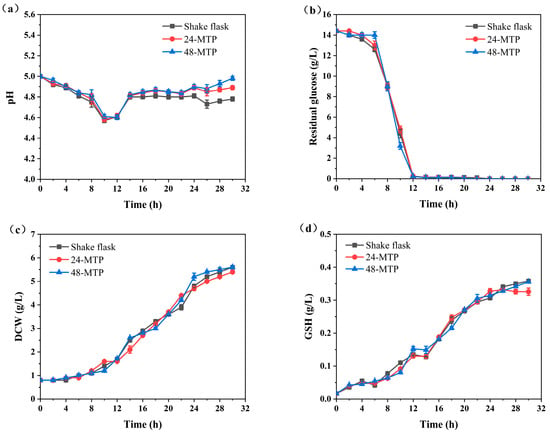
Figure 3.
Comparison of cell culture performance in shake flasks, 24-deep MTPs, and 48-deep MTPs: (a) pH; (b) residual glucose; (c) dry cell weight (DCW); and (d) GSH concentration.
The pH values in shake flasks were significantly lower than those in 24-deep MTPs and 48-deep MTPs (Figure 3a). This phenomenon was effectively explained using computational fluid dynamics (CFD) simulations. Simulations at the rotational speed of 220 rpm captured the variations in mass transfer, turbulence parameters, and shear environment within these three different bioreactors (Table 1).

Table 1.
The CFD parameters of shake flasks, 24-MTPs, and 48-MTPs.
Turbulence parameters, such as the energy dissipating rate (ε) and volumetric power consumption (P/V), are crucial for studying fermentation processes [27]. The P/V and ε values of the 500 mL shake flasks were considerably higher than those of deep MTPs. Conversely, the shear strain rate (SSR) provides a quantitative assessment of the shear environment within bioreactors. The oxygen volumetric mass transfer coefficient (KLa), which characterizes oxygen transfer at the gas–liquid interface, was highest in 48-deep MTPs (189.7 h−1), representing a 2.63-fold increase compared to shake flasks (72.1 h−1). This higher KLa value explained the slower pH recovery in shake flasks, attributed to limited oxygen availability, which affects microbial metabolism.
To mitigate the oxygen supply limitation in the shake flask, the filling volume was optimized to 30 mL, increasing the specific surface area of the gas–liquid interface and resulting in a higher KLa value of 125.1 h−1. Thus, CFD simulations provided hydrodynamic characteristic parameters that are difficult to obtain through traditional experimental techniques [20]. These parameters can explain the differential metabolism exhibited by microorganisms in varying bioreactor scales and guide the selection and design of suitable bioreactors for culturing diverse microbial species.
The results indicate that 48-deep MTPs show significant potential as an effective alternative to traditional shake flasks. In CFD simulations, 48-deep MTPs exhibited excellent oxygen transfer efficiency, which is more conducive to enhancing cellular metabolic activity and GSH production. This finding is consistent with the work of Tan et al., who reported similar improvements in oxygen transfer efficiency with 48-deep MTPs, which could serve as a better alternative to shake flasks for culturing high-aerobic microbes as a scale-down tool [15]. Moreover, the space required to cultivate one mutant in a shake flask is equivalent to the space needed to simultaneously cultivate 48 mutants in 48-deep MTPs, greatly saving cultivation space. Additionally, 48-deep MTPs required only a small amount of culture media (1 mL/well), significantly reducing the cost of culture media [28].
3.2. High-Throughput Qualitative Analysis of GSH
In mutation breeding processes, strains undergo random mutations without specific direction. Therefore, implementing appropriate screening methodologies after mutagenesis is crucial for improving the efficiency of mutant strain selection, increasing the probability of discovering target products, and enhancing experimental accuracy [29]. The ability to rapidly and accurately quantify GSH is essential for identifying high-yielding mutant strains.
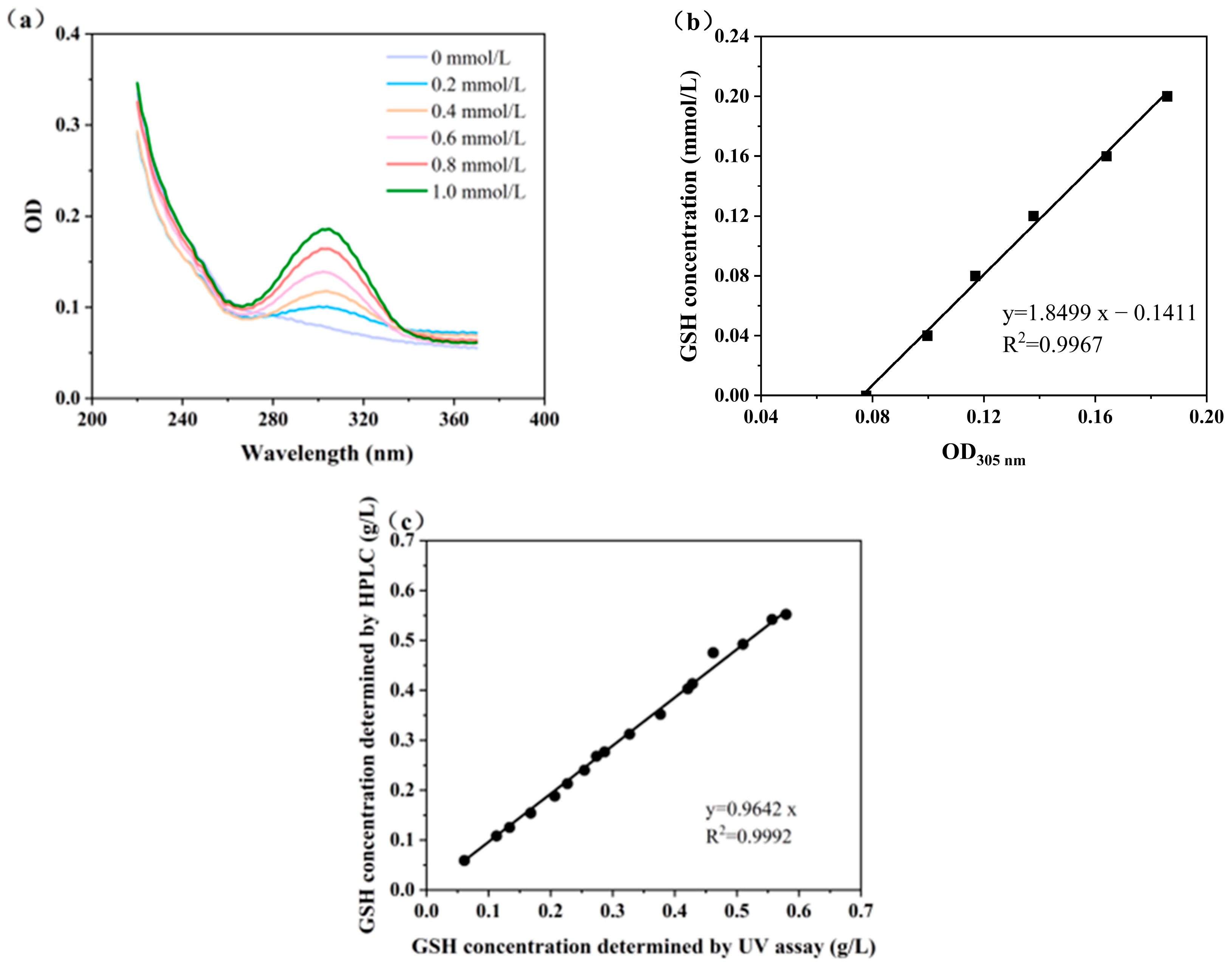
The alloxan molecule contains a highly reactive 5-carbonyl group, which can specifically react with the sulfhydryl group (-SH) in GSH to form derivatives with UV absorption properties. This derivative produces a characteristic absorption peak at 305 nm, which can be detected by a microplate reader. The specificity of this reaction minimizes interference from other compounds, ensuring accurate quantification even in complex biological samples. Figure 4a shows the characteristic absorption peak of GSH derivatives at 305 nm, using a microplate reader with full wavelength scan mode. This peak is indicative of the formation of a specific adduct resulting from the reaction between GSH and alloxan, which serves as the basis for GSH quantification. Figure 4b displays a standard curve with an R2 value of 0.9967, indicating an excellent correlation between OD305 values and GSH concentrations, following the equation GSH concentration (mmol/L) = 1.8499 OD305 − 0.141. This linear relationship enables the precise quantification of GSH with a microplate reader.
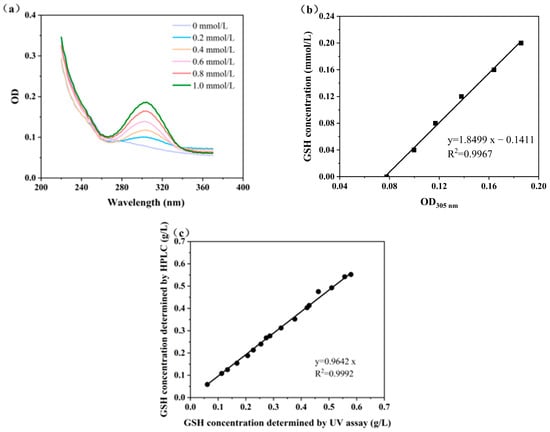
Figure 4.
High-throughput determination of GSH concentrations: (a) full wavelength scan of derivative GSH; (b) the standard curve for GSH rapid determination; and (c) GSH concentrations determined by microplate reader and HPLC.
To validate the reliability of alloxan method, 16 fermentation broth samples were randomly selected and analyzed for GSH concentrations using both the microplate reader and HPLC. The GSH concentrations measured by the microplate reader closely matched those determined by HPLC, with a correlation coefficient of 0.9992 (Figure 4c). This high concurrence confirms that the alloxan method combined with microplate detection is a reliable alternative for determining GSH concentrations. It requires fewer reagents and less sophisticated equipment compared to HPLC, making it accessible for both academic and industrial settings. Additionally, the microplate reader allows for the simultaneous detection of multiple samples, significantly increasing throughput [30]. This is particularly advantageous for large-scale screening applications, where rapid analysis is crucial.
While other methods, such as the DTNB method, offer high sensitivity and specificity, they often suffer from complex procedures and higher background interference [19,31]. In contrast, the alloxan method provides a balance between sensitivity, specificity, and practicality. This makes it an ideal choice for high-throughput screening, where the rapid and accurate identification of high-yielding mutant strains is essential. The alloxan method, combined with microplate reader detection, offers several advantages. For instance, the microplate reader’s capability to perform full wavelength scans ensures versatility and adaptability for various biochemical assays, further enhancing its utility in diverse research settings.
The alloxan method, when combined with microplate reader detection, is especially effective for high-throughput screening applications, where the rapid and accurate identification of high-yield mutant strains is essential. Research has shown that high-throughput screening methods based on UV/visible spectroscopy are more suitable for large-scale mutant screening than HPLC measurements [32]. For instance, Sarnaik et al. demonstrated the efficiency and accuracy of the microplate method in detecting microbial metabolites, showing results comparable to those obtained by HPLC, but with greater ease of operation and lower cost [33]. This highlights the potential of the alloxan method as a reliable and cost-effective alternative for GSH quantification in high-throughput screening. Numerous products can be quantified using the microplate reader, such as tylosin [34], L-lactic acid [8], and (2S)-naringenin [32], further underscoring its broad applicability.
Despite these advantages, HPLC remains a well-established analytical technique with irreplaceable benefits. It typically provides superior separation capabilities and detection precision, especially for a complex sample analysis [35]. However, the complexity and higher cost of HPLC operations limit its widespread application in high-throughput screening. Nevertheless, for a complex sample analysis that requires higher precision and separation capabilities, HPLC continues to be an indispensable tool in analytical chemistry.
3.3. High-Throughput Screening of High GSH-Producing Strains
Mutagenesis is a powerful tool for generating microbial strains with enhanced metabolic capabilities, but it often results in a wide range of genetic changes with varying impacts on cellular function. Various mutagenesis methods induce different degrees of DNA damage to microorganisms, leading to distinct phenotypic characteristics among the mutants. In this study, we employed ARTP mutagenesis coupled with ethionine-resistant plates to identify high GSH-producing strains of S. cerevisiae.
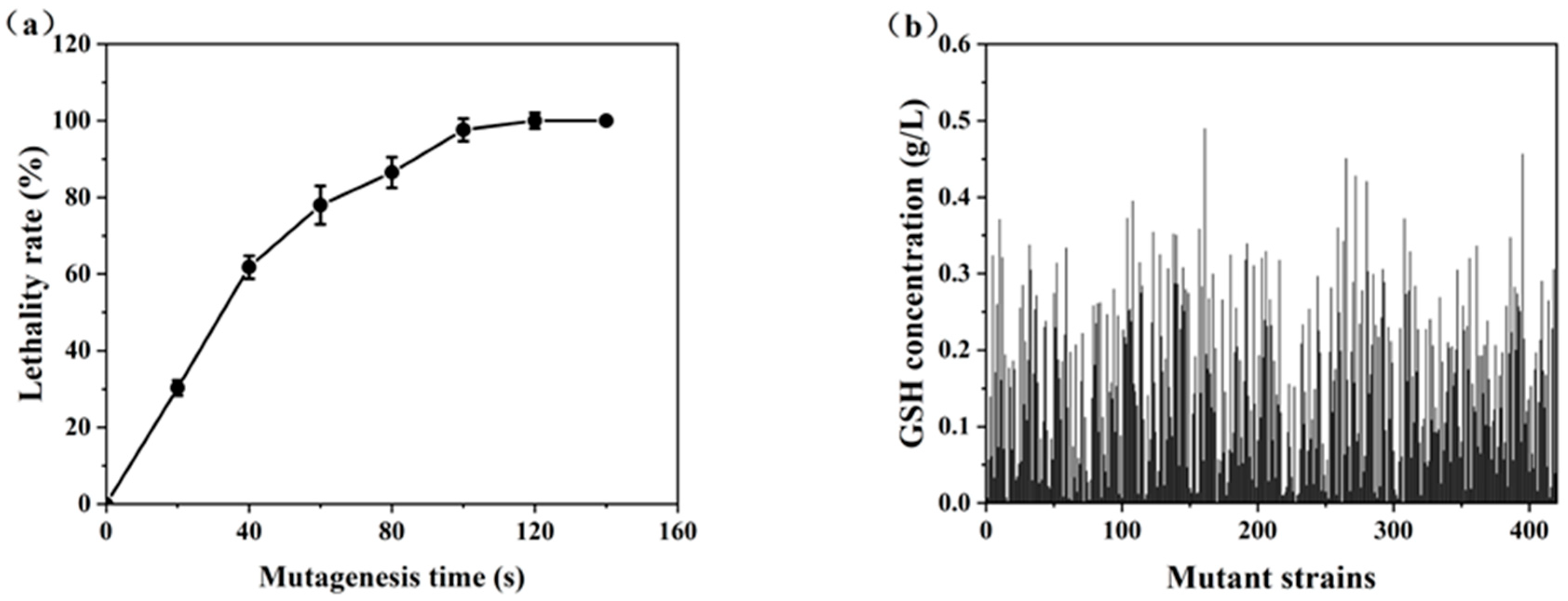
As depicted in Figure 5a, the lethality rate of the treated cells increased progressively with longer exposure times to the ARTP jet. Lethality rates reached 30.3%, 61.8%, 78.0%, 86.5%, 97.6%, and 100% after exposure for 20, 40, 60, 80, 100, and 120 s, respectively. An optimal lethality rate of approximately 85% is ideal for balancing genetic diversity and cellular viability [12]. Therefore, the treatment time of 80 s, which resulted in a lethality rate of 86.5%, was chosen as the mutagenesis time.
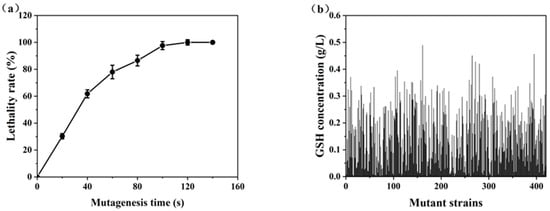
Figure 5.
High-throughput screening of high GSH-producing strains: (a) the lethality rate curve; (b) GSH concentrations of the mutant strains.
In the primary screening experiments, a total of 420 colonies were isolated from the mutagenized population. However, only 7.14% of these colonies displayed a positive mutation rate, indicating the rarity of desirable mutations. Notably, six strains (S-108, S-161, S-265, S-272, S-280, and S-395) exhibited significant GSH production, exceeding that of the parent strain by more than 20%. These strains were selected for further propagating stability tests in shake flasks to assess their potential for industrial application.
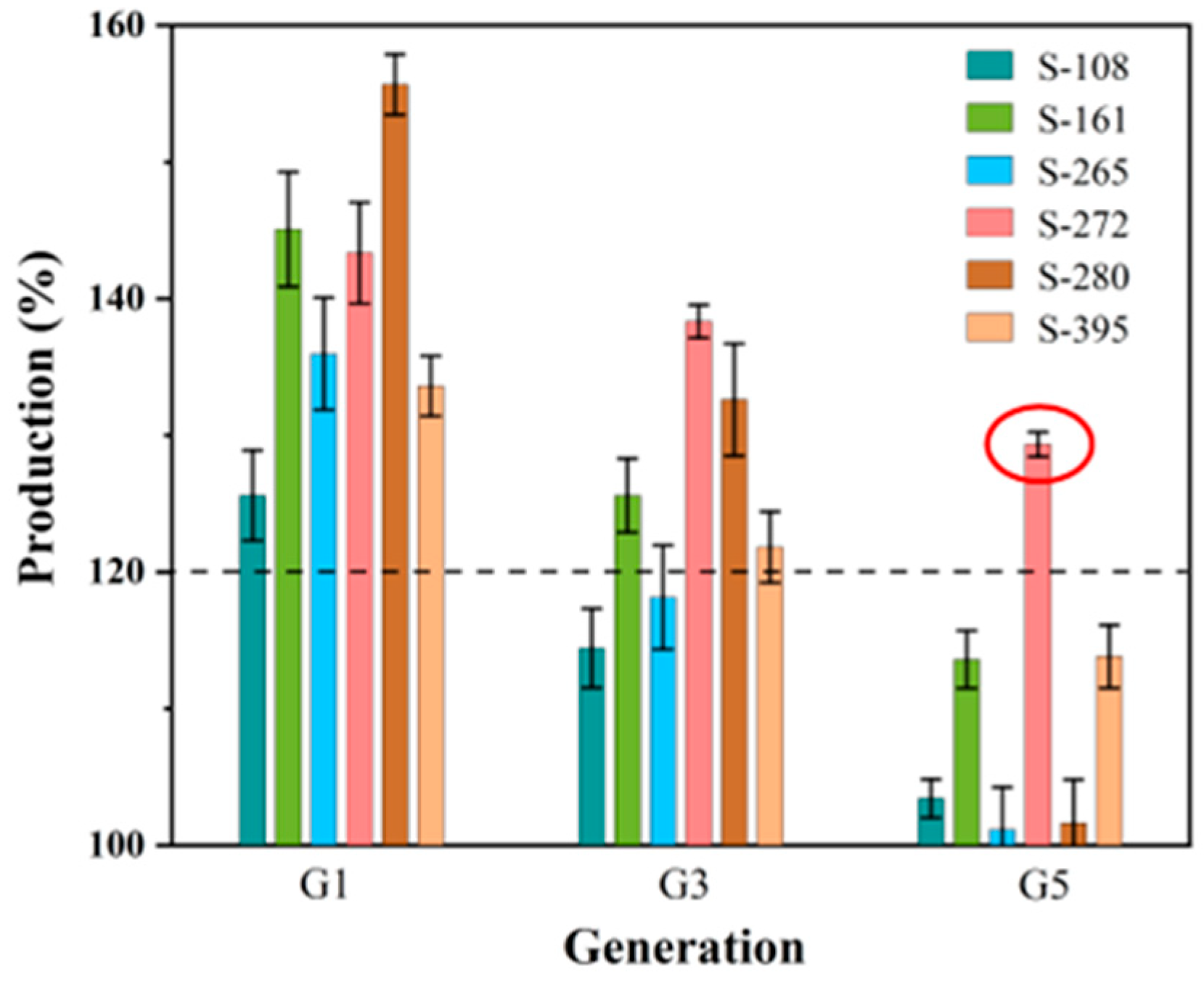
Before initiating fermentation verification, the propagating stability tests of the six selected strains over five generations were investigated (Figure 6). The results revealed a decline in GSH yield ranging from 9.77% to 34.73% across the strains. Strain S-272 demonstrated exceptional stability, with a minimal yield reduction of merely 9.77% by the fifth generation. Moreover, the GSH yield of S-272 was 29.33% higher than that of the parent strain, highlighting its potential for sustained high production. Based on these findings, S-272 was selected for further fermentation verification.
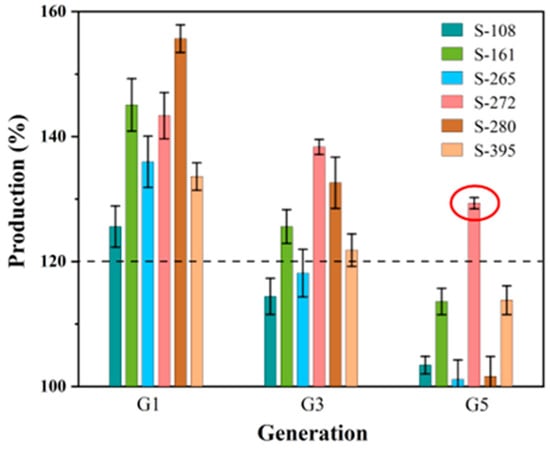
Figure 6.
Production variation in the propagating stability tests. (The red circle points out the production of strain S-272 selected for further fermentation verification).
Many studies have revealed that ARTP mutagenesis technology is an effective and convenient method to construct mutant libraries of both prokaryotes and eukaryotes [13,36,37]. The ARTP technology has been shown to enhance mutation rates and create diverse mutant libraries, which are crucial for identifying strains with improved metabolic capabilities [29]. The success of our screening strategy underscores the effectiveness of combining ARTP mutagenesis with targeted selection methods. The high lethality rate achieved with an 80 s ARTP treatment ensured a robust mutagenesis process, generating a diverse pool of genetic variants. The exceptional stability of S-272 over multiple generations suggests that the genetic changes induced by ARTP have led to stable and inheritable improvements in GSH production. This stability is crucial for industrial applications, where consistent performance is required for large-scale fermentation processes.
3.4. Fermentation Verification of the High-Yield Mutant S-272 in 5 L Bioreactors
The high-yield strain S-272 and its parental strain were cultured in 5 L bioreactors. To ensure the comparability of their respective fermentation processes, batch fermentations were performed for 24 h. The results were replicated in at least three independent experimental batches to confirm the consistency and reproducibility. Throughout these experiments, parameters such as inoculum volume, culture medium composition, and operating conditions were maintained consistently.
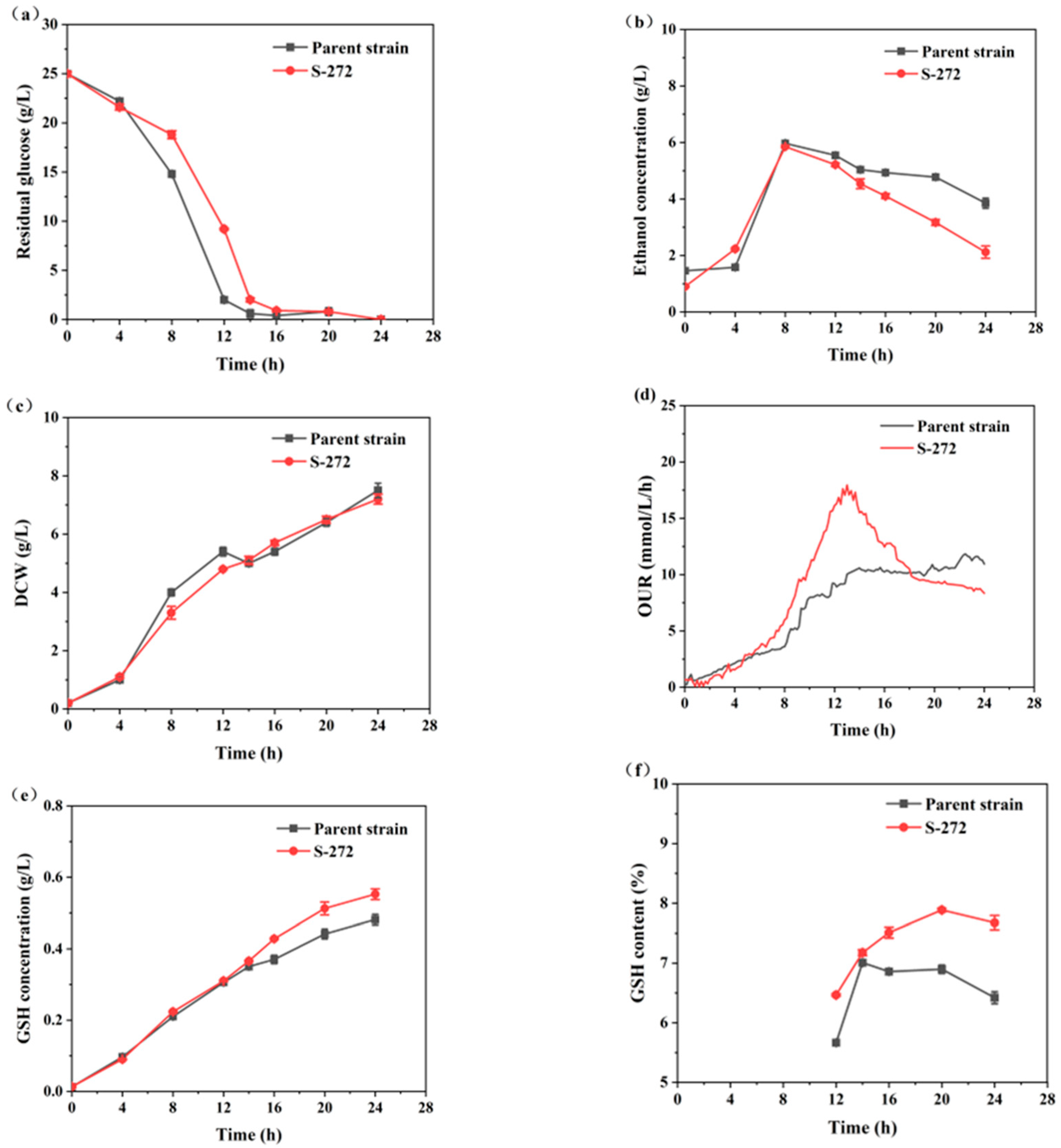
Figure 7 shows the curves for residual sugar, ethanol concentration, DCW, OUR, GSH concentration, and GSH content. The physiological characteristics of strain S-272 were significantly different from the parent strain. At the beginning of the fermentation, the glucose consumption rate of strain S-272 was slower than that of the parent strain (Figure 7a). Moreover, the DCW of strain S-272 was higher than that of the parent strain (Figure 7c). After 12 h, the ethanol concentrations of strain S-272 decreased rapidly, indicating its efficient synthesis of enzymes to utilize ethanol (Figure 7b). In contrast, the parent strain experienced a period of growth stagnation during 12–14 h. The plasma streams generated by ARTP mutagenesis significantly enhance the permeability of microbial cell membranes, leading to DNA damage. This damage triggers an SOS repair process, which is highly error-prone, introducing various discrepancies during the repair phase and consequently yielding a multitude of mutant strains for screening [38,39]. The selected strain S-272 was more capable of using ethanol than the parent strain. Figure 7d illustrates a reduction in OUR in the parent strain, which is attributed to a decline in respiratory intensity in response to an incremental rise in the ethanol concentration within the fermentation broth.
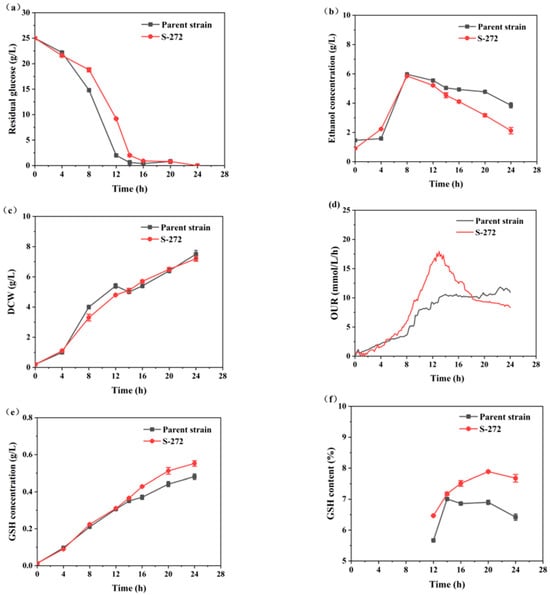
Figure 7.
Comparison of parameter profiles between the mutant S-272 and the parent strain during the fermentation processes: (a) residual glucose; (b) ethanol concentration; (c) DCW; (d) OUR; (e) GSH concentration; and (f) GSH content.
Table 2 summarizes the specific growth rate, specific ethanol consumption rate, specific product synthesis rate, final GSH yield, and intracellular GSH content during the rapid GSH synthesis phase (14–24 h). The specific growth rate of the parent strain was 0.04 h−1, exceeding that of S-272. However, S-272 demonstrated a significant improvement in metabolic efficiency, with a specific ethanol consumption rate of 0.046 g/g DCW/h and a specific product synthesis rate of 0.004 g/g DCW/h, representing increases of 84.0% and 3.3% compared to the parent strain, respectively. At the end of fermentation, S-272 achieved a GSH concentration of 0.553 g/L and an intracellular GSH content of 7.68%, which were 14.73% and 19.55% higher than those of the parent strain.

Table 2.
Comparison of fermentation performances with the mutant S-272 and the parent strain (14–24 h).
3.5. Exploration of the High GSH-Producing Mechanism in S-272
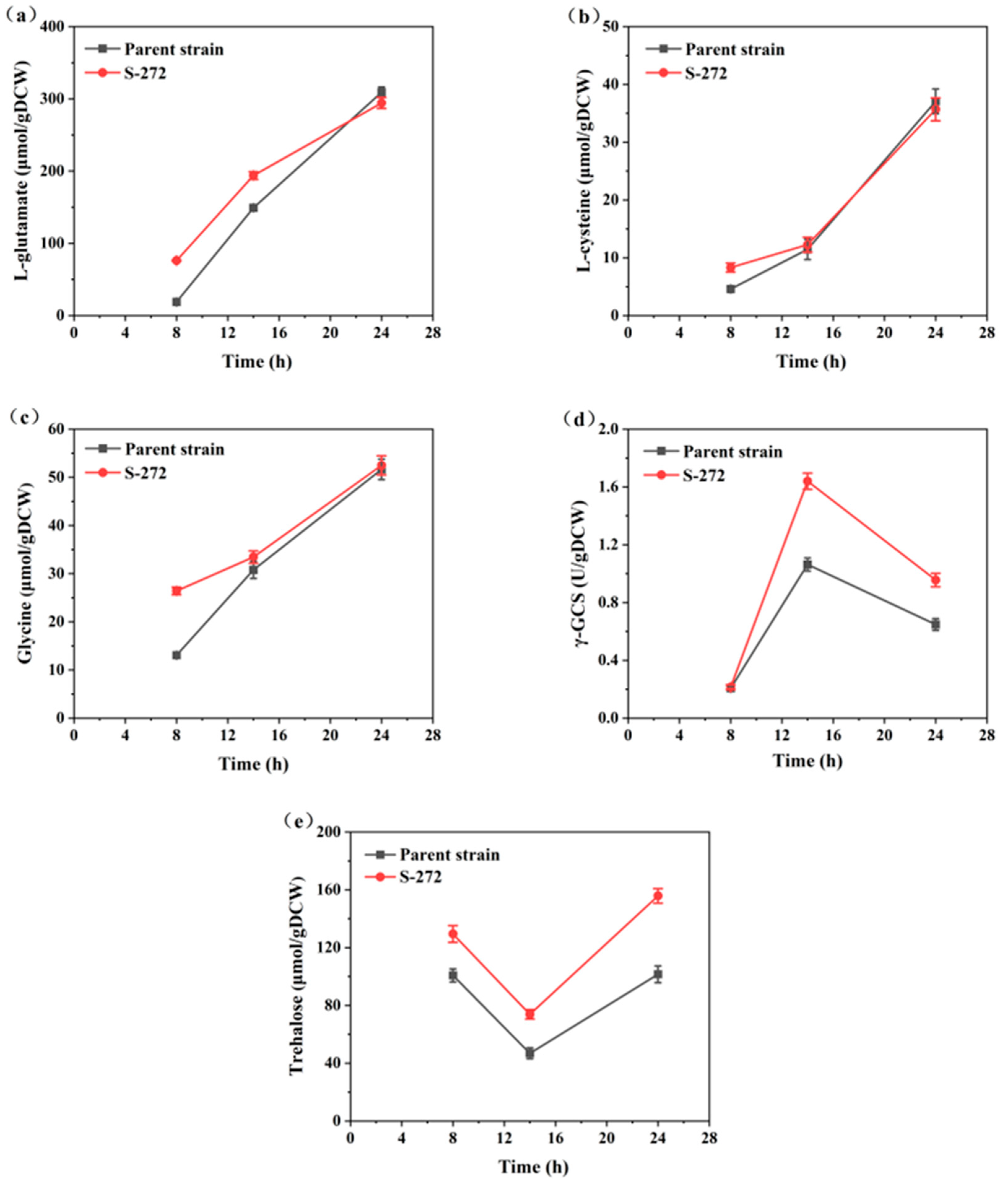
The synthesis of glutathione (GSH) is a two-step enzymatic process. The first step is catalyzed by γ-glutamylcysteine synthetase (γ-GCS), which synthesizes γ-glutamylcysteine (γ-GC) from glutamate and cysteine in the presence of ATP. This step is rate-limiting for GSH synthesis, and the activity of γ-GCS directly influences GSH production [40]. The second step involves GSH synthase, which adds glycine to γ-GC to form GSH. By assessing intracellular metabolite concentrations and enzyme activities, metabolic changes were observed between the parent strain and S-272 (Figure 8).
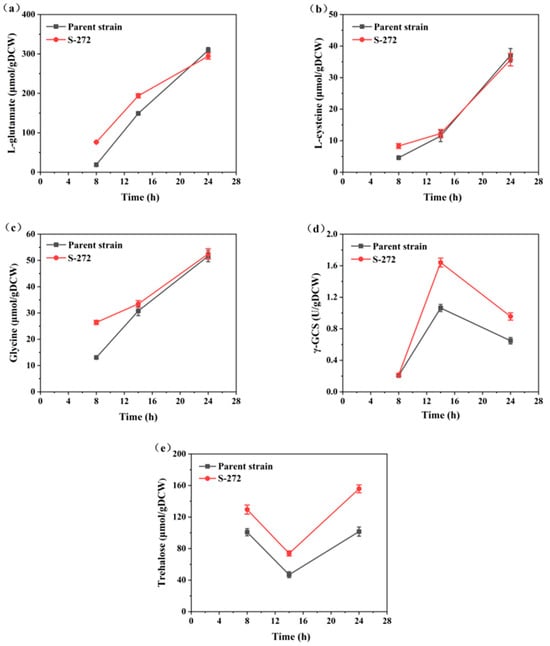
Figure 8.
Comparison of key intracellular substance concentrations and enzyme activities between S-272 and the parent strain: (a) L-glutamate concentration; (b) L-cysteine concentration; (c) glycine concentration; (d) γ-GCS activity; and (e) trehalose concentration.
Notably, intracellular precursor amino acids (L-glutamate, L-cysteine, and glycine) were increased significantly during the ethanol consumption phase (14–24 h) in contrast to the glucose consumption phase (Figure 8a–c). This increase likely reflects a metabolic shift towards GSH biosynthesis due to the decreased specific growth rate of yeast metabolized ethanol, thereby promoting the supply of these three precursor amino acids essential for GSH production. The elevated levels of these amino acids are crucial, as they serve as substrates for γ-GCS. The activity of γ-GCS peaked at 14 h in S-272, coinciding with the highest intracellular GSH content (Figure 8d). This suggests that the increased availability of precursor amino acids directly stimulates γ-GCS activity, enhancing GSH synthesis [41]. By 24 h, both γ-GCS activity and GSH content had declined; however, the concentrations of intracellular amino acids continued to rise, indicating a sustained metabolic drive towards GSH production.
A notable difference was observed between the two strains, with S-272 consistently exhibiting higher levels of γ-GCS activity than the parent strain during the rapid GSH synthesis phase. Furthermore, the trehalose concentration in S-272 initially decreased and then increased during fermentation, significantly higher than that of the parental strain at 28 h (Figure 8e). Trehalose, an important cytoprotective agent, likely contributed to the maintenance of higher GSH production by safeguarding cellular integrity against oxidative stress [42,43]. The superior performance of S-272 under high ethanol concentrations in the fermentation broth displayed rapid ethanol utilization. The genotypes and phenotypes of the S-272 are altered, potentially enhancing metabolic pathways favorable for GSH production.
4. Conclusions
In conclusion, this study successfully enhanced the GSH production in S. cerevisiae through ARTP mutagenesis and high-throughput screening. The employment of 48-deep MTPs provided a more efficient and scalable platform for mutant screening when compared to traditional shake flasks. The alloxan method, combined with microplate reader detection, proved to be a highly efficient, specific, and cost-effective approach for GSH quantification, making it particularly suitable for high-throughput screening. The mutant strain S-272 exhibited significant improvements in GSH production, achieving a final concentration of 0.553 g/L and an intracellular content of 7.68%, representing increases of 14.7% and 19.5% compared to the parent strain. These enhancements can be attributed to increased ethanol utilization, elevated γ-GCS activity, and higher intracellular trehalose content.
The enhanced metabolic capabilities of S-272 provide a strong foundation for further metabolic engineering and GSH production optimization. Future work will focus on elucidating the specific genetic modifications induced by ARTP mutagenesis and exploring additional strategies to further enhance GSH production. The findings of this study hold significant implications for the industrial-scale production of GSH and highlight the potential for applying similar mutagenesis and screening techniques to other biotechnological applications.
Author Contributions
L.L.: investigation, methodology, data curation, conceptualization, writing—original draft, validation, visualization, and formal analysis; Z.W.: writing—review and editing, supervision, resources, project administration, and funding acquisition. A.M.: writing—review and editing; Y.Z.: writing—review and editing, supervision, and resources. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the financial support of research projects from the National Key R&D Program of China (No.2021YFC2101000), High-quality development project CEIEC-2022-ZM02-0259, Shanghai Scientific and Technological Innovation Action Plans—Scientific Instrument Development, China (grant No. 22142201000), and National Natural Science Foundation of China (No. 32071471).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors acknowledge the funding support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| GSH | Glutathione |
| ARTP | Atmospheric and room temperature plasma |
| AC | Alternating current |
| rms | root mean square |
| MTPs | Microtiter plates |
| CFD | Computational fluid dynamics |
| HPC | High-performance liquid chromatography |
| OUR | Oxygen uptake rate |
| DO | Dissolved oxygen |
| MPMS | Multifunction plasma mutagenesis system |
| GC | Gas chromatography |
| GC/MS | Gas chromatography/mass spectrometry |
| ε | Energy dissipating rate |
| P/V | Volumetric power consumption |
| SSR | Shear strain rate |
| KLa | Oxygen volumetric mass transfer coefficient |
| DTNB | 2-nitrobenzoic acid |
References
- Schmacht, M.; Lorenz, E.; Senz, M. Microbial production of glutathione. World J. Microbiol. Biotechnol. 2017, 33, 106. [Google Scholar] [CrossRef] [PubMed]
- Do, D.; Fickers, P.; Ben, T.I. Improvement of glutathione production by a metabolically engineered Yarrowia lipolytica strain using a small-scale optimization approach. Biotechnol. Lett. 2021, 43, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lemos Junior, W.J.F.; Binati, R.L.; Bersani, N.; Torriani, S. Investigating the glutathione accumulation by non-conventional wine yeasts in optimized growth conditions and multi-starter fermentations. LWT 2021, 142, 110990. [Google Scholar] [CrossRef]
- Li, Y.; Wei, G.; Chen, J. Glutathione: A review on biotechnological production. Appl. Microbiol. Biotechnol. 2004, 66, 233–242. [Google Scholar] [CrossRef]
- Santos, L.O.; Silva, P.G.P.; Lemos Junior, W.J.F.; de Oliveira, V.S.; Anschau, A. Glutathione production by Saccharomyces cerevisiae: Current state and perspectives. Appl. Microbiol. Biot. 2022, 106, 1879–1894. [Google Scholar] [CrossRef]
- Malairuang, K.; Krajang, M.; Sukna, J.; Rattanapradit, K.; Chamsart, S. High cell density cultivation of Saccharomyces cerevisiae with intensive multiple sequential batches together with a novel technique of fed-batch at cell level (FBC). Processes 2020, 8, 1321. [Google Scholar] [CrossRef]
- Zhong, L.; Carere, J.; Mats, L.; Lu, Z.; Lu, F.; Zhou, T. Formation of glutathione patulin conjugates associated with yeast fermentation contributes to patulin reduction. Food Control 2021, 123, 107334. [Google Scholar] [CrossRef]
- Lv, X.; Song, J.; Yu, B.; Liu, H.; Li, C.; Zhuang, Y.; Wang, Y. High-throughput system for screening of high l-lactic acid-productivity strains in deep-well microtiter plates. Bioprocess. Biosyst. Eng. 2016, 39, 1737–1747. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y.; Chen, X.; Liu, L. Kick-starting evolution efficiency with an autonomous evolution mutation system. Metab. Eng. 2019, 54, 127–136. [Google Scholar] [CrossRef]
- Wen, Y.; Zang, R.; Zhang, X.; Yang, S. A 24-microwell plate with improved mixing and scalable performance for high throughput cell cultures. Process Biochem. 2012, 47, 612–618. [Google Scholar] [CrossRef]
- Ottenheim, C.; Nawrath, M.; Wu, J.C. Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): The latest development. Bioresour. Bioprocess. 2018, 5, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zhou, Q.Q.; Zhang, X.F.; Wang, L.Y.; Chang, H.B.; Li, H.P.; Oda, Y.; Xing, X.H. Quantitative evaluation of DNA damage and mutation rate by atmospheric and room-temperature plasma (ARTP) and conventional mutagenesis. Appl. Microbiol. Biotechnol. 2015, 99, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.F.; Li, H.P.; Wang, L.Y.; Zhang, C.; Xing, X.H.; Bao, C.Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Y.; Xue, J.; Li, C.; Wang, Z.; Wang, Y. Enhancing nemadectin production by Streptomyces cyaneogriseus ssp. Noncyanogenus through quantitative evaluation and optimization of dissolved oxygen and shear force. Bioresour. Technol. 2018, 255, 180–188. [Google Scholar] [CrossRef]
- Tan, J.; Chu, J.; Hao, Y.; Guo, Y.; Zhuang, Y.; Zhang, S. High-throughput system for screening of Cephalosporin C high-yield strain by 48-deep-well microtiter plates. Appl. Biochem. Biotechnol. 2013, 169, 1683–1695. [Google Scholar] [CrossRef]
- Yu, L.; Li, F.; Ni, J.; Qin, X.; Lai, J.; Su, X.; Li, Z.; Zhang, M. UV-ARTP compound mutagenesis breeding improves macrolactins production of Bacillus siamensis and reveals metabolism changes by proteomic. J. Biotechnol. 2024, 381, 36–48. [Google Scholar] [CrossRef]
- Gao, S.; Li, L.; Wei, Y.; Wen, L.; Shao, S.; Wu, J.; Zong, X. Research progress of ARTP mutagenesis technology based on citespace visualization analysis. Mol. Biotechnol. 2024, 1–11, online ahead of print. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, J.; Yang, J.; Wei, M.; Zhao, J.; Xu, H.; Xie, J.; Tong, Y.; Yu, L. Citric acid production from acorn starch by tannin tolerance mutant Aspergillus niger AA120. Appl. Biochem. Biotech. 2019, 188, 1–11. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, S.; Tan, T. Optimization of the medium for glutathione production in Saccharomyces cerevisiae. Process Biochem. 2007, 42, 454–458. [Google Scholar] [CrossRef]
- Li, C.; Xia, J.; Chu, J.; Wang, Y.; Zhuang, Y.; Zhang, S. CFD analysis of the turbulent flow in baffled shake flasks. Biochem. Eng. J. 2013, 70, 140–150. [Google Scholar] [CrossRef]
- Wang, P.; Yin, Y.; Wang, X.; Wen, J. Enhanced ascomycin production in Streptomyces hygroscopicus var. Ascomyceticus by employing polyhydroxybutyrate as an intracellular carbon reservoir and optimizing carbon addition. Microb. Cell Fact. 2021, 20, 70. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, M.; Zhou, S.; You, J.; Zhao, Z.; Liu, Z.; Xu, M.; Rao, Z. High-efficient production of l-homoserine in Escherichia coli through engineering synthetic pathway combined with regulating cell division. Bioresour. Technol. 2023, 389, 129828. [Google Scholar] [CrossRef]
- Singh, N.; Akhtar, M.; Anchliya, A. Development and validation of HPLC method for simultaneous estimation of reduced and oxidized glutathione in bulk pharmaceutical formulation. Austin J. Anal. Pharm. Chem. 2021, 8, 1129. [Google Scholar]
- Mohammed, A.H.; Mohammed, A.K.; Kamar, F.H.; Abbas, A.A.; Nechifor, G. Determination of ethanol in fermented broth by headspace gas chromatography using capillary column. Rev. Chim. - Buchar. 2018, 69, 2969–2972. [Google Scholar] [CrossRef]
- de Jonge, L.; Buijs, N.A.; Heijnen, J.J.; van Gulik, W.M.; Abate, A.; Wahl, S.A. Flux response of glycolysis and storage metabolism during rapid feast/famine conditions in Penicillium chrysogenum using dynamic (13)C labeling. Biotechnol. J. 2014, 9, 372–385. [Google Scholar] [CrossRef]
- Wang, D.H.; Zhang, J.L.; Dong, Y.Y.; Wei, G.Y.; Qi, B. Glutathione is involved in physiological response of Candida utilis to acid stress. Appl. Microbiol. Biotechnol. 2015, 99, 10669–10679. [Google Scholar] [CrossRef]
- Zhang, H.; Williams-Dalson, W.; Keshavarz-Moore, E.; Shamlou, P.A. Computational-fluid-dynamics (CFD) analysis of mixing and gas-liquid mass transfer in shake flasks. Biotechnol. Appl. Biochem. 2005, 41, 1–8. [Google Scholar] [CrossRef]
- Luo, Z.; Zeng, W.; Du, G.; Liu, S.; Fang, F.; Zhou, J.; Chen, J. A high-throughput screening procedure for enhancing pyruvate production in Candida glabrata by random mutagenesis. Bioprocess. Biosyst. Eng. 2017, 40, 693–701. [Google Scholar] [CrossRef]
- Li, D.; Shen, J.; Ding, Q.; Wu, J.; Chen, X. Recent progress of atmospheric and room-temperature plasma as a new and promising mutagenesis technology. Cell Biochem. Funct. 2024, 42, e3991. [Google Scholar] [CrossRef]
- Kalogerakis, G.C.; Boparai, H.K.; Yang, M.I.; Sleep, B.E. A high-throughput and cost-effective microplate reader method for measuring persulfates (peroxydisulfate and peroxymonosulfate). Talanta 2022, 240, 123170. [Google Scholar] [CrossRef]
- Ellman, G.L. Reprint of: Tissue sulfhydryl groups. Arch. Biochem. Biophys. 2022, 726, 109245. [Google Scholar] [CrossRef]
- Gao, Q.; Gao, S.; Zeng, W.; Li, J.; Zhou, J. Enhancing (2s)-naringenin production in Saccharomyces cerevisiae by high-throughput screening method based on ARTP mutagenesis. 3 Biotech. 2024, 14, 85. [Google Scholar] [CrossRef]
- Sarnaik, A.; Liu, A.; Nielsen, D.; Varman, A.M. High-throughput screening for efficient microbial biotechnology. Curr. Opin. Biotechnol. 2020, 64, 141–150. [Google Scholar] [CrossRef]
- Yao, Z.; Fan, J.; Dai, J.; Yu, C.; Zeng, H.; Li, Q.; Hu, W.; Yan, C.; Hao, M.; Li, H.; et al. A high-throughput method based on microculture technology for screening of high-yield strains of tylosin-producing Streptomyces fradiae. J. Microbiol. Biotechnol. 2023, 33, 831–839. [Google Scholar] [CrossRef]
- Zeng, W.; Guo, L.; Xu, S.; Chen, J.; Zhou, J. High-throughput screening technology in industrial biotechnology. Trends Biotechnol. 2020, 38, 888–906. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Y.; Qi, H.; He, J.; Wu, Z.; Xu, H.; Qiao, M. Improving the level of the tyrosine biosynthesis pathway in Saccharomyces cerevisiae through htz1 knockout and atmospheric and room temperature plasma (ARTP) mutagenesis. ACS Synth. Biol. 2021, 10, 49–62. [Google Scholar] [CrossRef]
- Jin, J.; Wang, Y.; Yao, M.; Gu, X.; Li, B.; Liu, H.; Ding, M.; Xiao, W.; Yuan, Y. Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol. Biofuels 2018, 11, 230. [Google Scholar] [CrossRef]
- Li, H.P.; Wang, L.Y.; Li, G.; Jin, L.H.; Le, P.S.; Zhao, H.X.; Xing, X.H.; Bao, C.Y. Manipulation of lipase activity by the helium radio-frequency, atmospheric-pressure glow discharge plasma jet. Plasma Process. Polym. 2011, 8, 224–229. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S. Breeding of high-yield alkaline protease producing strain by atmospheric and room temperature plasma mutagenesis. IOP Conf. Ser. Earth Environ. Sci. 2020, 453, 12089. [Google Scholar] [CrossRef]
- Hu, X.; Shen, X.; Zhu, S.; Zeng, H.; Shuai, Y. Optimization of glutathione production in Saccharomyces cerevisiae HBSD-W08 using plackett-burman and central composite rotatable designs. BMC Microbiol. 2023, 23, 11. [Google Scholar] [CrossRef]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, Q.; Jia, S.; Qiao, C. Effects of high pressure on the accumulation of trehalose and glutathione in the Saccharomyces cerevisiae cells. Biochem. Eng. J. 2007, 37, 226–230. [Google Scholar] [CrossRef]
- Camara, A.J.; Marechal, P.A.; Tourdot-Marechal, R.; Husson, F. Dehydration stress responses of yeasts Torulaspora delbrueckii, Metschnikowia pulcherrima and Lachancea thermotolerans: Effects of glutathione and trehalose biosynthesis. Food Microbiol. 2019, 79, 137–146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).