Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions
Abstract
:1. Introduction
2. Materials and Methods
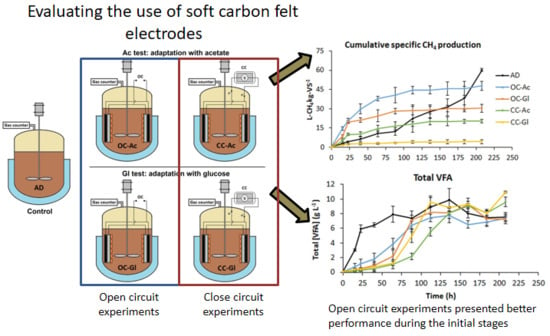
2.1. Reactor Design
2.2. Electrode Preparation and Operation
2.3. Analytical Measurements and Calculations
3. Results and Discussion
3.1. Batch Digestion Tests
3.2. Combined AD-BES Reactors
3.2.1. Methane Production and Current Profiles
3.2.2. Analysis of Volatile Fatty Acids
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saleh, B. Parametric and working fluid analysis of a combined organic Rankine-vapor compression refrigeration system activated by low-grade thermal energy. J. Adv. Res. 2016, 7, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, T.C. Production of Bio-Derived Fuels and Chemicals. Fermentation 2017, 3, 42. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M. Biomass of invasive plant species as a potential feedstock for bioenergy production. Biofuel. Bioprod. Bior. 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Martinez, E.J.; Moreno, R.; González, R.; Otero, M.; Gómez, X. Enhancing anaerobic digestion of poultry blood using activated carbon. J. Adv. Res. 2017, 8, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Fantozzi, F. Codigestion of untreated and treated sewage sludge with the organic fraction of municipal solid wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef]
- Westman, S.Y.; Chandolias, K.; Taherzadeh, M.J. Syngas Biomethanation in a Semi-Continuous Reverse Membrane Bioreactor (RMBR). Fermentation 2016, 2, 8. [Google Scholar] [CrossRef]
- Martin, I.; Pidou, M.; Soares, A.; Judd, S.; Jefferson, B. Modelling the energy demands of aerobic and anaerobic membrane bioreactors for wastewater treatment. Environ. Technol. 2011, 32, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Tartakovsky, B.; Mehta, P.; Bourque, J.S.; Guiot, S.R. Electrolysis-enhanced anaerobic digestion of wastewater. Bioresour. Technol. 2011, 102, 5685–5691. [Google Scholar] [CrossRef] [PubMed]
- Aslanzadeh, S.; Rajendran, K.; Jeihanipour, A.; Taherzadeh, M.J. The effect of effluent recirculation in a semi-continuous two-stage anaerobic digestion system. Energies 2013, 6, 2966–2981. [Google Scholar] [CrossRef]
- Bialek, K.; Cysneiros, D.; O’Flaherty, V. Hydrolysis, acidification and methanogenesis during low-temperature anaerobic digestion of dilute dairy wastewater in an inverted fluidised bioreactor. Appl. Microbiol. Biot. 2014, 98, 8737–8750. [Google Scholar] [CrossRef] [PubMed]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Ziganshina, E.E.; Belostotskiy, D.E.; Ilinskaya, O.N.; Boulygina, E.A.; Grigoryeva, T.V.; Ziganshin, A.M. Effect of the organic loading rate increase and the presence of zeolite on microbial community composition and process stability during anaerobic digestion of chicken wastes. Microbial. Ecol. 2015, 70, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Quan, X.; Chen, S. Effects of ferric iron on the anaerobic treatment and microbial biodiversity in a coupled microbial electrolysis cell (MEC)—Anaerobic reactor. Water Res. 2013, 47, 5719–5728. [Google Scholar] [CrossRef] [PubMed]
- Sleutels, T.H.J.A.; Ter Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Bioelectrochemical systems: An outlook for practical applications. ChemSusChem 2012, 5, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Microbial electrolysis cells turning to be versatile technology: Recent advances and future challenges. Water Res. 2014, 56, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Abu-Reesh, I.M.; He, Z. Development of bioelectrochemical systems to promote sustainable agriculture. Agriculture 2015, 5, 367–388. [Google Scholar] [CrossRef]
- Moreno, R.; Escapa, A.; Cara, J.; Carracedo, B.; Gómez, X. A two-stage process for hydrogen production from cheese whey: Integration of dark fermentation and biocatalyzed electrolysis. Int. J. Hydrog. Energy 2015, 40, 168–175. [Google Scholar] [CrossRef]
- ElMekawy, A.; Srikanth, S.; Vanbroekhoven, K.; De Wever, H.; Pant, D. Bioelectro-catalytic valorization of dark fermentation effluents by acetate oxidizing bacteria in bioelectrochemical system (BES). J. Power Sources 2014, 262, 183–191. [Google Scholar] [CrossRef]
- Liu, H.; Leng, F.; Guan, Y.; Yao, Y.; Li, Y.; Xu, S. Simultaneous Pollutant Removal and Electricity Generation in a Combined ABR-MFC-MEC System Treating Fecal Wastewater. Water Air Soil. Pollut. 2017, 228, 179. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron-graphite electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Liu, W.; He, Z.; Yang, C.; Zhou, A.; Guo, Z.; Liang, B.; Varrone, C.; Wang, A.-J. Microbial network for waste activated sludge cascade utilization in an integrated system of microbial electrolysis and anaerobic fermentation. Biotechnol. Biofuels 2016, 9, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vrieze, J.; Gildemyn, S.; Arends, J.B.A.; Vanwonterghem, I.; Verbeken, K.; Boon, N.; Verstraete, W.; Tyson, G.W.; Hennebel, T.; Rabaey, K. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res. 2014, 54, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ahn, Y.; Logan, B.E. A two-stage microbial fuel cell and anaerobic fluidized bed membrane bioreactor (MFC-AFMBR) system for effective domestic wastewater treatment. Environ. Sci. Technol. 2014, 48, 4199–4206. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, X.; Jin, J.; Vidonish, J.; Zhu, L. A novel bioelectrode and anaerobic sludge coupled system for p-ClNB degradation by magnetite nanoparticles addition. Environ. Sci. Pollut. Res. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.; San-Martín, M.I.; Escapa, A.; Morán, A. Domestic wastewater treatment in parallel with methane production in a microbial electrolysis cell. Renew. Energy 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Deng, L.; Chen, C.; Zheng, D.; Yang, H.; Liu, Y.; Chen, Z. Effect of temperature on continuous dry fermentation of swine manure. J. Environ. Manag. 2016, 177, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; San-Martín, M.I.; Mateos, R.; Morán, A. Scaling-up of membraneless microbial electrolysis cells (MECs) for domestic wastewater treatment: Bottlenecks and limitations. Bioresour. Technol. 2015, 180, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.J.; Fierro, J.; Sánchez, M.E.; Gómez, X. Anaerobic co-digestion of FOG and sewage sludge: Study of the process by Fourier transform infrared spectroscopy. Int. Biodeterior. Biodegrad. 2012, 75, 1–6. [Google Scholar] [CrossRef]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environmental Federation (WEF). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem. 2015, 40, 3412–3418. [Google Scholar] [CrossRef]
- Zinder, S.H.; Koch, M. Non-aceticlastic methanogenesis from acetate: Acetate oxidation by a thermophilic syntrophic coculture. Arch. Microbiol. 1984, 138, 263–272. [Google Scholar] [CrossRef]
- Westerholm, M.; Müller, B.; Arthurson, V.; Schnürer, A. Changes in the acetogenic population in a mesophilic anaerobic digester in response to increasing ammonia concentration. Microbes Environ. 2011, 26, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Ma, J.; Carballa, M.; Van De Caveye, P.; Verstraete, W. Enhanced propionic acid degradation (EPAD) system: Proof of principle and feasibility. Water Res. 2009, 43, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Pullammanappallil, P.C.; Chynoweth, D.P.; Lyberatos, G.; Svoronos, S.A. Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. Bioresour. Technol. 2001, 78, 165–169. [Google Scholar] [CrossRef]
- Fierro, J.; Martinez, E.J.; Rosas, J.G.; Fernández, R.A.; López, R.; Gómez, X. Co-Digestion of swine manure and crude glycerine: Increasing glycerine ratio results in preferential degradation of labile compounds. Water Air Soil Pollut. 2016, 227, 78. [Google Scholar] [CrossRef]
- Fukuzaki, S.; Nishio, N.; Shobayashi, M.; Nagai, S. Inhibition of the fermentation of propionate to methane by hydrogen, acetate, and propionate. Appl. Environ. Microb. 1990, 56, 719–723. [Google Scholar]
- Cuetos, M.J.; Gómez, X.; Escapa, A.; Moran, A. Evaluation and simultaneous optimization of bio-hydrogen production using 32 factorial design and the desirability function. J. Power Sources 2007, 169, 131–139. [Google Scholar] [CrossRef]
- Fernández, C.; Carracedo, B.; Martínez, E.J.; Gómez, X.; Morán, A. Application of a packed bed reactor for the production of hydrogen from cheese whey permeate: Effect of organic loading rate. J. Environ. Sci. Heal. A 2014, 49, 210–217. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Value |
|---|---|
| pH | 7.36 |
| Organic matter (%) | 1.12 ± 0.05 |
| Total nitrogen (%) | 0.21 ± 0.01 |
| C/N ratio | 3.15 |
| Total solids (g L−1) | 16.71 ± 0.80 |
| Volatile solids (g L−1) | 11.93 ± 0.59 |
| NH4+ (g L−1) | 1.13 ± 0.05 |
| Alcalinity (g-CaCO3 L−1) | 2.32 ± 0.10 |
| VFA (g L−1) | 0.5 ± 0.001 |
| Acetate (g L−1) | 0.030 ± 0.001 |
| PO43− (ppm) | 440 ± 13 |
| Ca2+ (ppm) | 576 ± 17 |
| Mg2+ (ppm) | 108 ± 3 |
| K+ (ppm) | 232 ± 7 |
| Na+ (ppm) | 48.4 ± 1.0 |
| Mn (ppm) | 3.4 ± 0.1 |
| Fe (ppm) | 263 ± 7 |
| Cu (ppm) | 3.4 ± 0.1 |
| Zn (ppm) | 17.1 ± 0.5 |
| Time (h) | Specific CH4 Production (L-CH4 kgVS−1 ) | VFA Production (mg L−1) | |||||
|---|---|---|---|---|---|---|---|
| Ratio 0.25 | Ratio 2.0 | Ratio 0.25 | Ratio 2.0 | ||||
| TVFA | Acetate | Butyrate | Propionate | TVFA | |||
| 0 | 0 ± 0 | 0 ± 0 | 37 | 36 | 6 | 0 | 42 |
| 15 | 105 ± 15 | 11 ± 3 | 225 | 491 | 1043 | 18 | 1560 |
| 23 | 153 ± 24 | 23 ± 2 | 170 | 820 | 2139 | 24 | 2988 |
| 40 | 234 ± 22 | 30 ± 10 | 240 | 1190 | 2372 | 173 | 3814 |
| 64 | 240 ± 15 | 34 ± 2 | 4 | 1496 | 2415 | 364 | 4513 |
| 88 | 245 ± 17 | 37 ± 14 | 3 | 1902 | 2942 | 356 | 5576 |
| 112 | 253 ± 15 | 39 ± 0 | - | - | - | - | - |
| 136 | 268 ± 15 | 43 ± 16 | 84 | 2300 | 3280 | 415 | 6458 |
| 160 | 283 ± 15 | 47 ± 16 | 6 | 2298 | 3325 | 413 | 6383 |
| 184 | 293 ± 25 | 48 ± 18 | n.d. | - | - | - | - |
| 208 | 299 ± 15 | 50 ± 15 | n.d. | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, R.; Martínez, E.J.; Escapa, A.; Martínez, O.; Díez-Antolínez, R.; Gómez, X. Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions. Fermentation 2018, 4, 2. https://doi.org/10.3390/fermentation4010002
Moreno R, Martínez EJ, Escapa A, Martínez O, Díez-Antolínez R, Gómez X. Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions. Fermentation. 2018; 4(1):2. https://doi.org/10.3390/fermentation4010002
Chicago/Turabian StyleMoreno, Rubén, Elia J. Martínez, Adrián Escapa, Olegario Martínez, Rebeca Díez-Antolínez, and Xiomar Gómez. 2018. "Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions" Fermentation 4, no. 1: 2. https://doi.org/10.3390/fermentation4010002
APA StyleMoreno, R., Martínez, E. J., Escapa, A., Martínez, O., Díez-Antolínez, R., & Gómez, X. (2018). Mitigation of Volatile Fatty Acid Build-Up by the Use of Soft Carbon Felt Electrodes: Evaluation of Anaerobic Digestion in Acidic Conditions. Fermentation, 4(1), 2. https://doi.org/10.3390/fermentation4010002