Hydrotalcites as a Promising Adsorbent for Hemicellulose Hydrolysate Detoxification in Xylitol Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sugarcane Straw Hemicellulosic Hydrolysate (SSHH)
2.2. Detoxification of the Sugarcane Straw Hemicellulosic Hydrolysate
Experimental Design: 22 Full Factorial Approach
2.3. Microorganism and Inoculum Preparation
2.4. Medium and Fermentation Conditions
2.5. Analytical Methods
3. Results and Discussion
3.1. Characterization of the Sugarcane Straw Hemicellulosic Hydrolysate
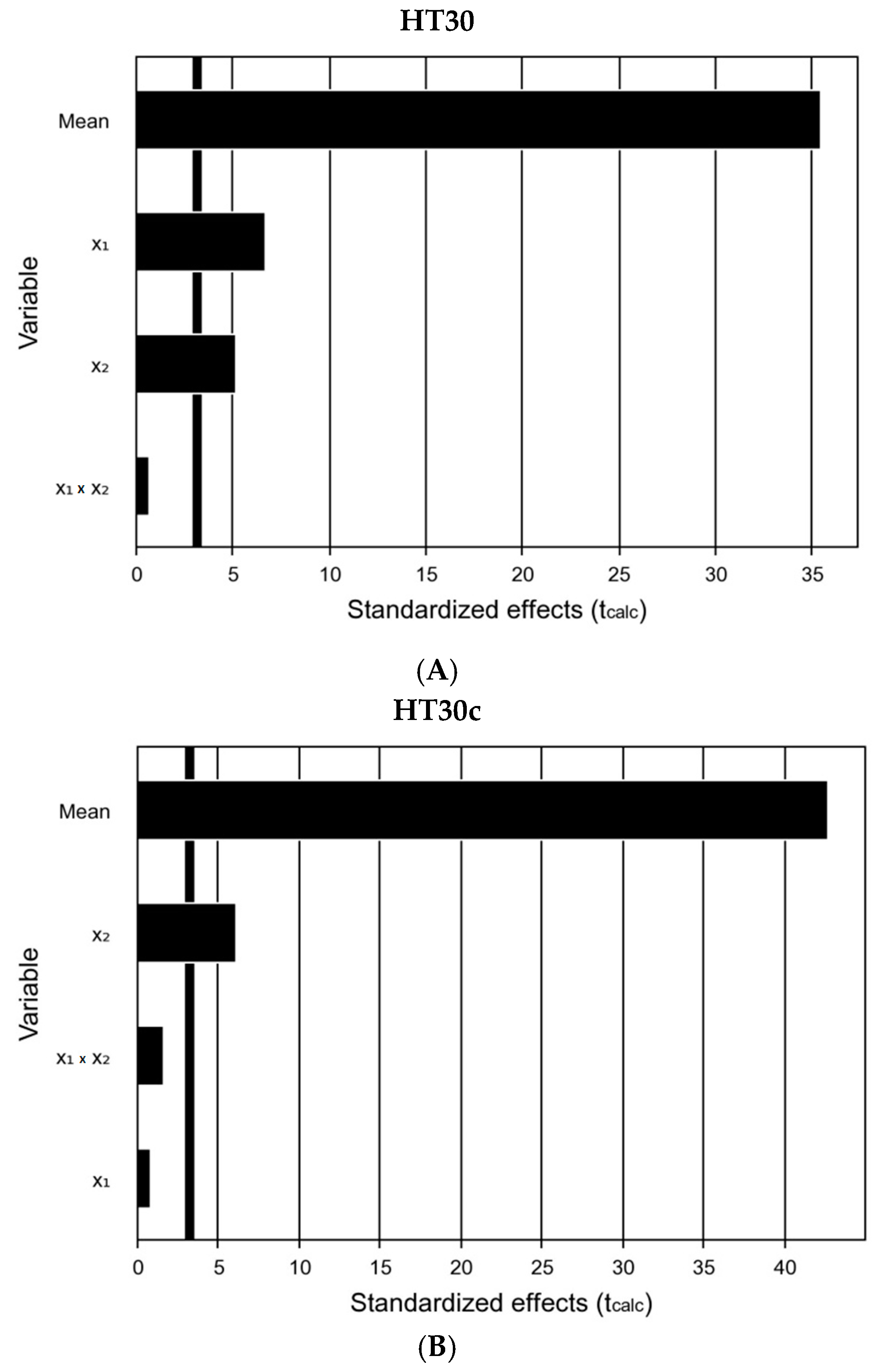
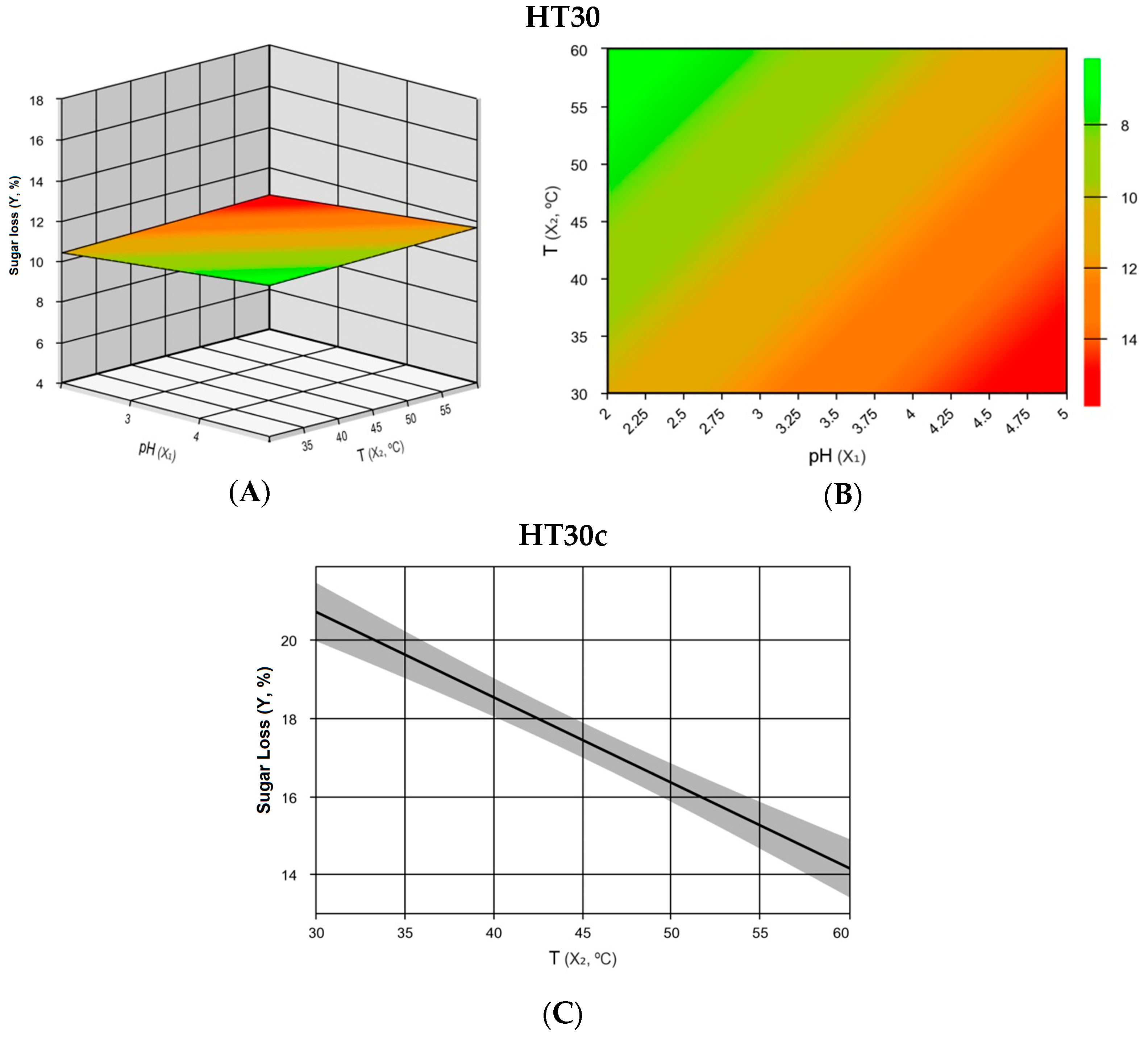
3.2. Influence of pH and Temperature at Different Residence Times
3.3. Kinetic Models for Sugarcane Straw Hemicellulosic Hydrolysate Detoxification
3.4. Fermentation of Detoxified Sugarcane Straw Hemicellulosic Hydrolysate
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HMF | 5-Hydroxymethylfurfural |
| ADH | Alcohol dehydrogenase |
| DOE | Design of experiment |
| FFD | Full factorial design |
| HPLC | High-Performance Liquid Chromatography |
| HT30 | [Mg-Al-CO3], PURAL®MG 30, 30% MgO composition uncalcined |
| HT30c | [Mg-Al-CO3], PURAL®MG 30, 30% MgO composition calcined |
| ICUMSA | International Commission for Uniform Methods of Sugar Analysis |
| OD600 | Optical density measured at 600 nm |
| PPP | Pentose phosphate pathway |
| PR | Phenol removal |
| SL | Sugar loss |
| SSHH | Sugarcane straw hemicellulosic hydrolysate |
| XR | Xylose reductase |
| XDH | Xylitol dehydrogenase |
References
- Park, Y.-C.; Oh, E.J.; Jo, J.-H.; Jin, Y.-S.; Seo, J.-H. Recent advances in biological production of sugar alcohols. Curr. Opin. Biotechnol. 2016, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M. Sugar alcohols—Their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef]
- Cardoso, C.A.B.; Cassiano, L.P.S.; Costa, E.N.; Souza-e-Silva, C.M.; Magalhães, A.C.; Grizzo, L.T.; Caldana, M.L.; Bastos, J.R.M.; Buzalaf, M.A.R. Effect of xylitol varnishes on remineralization of artificial enamel caries lesions in situ. J. Dent. 2016, 50, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K. Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med. Princ. Pract. 2011, 20, 303–320. [Google Scholar] [CrossRef]
- Söderling, E.; Pienihäkkinen, K. Effects of xylitol and erythritol consumption on mutans streptococci and the oral microbiota: A systematic review. Acta Odontol. Scand. 2020, 78, 599–608. [Google Scholar] [CrossRef]
- Marom, T.; Marchisio, P.; Tamir, S.O.; Torretta, S.; Gavriel, H.; Esposito, S. Complementary and alternative medicine treatment options for otitis media: A systematic review. Medicine 2016, 95, e2695. [Google Scholar] [CrossRef]
- Xu, M.L.; Wi, G.R.; Kim, H.J.; Kim, H.-J. Ameliorating effect of dietary xylitol on human respiratory syncytial virus (hrsv) infection. Biol. Pharm. Bull. 2016, 39, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef]
- Rahman, M.A.; Islam, M.S. Xylitol improves pancreatic islets morphology to ameliorate type 2 diabetes in rats: A dose response study. J. Food Sci. 2014, 79, H1436–H1442. [Google Scholar] [CrossRef]
- Kriz, D.; Ansari, D.; Andersson, R. Potential biomarkers for early detection of pancreatic ductal adenocarcinoma. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2020, 22, 2170–2174. [Google Scholar] [CrossRef]
- Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and pharmacological potential of xylitol: A molecular insight of unique metabolism. Foods 2020, 9, 1592. [Google Scholar] [CrossRef]
- Cheudjeu, A. Correlation of d-xylose with severity and morbidity-related factors of covid-19 and possible therapeutic use of d-xylose and antibiotics for covid-19. Life Sci. 2020, 260, 118335. [Google Scholar] [CrossRef]
- Dasgupta, D.; Bandhu, S.; Adhikari, D.K.; Ghosh, D. Challenges and prospects of xylitol production with whole cell bio-catalysis: A review. Microbiol. Res. 2017, 197, 9–21. [Google Scholar] [CrossRef]
- Mpabanga, T.P.; Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification strategies applied to lignocellulosic hydrolysates for improved xylitol production. In D-Xylitol: Fermentative Production, Application and Commercialization; da Silva, S.S., Chandel, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 63–82. [Google Scholar]
- Canilha, L.; Chandel, A.K.; Suzane dos Santos Milessi, T.; Antunes, F.A.F.; Luiz da Costa Freitas, W.; das Gracas Almeida Felipe, M.; da Silva, S.S. Bioconversion of sugarcane biomass into ethanol: An overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef]
- Misra, S.; Raghuwanshi, S.; Gupta, P.; Dutt, K.; Saxena, R.K. Fermentation behavior of osmophilic yeast candida tropicalis isolated from the nectar of hibiscus rosa sinensis flowers for xylitol production. Antonie Van Leeuwenhoek 2012, 101, 393–402. [Google Scholar] [CrossRef]
- Carneiro, C.; de Paula, E.S.F.C.; Almeida, J.R.M. Xylitol production: Identification and comparison of new producing yeasts. Microorganisms 2019, 7, 484. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.F.; Chaves-Villamil, A.C.; de Arruda, P.V.; dos Santos, J.C.; Felipe, M.d.G.d.A. Sugarcane syrup improves xylitol bioproduction from sugarcane bagasse and straw hemicellulosic hydrolysate. Waste Biomass Valoriz. 2020, 11, 4215–4224. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Romero, I.; Cara, C.; Castro, E.; Mussatto, S. Xylitol production by debaryomyces hansenii and candida guilliermondii from rapeseed straw hemicellulosic hydrolysate. Bioresour. Technol. 2018, 247, 736–743. [Google Scholar] [CrossRef]
- Leonel, L.V.; Sene, L.; da Cunha, M.A.A.; Dalanhol, K.C.F.; de Almeida Felipe, M.d.G. Valorization of apple pomace using bio-based technology for the production of xylitol and 2g ethanol. Bioprocess Biosyst. Eng. 2020, 43, 2153–2163. [Google Scholar] [CrossRef]
- Dalli, S.S.; Patel, M.; Rakshit, S.K. Development and evaluation of poplar hemicellulose prehydrolysate upstream processes for the enhanced fermentative production of xylitol. Biomass Bioenergy 2017, 105, 402–410. [Google Scholar] [CrossRef]
- Moraes, E.J.C.; Silva, D.D.V.; Dussán, K.J.; Tesche, L.Z.; de Almeida Silva, J.B.; Rai, M.; Felipe, M.G.A. Xylitol-sweetener production from barley straw: Optimization of acid hydrolysis condition with the energy consumption simulation. Waste Biomass Valorization 2020, 11, 1837–1849. [Google Scholar] [CrossRef]
- Kelly, C.; Jones, O.; Barnhart, C.; Lajoie, C. Effect of furfural, vanillin and syringaldehyde on candida guilliermondii growth and xylitol biosynthesis. Appl. Biochem. Biotechnol. 2008, 148, 97–108. [Google Scholar] [CrossRef]
- Silva, D.D.V.; Felipe, M.G.A.; Mancilha, I.M.; Luchese, R.H.; Silva, S.S. Inhibitory effect of acetic acid on bioconversion of xylose in xylitol by candida guilliermondii in sugarcane bagasse hydrolysate. Braz. J. Microbiol. 2004, 35, 248–254. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Felipe, M.G.; Vieira, D.C.; Vitolo, M.; Silva, S.S.; Roberto, I.C.; Manchilha, I.M. Effect of acetic acid on xylose fermentation to xylitol by candida guilliermondii. J. Basic Microbiol. 1995, 35, 171–177. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Costa, C.E.; Sá-Correia, I.; Domingues, L. Molecular and physiological basis of saccharomyces cerevisiae tolerance to adverse lignocellulose-based process conditions. Appl. Microbiol. Biotechnol. 2019, 103, 159–175. [Google Scholar] [CrossRef]
- Bianchini, I.d.A.; Jofre, F.M.; Queiroz, S.d.S.; Lacerda, T.M.; Felipe, M.d.G.d.A. Relation of xylitol formation and lignocellulose degradation in yeast. Appl. Microbiol. Biotechnol. 2023, 107, 3143–3151. [Google Scholar] [CrossRef]
- Luo, X.; Zeng, B.; Zhong, Y.; Chen, J. Production and detoxification of inhibitors during the destruction of lignocellulose spatial structure. BioResources 2022, 17, 1939–1961. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.-S.; Lee, D.-J. Inhibitor formation and detoxification during lignocellulose biorefinery: A review. Bioresour. Technol. 2022, 361, 127666. [Google Scholar] [CrossRef] [PubMed]
- Marton, J.M.; Felipe, M.G.A.; Almeida e Silva, J.B.; Pessoa Júnior, A. Evaluation of the activated charcoals and adsorption conditions used in the treatment of sugarcane bagasse hydrolysate for xylitol production. Braz. J. Chem. Eng. 2006, 23, 9–21. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Asghar, N.; Waheed, M. Comparative evaluation of detoxification strategies for sugarcane bagasse hydrolysate. J. Anim. Plant Sci. 2019, 29, 1775–1783. [Google Scholar]
- Kumar, V.; Krishania, M.; Sandhu, P.P.; Ahluwalia, V.; Gnansounou, E.; Sangwan, R.S.J.B.t. Efficient detoxification of corn cob hydrolysate with ion-exchange resins for enhanced xylitol production by candida tropicalis mtcc 6192. Bioresour. Technol. 2018, 251, 416–419. [Google Scholar] [CrossRef]
- Santana, N.B.; Dias, J.C.T.; Rezende, R.P.; Franco, M.; Oliveira, L.K.S.; Souza, L.O. Production of xylitol and bio-detoxification of cocoa pod husk hemicellulose hydrolysate by candida boidinii xm02g. PLoS ONE 2018, 13, e0195206. [Google Scholar] [CrossRef]
- Candido, J.P.; Claro, E.M.T.; de Paula, C.B.C.; Shimizu, F.L.; de Oliveria Leite, D.A.N.; Brienzo, M.; de Angelis, D.F. Detoxification of sugarcane bagasse hydrolysate with different adsorbents to improve the fermentative process. World J. Microbiol. Biotechnol. 2020, 36, 43. [Google Scholar] [CrossRef]
- Toledo, T.V.; Bellato, C.R.; Pessoa, K.D.; Fontes, M.P.F. Remoção de cromo (vi) de soluções aquosas utilizando o compósito magnético calcinado hidrotalcita-óxido de ferro: Estudo cinético e de equilíbrio termodinâmico. Química Nova 2013, 36, 419–425. [Google Scholar] [CrossRef]
- Forte, M.B.S.; Elias, É.C.L.; Pastore, H.O.; Filho, F.M.; Rodrigues, M.I. Evaluation of clavulanic acid adsorption in mgal-layered double hydroxides: Kinetic, equilibrium and thermodynamic studies. Adsorpt. Sci. Technol. 2012, 30, 65–80. [Google Scholar] [CrossRef]
- Mallakpour, S.; Hatami, M.; Hussain, C.M. Recent innovations in functionalized layered double hydroxides: Fabrication, characterization, and industrial applications. Adv. Colloid Interface Sci. 2020, 283, 102216. [Google Scholar] [CrossRef]
- Schöwe, N.; Bretz, K.; Hennig, T.; Schlüter, S.; Deerberg, G. Succinic acid removal and recovery from aqueous solution using hydrotalcite granules: Experiments and modeling. Ind. Eng. Chem. Res. 2015, 54, 1123–1130. [Google Scholar] [CrossRef]
- Kuzawa, K.; Jung, Y.-J.; Kiso, Y.; Yamada, T.; Nagai, M.; Lee, T.-G. Phosphate removal and recovery with a synthetic hydrotalcite as an adsorbent. Chemosphere 2006, 62, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Travália, B.; Santos, N.; Vieira, M.; Forte, M. Adsorption of fermentation inhibitors by layered double hydroxides in synthetic hemicellulose hydrolysate: A batch multicomponent analysis. Ind. Eng. Chem. Res. 2019, 58, 18822–18828. [Google Scholar] [CrossRef]
- Travália, B.M.; Soares Forte, M.B. New proposal in a biorefinery context: Recovery of acetic and formic acids by adsorption on hydrotalcites. J. Chem. Eng. Data 2020, 65, 4503–4511. [Google Scholar] [CrossRef]
- Stawiński, W.; Węgrzyn, A.; Freitas, O.; Chmielarz, L.; Figueiredo, S. Dual-function hydrotalcite-derived adsorbents with sulfur storage properties: Dyes and hydrotalcite fate in adsorption-regeneration cycles. Microporous Mesoporous Mater. 2017, 250, 72–87. [Google Scholar] [CrossRef]
- Benali, M.; Oulmekki, A.; Toyir, J. Enhancing the selective catalytic oxidation of lignocellulosic biomass to formic acid using hydrogen peroxide and a reusable mgal hydrotalcite-derived as a catalyst in a green solvent. Biomass Bioenergy 2024, 191, 107440. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.F.; Costa, I.A.L.; Silva, D.D.V.; Dussán, K.J.; Villela, T.R.; Canettieri, E.V.; Carvalho, J.A.; Soares Neto, T.G.; Felipe, M.G.A. Biochemical conversion of sugarcane straw hemicellulosic hydrolyzate supplemented with co-substrates for xylitol production. Bioresour. Technol. 2016, 200, 1085–1088. [Google Scholar] [CrossRef]
- Rodrigues, M.I.; Iemma, A.F. Experimental Design and Process Optimization; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lima, L.; Felipe, M.; Torres, F. Reclassification of candida guilliermondii fti 20037 as candida tropicalis based on molecular phylogenetic analysis. Braz. J. Microbiol. 2003, 34, 96–98. [Google Scholar] [CrossRef]
- Silva, D.D.; Candido, E.J.; Arruda, P.V.; Silva, S.S.; Felipe, M.G. New cultive medium for bioconversion of c5 fraction from sugarcane bagasse using rice bran extract. Braz. J. Microbiol. 2014, 45, 1469–1475. [Google Scholar] [CrossRef]
- Villarreal, M.L.M.; Prata, A.M.R.; Felipe, M.G.A.; Silva, J.A.E.B. Detoxification procedures of eucalyptus hemicellulose hydrolysate for xylitol production by candida guilliermondii. Enzym. Microb. Technol. 2006, 40, 17–24. [Google Scholar] [CrossRef]
- Dussán, K.J.; Silva, D.D.V.; Perez, V.H.; da Silva, S.S. Evaluation of oxygen availability on ethanol production from sugarcane bagasse hydrolysate in a batch bioreactor using two strains of xylose-fermenting yeast. Renew. Energy 2016, 87, 703–710. [Google Scholar] [CrossRef]
- Gouveia, E.R.; Nascimento, R.T.; Souto-Maior, A.M.; Rocha, G.J.M. Validação de metodologia para a caracterização química de bagaço de cana-de-açúcar. Quim. Nova 2009, 32, 1500–1503. [Google Scholar] [CrossRef]
- ICUMSA. Icumsa Method gs 1/3-7 (2011) Determination of the Solution Colour of Raw Sugars, Brown Sugars and Coloured Syrups at ph 7.0 Single Method. Verlag Dr. Albert Bartens KG: Berlin, Germany, 2011. [Google Scholar]
- Silva, D.D.V.; Felipe, M.d.G.d.A. Effect of glucose:Xylose ratio on xylose reductase and xylitol dehydrogenase activities from candida guilliermondii in sugarcane bagasse hydrolysate. J. Chem. Technol. Biotechnol. 2006, 81, 1294–1300. [Google Scholar] [CrossRef]
- Ingle, A.P.; Philippini, R.R.; de Souza Melo, Y.C.; da Silva, S.S. Acid-functionalized magnetic nanocatalysts mediated pretreatment of sugarcane straw: An eco-friendly and cost-effective approach. Cellulose 2020, 27, 7067–7078. [Google Scholar] [CrossRef]
- Villa, P.; Felipe, M.G.A.; Rodriguez, R.C.L.; Vitolo, M.; Reis, E.L.; Silva, S.S. Influence of phenolic compounds on the bioprocess of xylitol production by candida guilliermondii. In Proceedings of the Esbes-2 European Symposium on Biochemical Engineering Science, Porto, Portugal, 16–19 September 1998. [Google Scholar]
- Yang, L.; Shahrivari, Z.; Liu, P.K.T.; Sahimi, M.; Tsotsis, T.T. Removal of trace levels of arsenic and selenium from aqueous solutions by calcined and uncalcined layered double hydroxides (LDH). Ind. Eng. Chem. Res. 2005, 44, 6804–6815. [Google Scholar] [CrossRef]
- Yu, S.; Kim, E.; Park, S.; Song, I.K.; Jung, J.C. Isomerization of glucose into fructose over mg–al hydrotalcite catalysts. Catal. Commun. 2012, 29, 63–67. [Google Scholar] [CrossRef]
- Steinbach, D.; Klier, A.; Kruse, A.; Sauer, J.; Wild, S.; Zanker, M. Isomerization of glucose to fructose in hydrolysates from lignocellulosic biomass using hydrotalcite. Processes 2020, 8, 644. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Structure–performance correlations of mg–al hydrotalcite catalysts for the isomerization of glucose into fructose. J. Catal. 2015, 327, 1–9. [Google Scholar] [CrossRef]
- Souzanchi, S.; Nazari, L.; Rao, K.T.V.; Tan, Z.; Xu, C. Continuous isomerization of glucose to fructose using activated hydrotalcite catalyst: Effects of reaction conditions. Appl. Catal. O Open 2024, 190, 206954. [Google Scholar] [CrossRef]
- Kang, M.J.; Chun, K.S.; Rhee, S.W.; Do, Y. Comparison of sorption behavior of i- and tco-4 on mg/al layered double hydroxide. Radiochim. Acta 1999, 85, 57–64. [Google Scholar] [CrossRef]
- Seida, Y.; Nakano, Y.; Nakamura, Y. Rapid removal of dilute lead from water by pyroaurite-like compound. Water Res. 2001, 35, 2341–2346. [Google Scholar] [CrossRef]
- Tabana, L.S.; Adekoya, G.J.; Tichapondwa, S.M. Integrated study of antiretroviral drug adsorption onto calcined layered double hydroxide clay: Experimental and computational analysis. Environ. Sci. Pollut. Res. 2024, 31, 32282–32300. [Google Scholar] [CrossRef] [PubMed]
- Khraisheh, M.A.M.; Al-Degs, Y.S.; Allen, S.J.; Ahmad, M.N. Elucidation of controlling steps of reactive dye adsorption on activated carbon. Ind. Eng. Chem. Res. 2002, 41, 1651–1657. [Google Scholar] [CrossRef]
- Artifon, W.; Bonatto, C.; Bordin, E.R.; Bazoti, S.F.; Dervanoski, A.; Alves, S.L.; Treichel, H. Bioethanol production from hydrolyzed lignocellulosic after detoxification via adsorption with activated carbon and dried air stripping. Bioeng. Biotechnol. 2018, 6, 107. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Martinez, A.; Rodriguez, M.E.; Wells, M.L.; York, S.W.; Preston, J.F.; Ingram, L.O. Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol. Prog. 2001, 17, 287–293. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Sganzerla, W.G.; Costa, J.M.; Souza, F.M.; Rostagno, M.A.; Forster-Carneiro, T. Adsorbents for the purification and recovery of biocompounds: An updated review. Biofuels Bioprod. Biorefining 2024, 18, 265–290. [Google Scholar] [CrossRef]
- Mohamed Nasser, S.; Abbas, M.; Trari, M. Understanding the rate-limiting step adsorption kinetics onto biomaterials for mechanism adsorption control. Prog. React. Kinet. Mech. 2024, 49, 14686783241226858. [Google Scholar] [CrossRef]
- Magalhães, B.L.; Grassi, M.C.B.; Pereira, G.A.; Brocchi, M.J.B. Improved n-butanol production from lignocellulosic hydrolysate by clostridium strain screening and culture-medium optimization. Biomass Bioenergy 2018, 108, 157–166. [Google Scholar] [CrossRef]
- Suko, A.V.; Bura, R.J.I.B. Enhanced xylitol and ethanol yields by fermentation inhibitors in steam-pretreated lignocellulosic biomass. Ind. Biotechnol. 2016, 12, 187–194. [Google Scholar] [CrossRef]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and applications—A review. Front. Sustain. 2022, 3, 826190. [Google Scholar] [CrossRef]
- Kumari, P.; Mathur, P.; Sharma, C.; Chaturvedi, P. Xylitol production from lignocellulosic biowastes. Bioresour. Technol. Rep. 2025, 29, 102025. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J. Biotechnological production of xylitol. Part 3: Operation in culture media made from lignocellulose hydrolysates. Bioresour. Technol. 1998, 66, 25–40. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Chade, M.; Mereles, E.B.; Bengoechea, D.I.; Brizuela, J.G.; Felissia, F.E.; Area, M.C. Strategies of detoxification and fermentation for biotechnological production of xylitol from sugarcane bagasse. Ind. Crops Prod. 2016, 91, 161–169. [Google Scholar] [CrossRef]

| Characteristics | Sugarcane Straw Hemicellulosic Hydrolysate | ||
|---|---|---|---|
| Original | Concentrated 4× | ||
| Sugars (g/L) | Glucose | 2.47 | 12.39 |
| Xylose | 15.63 | 72.65 | |
| Arabinose | 2.83 | 11.99 | |
| Toxic compounds (g/L) | Acetic acid | 2.50 | 2.95 |
| Furfural | 0.147 | 0.165 | |
| 5-HMF | 0.302 | 1.12 | |
| Total phenols | 2.61 | 6.82 | |
| Color | Absorbance (420 nm) | 0.240 | 0.873 |
| ICUMSA Method | 0.099 | 0.362 | |
| °Brix | 4.0 | 15.6 | |
| pH | 0.79 | 0.29 | |
| HT30 | 15 min | 30 min | 45 min | |||||
|---|---|---|---|---|---|---|---|---|
| Assay | pH (x1) | T (°C) (x2) | SL (%) | PR (%) | SL (%) | PR (%) | SL (%) | PR (%) |
| 1 | 2 (−1) | 30 (−1) | 9.80 | 65.27 | 11.11 | 66.01 | 9.98 | 68.43 |
| 2 | 5 (+1) | 30 (−1) | 15.37 | 63.68 | 16.10 | 67.75 | 23.78 | 67.43 |
| 3 | 2 (−1) | 60 (+1) | 6.60 | 63.69 | 6.32 | 67.27 | 7.56 | 64.94 |
| 4 | 5 (+1) | 60 (+1) | 11.20 | 69.75 | 12.35 | 66.93 | 17.46 | 69.73 |
| * 5 | 3.5 (0) | 45 (0) | 9.72 | 57.77 | 10.07 | 62.61 | 14.29 | 63.21 |
| * 6 | 3.5 (0) | 45 (0) | 16.66 | 63.65 | 10.76 | 64.14 | 16.45 | 64.52 |
| * 7 | 3.5 (0) | 45 (0) | 16.60 | 68.64 | 10.52 | 67.30 | 26.49 | 58.82 |
| HT30c | 15 min | 30 min | 45 min | |||||
| Assay | pH (x1) | T (°C) (x2) | SL (%) | PR (%) | SL (%) | PR (%) | SL (%) | PR (%) |
| 1 | 2 (−1) | 30 (−1) | 20.74 | 65.83 | 19.90 | 75.69 | 20.59 | 66.39 |
| 2 | 5 (+1) | 30 (−1) | 20.47 | 72.09 | 22.42 | 79.10 | 19.03 | 77.96 |
| 3 | 2 (−1) | 60 (+1) | 15.53 | 68.66 | 15.06 | 65.36 | 14.80 | 67.10 |
| 4 | 5 (+1) | 60 (+1) | 19.29 | 79.98 | 14.20 | 73.45 | 17.53 | 74.73 |
| * 5 | 3.5 (0) | 45 (0) | 15.82 | 64.45 | 15.87 | 65.41 | 16.65 | 72.19 |
| * 6 | 3.5 (0) | 45 (0) | 32.61 | 72.37 | 17.50 | 75.12 | 30.05 | 80.08 |
| * 7 | 3.5 (0) | 45 (0) | 16.71 | 66.31 | 17.00 | 66.67 | 16.15 | 65.77 |
| Variation Source | Sum of Squares | Degrees of Freedom | Mean Square | Fcalc|Flist | R2 (%) |
|---|---|---|---|---|---|
| HT30 | |||||
| Regression | 48.86 | 3 | 16.29 | 24.08 | 9.28 | 95.5 |
| Residues | 2.03 | 3 | 0.68 | ||
| Total | 50.89 | 6 | |||
| HT30c | |||||
| Regression | 42.64 | 1 | 42.64 | 30.32 | 6.61 | 85.8 |
| Residues | 7.03 | 5 | 1.41 | ||
| Total | 49.67 | 6 | |||
| Model | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion |
|---|---|---|---|
| Assay 1 pH: 2.0 Temperature: 30 °C | k1 = 0.0763 /min qe = 236.75 mg/g R2 = negative | k2 = 56865.12 g/mg·min qe = 221.12 mg/g R2 = 0.992 | kid = −7.38 mg/g·min0.5 C = 267.39 mg/g R2 = 0.867 |
| Assay 2 pH: 5.0 Temperature: 30 °C | k1 = 0.0574 /min qe = 247.57 mg/g R2 = negative | k2 = 56366.61 g/mg·min qe = 223.04 mg/g R2 = 0.995 | kid = −9.41 mg/g·min0.5 C = 280.19 mg/g R2 = 0.753 |
| Assay 3 pH: 2.0 Temperature: 60 °C | k1 = 0.0756 /min qe = 247.51 mg/g R2 = 0.993 | k2 = 849.60 g/mg·min qe = 234.80 mg/g R2 = 0.992 | kid = −3.61 mg/g·min0.5 C = 255.86 mg/g R2 = 0.172 |
| Assay 4 pH: 5.0 Temperature: 60 °C | k1 = 0.0683 /min qe = 225.45 mg/g R2 = negative | k2 = −0.0058 g/mg·min qe = 206.36 mg/g R2 = 0.990 | kid = −0.62 mg/g·min0.5 C = 209.33 mg/g R2 = 0.006 |
| Assays 5, 6 and 7 pH: 3.5 Temperature: 45 °C | k1 = 0.1172 /min qe = 257.79 mg/g R2 = negative | k2 = −103845.38 g/mg·min qe = 252.11 mg/g R2 = 0.993 | kid = 2.39 mg/g·min0.5 C = 209.33 mg/g R2 = 0.159 |
| Model | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion |
|---|---|---|---|
| Assay 1 pH: 2.0 Temperature: 30 °C | k1 = 0.0776 /min qe = 232.91 mg/g R2 = 0.632 | k2 = −17213.52 g/mg·min qe = 206.74 mg/g R2 = 0.826 | kid = −3.34 mg/g·min0.5 C = 227.14 mg/g R2 = 0.016 |
| Assay 2 pH: 5.0 Temperature: 30 °C | k1 = 0.0449 /min qe = 190.29 mg/g R2 = negative | k2 = 17853.08 g/mg·min qe = 150.14 mg/g R2 = 0.977 | kid = −14.90 mg/g·min0.5 C = 240.76 mg/g R2 = 0.681 |
| Assay 3 pH: 2.0 Temperature: 60 °C | k1 = 0.0767 /min qe = 236.15 mg/g R2 = negative | k2 = −123535.14 g/mg·min qe = 226.71 mg/g R2 = 0.994 | kid = 4.26 mg/g·min0.5 C = 201.87 mg/g R2 = 0.289 |
| Assay 4 pH: 5.0 Temperature: 60 °C | k1 = 0.0783 /min qe = 180.99 mg/g R2 = 0.754 | k2 = −70923.64 g/mg·min qe = 171.40 mg/g R2 = 0.950 | kid = 13.37 mg/g·min0.5 C = 91.68 mg/g R2 = 0.649 |
| Assays 5, 6 and 7 pH: 3.5 Temperature: 45 °C | k1 = 0.0643 /min qe = 220.12 mg/g R2 = negative | k2 = 31521.62 g/mg·min qe = 194.88 mg/g R2 = 0.978 | kid = −11.65 mg/g·min0.5 C = 268.08 mg/g R2 = 0.894 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.D.V.d.; Dussán, K.J.; Costa, I.A.L.; Forte, M.B.S.; Felipe, M.G.A. Hydrotalcites as a Promising Adsorbent for Hemicellulose Hydrolysate Detoxification in Xylitol Production. Fermentation 2025, 11, 243. https://doi.org/10.3390/fermentation11050243
Silva DDVd, Dussán KJ, Costa IAL, Forte MBS, Felipe MGA. Hydrotalcites as a Promising Adsorbent for Hemicellulose Hydrolysate Detoxification in Xylitol Production. Fermentation. 2025; 11(5):243. https://doi.org/10.3390/fermentation11050243
Chicago/Turabian StyleSilva, Débora D. V. da, Kelly J. Dussán, Isabela A. L. Costa, Marcus B. S. Forte, and Maria G. A. Felipe. 2025. "Hydrotalcites as a Promising Adsorbent for Hemicellulose Hydrolysate Detoxification in Xylitol Production" Fermentation 11, no. 5: 243. https://doi.org/10.3390/fermentation11050243
APA StyleSilva, D. D. V. d., Dussán, K. J., Costa, I. A. L., Forte, M. B. S., & Felipe, M. G. A. (2025). Hydrotalcites as a Promising Adsorbent for Hemicellulose Hydrolysate Detoxification in Xylitol Production. Fermentation, 11(5), 243. https://doi.org/10.3390/fermentation11050243







