Microwave-Assisted Extraction Applied to Merlot Grapes with Contrasting Maturity Levels: Effects on Phenolic Chemistry and Wine Color
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapes
2.2. Winemaking and Application of Microwave-Assisted Extraction
2.3. Wine Basic Analysis
2.4. Spectrophotometric Analysis of the Wines
2.5. HPLC-DAD-Mass Spectrometry (MS) Analysis of the Wines
2.6. Data Analysis
3. Results and Discussion
3.1. Basic Chemical Composition of the Grapes
3.2. Basic Chemical Composition of the Finished Wines
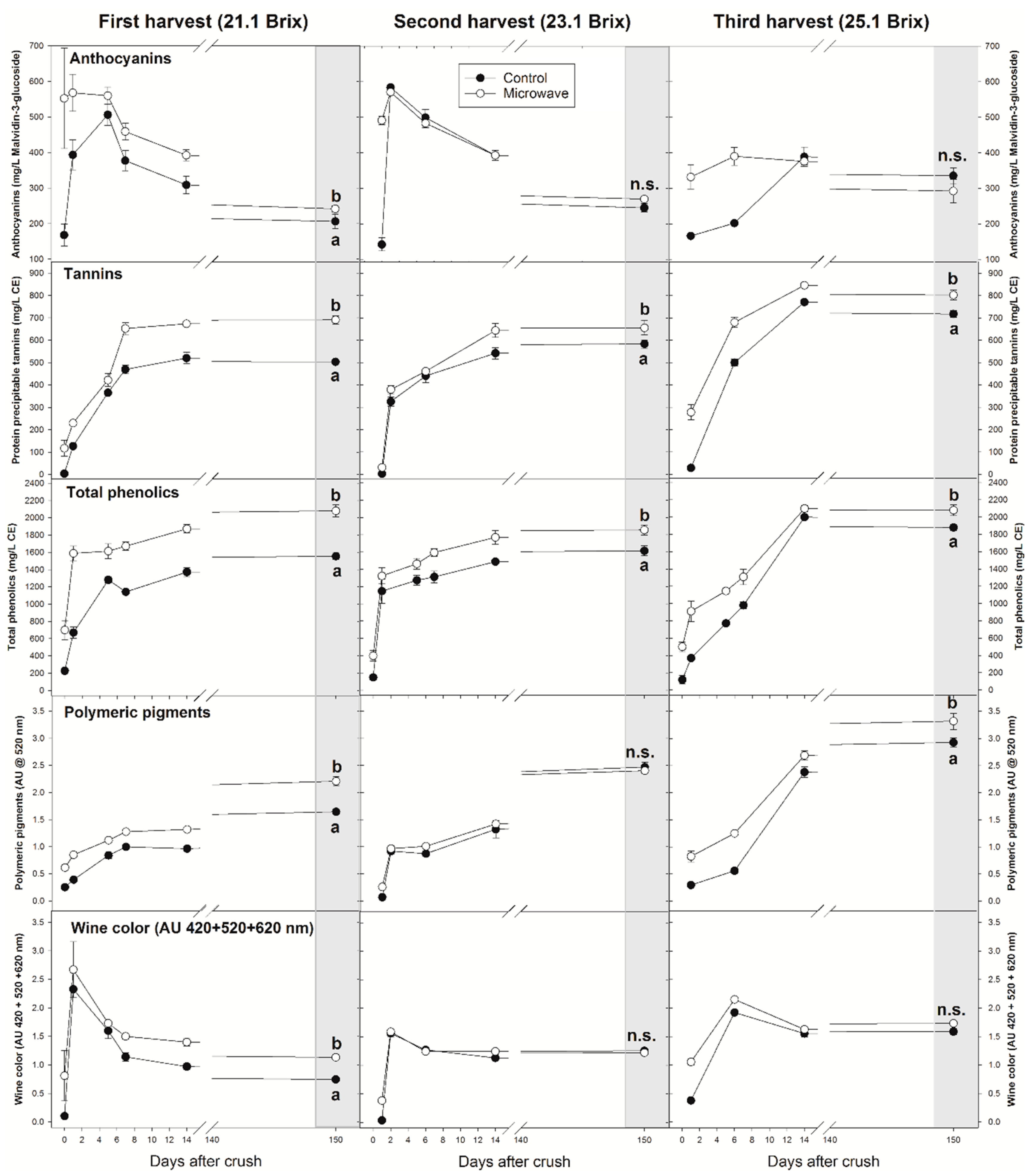
3.3. Phenolic and Chromatic Composition of the Wines
3.4. Detailed Anthocyanin and Anthocyanin-Derived Pigment Composition of the Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bindon, K.; Holt, H.; Williamson, P.O.; Varela, C.; Herderich, M.; Francis, I.L. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 2. Wine sensory properties and consumer preference. Food Chem. 2014, 154, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Casassa, L.F. Phenolic management in red wines: Investigation of the timing and severity of Regulated Deficit Irrigation (RDI), grape maturity and selected maceration conditions by HPLC-MS and sensory techniques. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 2013. [Google Scholar]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.; Larsen, R.C.; Hopfer, H.; Heymann, H.; Harbertson, J.F. Influence of fruit maturity, maceration length, and ethanol amount on chemical and sensory properties of Merlot wines. Am. J. Enol. Vitic. 2013, 64, 437–449. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.O. Phenolics and Ripening in Grape Berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar]
- Chen, J.-Y.; Wen, P.-F.; Kong, W.-F.; Pan, Q.-H.; Wan, S.-B.; Huang, W.-D. Changes and subcellular localizations of the enzymes involved in phenylpropanoid metabolism during grape berry development. J. Plant Physiol. 2006, 163, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Casassa, L.F. Flavonoid Phenolics in Red Winemaking. In Phenolic Compounds—Natural Sources, Importance and Applications; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Gil, M.; Kontoudakis, N.; González, E.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of Grape Maturity and Maceration Length on Color, Polyphenolic Composition, and Polysaccharide Content of Cabernet Sauvignon and Tempranillo Wines. J. Agric. Food Chem. 2012, 60, 7988–8001. [Google Scholar] [CrossRef]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 1. Grape and wine chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors affecting extraction and evolution of phenolic compounds during red wine maceration and the role of process modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar] [CrossRef]
- Koyama, K.; Goto-Yamamoto, N.; Hashizume, K. Influence of Maceration Temperature in Red Wine Vinification on Extraction of Phenolics from Berry Skins and Seeds of Grape (Vitis vinifera). Biosci. Biotechnol. Biochem. 2007, 71, 958–965. [Google Scholar] [CrossRef]
- Doco, T.; Williams, P.; Cheynier, V. Effect of Flash Release and Pectinolytic Enzyme Treatments on Wine Polysaccharide Composition. J. Agric. Food Chem. 2007, 55, 6643–6649. [Google Scholar] [CrossRef]
- Morel-Salmi, C.; Souquet, J.-M.; Bes, M.; Cheynier, V. Effect of Flash Release Treatment on Phenolic Extraction and Wine Composition. J. Agric. Food Chem. 2006, 54, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, T.; Loyola, C.; de Bruijn, J.; Bustamante, L.; Vergara, C.; von Baer, D.; Mardones, C.; Serra, I. Effect of thermomaceration and enzymatic maceration on phenolic compounds of grape must enriched by grape pomace, vine leaves and canes. Eur. Food Res. Technol. 2016, 242, 1149–1158. [Google Scholar] [CrossRef]
- Carew, A.L.; Gill, W.; Close, D.C.; Dambergs, R.G. Microwave Maceration with Early Pressing Improves Phenolics and Fermentation Kinetics in Pinot noir. Am. J. Enol. Vitic. 2014, 65, 401–406. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Casassa, L.F.; Huff, R.; Miller, E. Effect of Stem Additions and Microwave Extraction of Musts and Stems on Syrah, Merlot, and Cabernet Sauvignon Wines. In Proceedings of the 68th American Society for Enology and Viticulture National Conference, Seattle, WA, USA, 26–29 June 2017. [Google Scholar]
- Carew, A.L.; Sparrow, A.; Curtin, C.D.; Close, D.C.; Dambergs, R.G. Microwave Maceration of Pinot Noir Grape Must: Sanitation and Extraction Effects and Wine Phenolics Outcomes. Food Bioprocess Technol. 2013. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Edwards, G.; Caloghiris, S.; Wilkes, E. Chemical Analysis of Grapes and Wine Techniques and Concepts, 2nd ed.; Patrick Iland Wine Promotions Pty Ltd.: Adelaide, Australia, 2012; p. 118. [Google Scholar]
- Glories, Y. La couleur des vins rouges. 2ème partie. Mesure, origine et interprétation. Connaissance de la Vigne et du Vin 1984, 18, 253–271. [Google Scholar]
- Harbertson, J.F.; Picciotto, E.A.; Adams, D.O. Measurement of polymeric pigments in grape berry extracts and wines using a protein precipitation assay combined with bisulfite bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar]
- Blanco-Vega, D.; López-Bellido, F.J.; Alía-Robledo, J.M.; Hermosín-Gutiérrez, I. HPLC–DAD–ESI-MS/MS Characterization of Pyranoanthocyanins Pigments Formed in Model Wine. J. Agric. Food Chem. 2011, 59, 9523–9531. [Google Scholar] [CrossRef]
- Rankine, B.C.; Bridson, D.A. Glycerol in Australian Wines and Factors Influencing Its Formation. Am. J. Enol. Vitic. 1971, 22, 6–12. [Google Scholar]
- El Darra, N.; Turk, M.F.; Ducasse, M.-A.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Changes in polyphenol profiles and color composition of freshly fermented model wine due to pulsed electric field, enzymes and thermovinification pretreatments. Food Chem. 2016, 194, 944–950. [Google Scholar] [CrossRef]
- de Andrade Neves, N.; de Araújo Pantoja, L.; dos Santos, A. Thermovinification of grapes from the Cabernet Sauvignon and Pinot Noir varieties using immobilized yeasts. Eur. Food Res. Technol. 2014, 238, 79–84. [Google Scholar] [CrossRef]
- Robinson, S.P.; Davies, C. Molecular biology of grape berry ripening. Aust. J. Grape Wine Res. 2000, 6, 175–188. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between cell wall polysaccharide composition and the extractability of grape phenolic compounds into Shiraz wines. Part II: Extractability during fermentation into wines made from grapes of different ripeness levels. Food Chem. 2019, 278, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. The specificity of proanthocyanidin-protein interactions. J. Biol. Chem. 1981, 256, 4494–4497. [Google Scholar] [PubMed]
- Peyrot des Gachons, C.; Kennedy, J.A. Direct Method for Determining Seed and Skin Proanthocyanidin Extraction into Red Wine. J. Agric. Food Chem. 2003, 51, 5877–5881. [Google Scholar] [CrossRef] [PubMed]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in skins and seeds of Cabernet Sauvignon, Syrah, and Pinot Noir berries during ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.S.; Harbertson, J.F. Effect of extended maceration and ethanol concentration on the extraction and evolution of phenolics, colour components and sensory attributes of Merlot wines. Aust. J. Grape Wine Res. 2013, 19, 25–39. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.S.; Harwood, E.D.; Weller, K.M.; Ross, C.F. Chemical and sensory effects of saignée, water addition, and extended maceration on high Brix must. Am. J. Enol. Vitic. 2009, 60, 450–460. [Google Scholar]
- Harbertson, J.F.; Spayd, S. Measuring Phenolics in the Winery. Am. J. Enol. Vitic. 2006, 57, 280–288. [Google Scholar]
- Adams, D.O.; Harbertson, J.F.; Picciotto, E.A. Fractionation of red wine polymeric pigments by protein precipitation and bisulfite bleaching. In Red Wine Color; American Chemical Society: Washington, DC, USA, 2004; Vol. 886, pp. 275–288. [Google Scholar]
- Garrido-Bañuelos, G.; Buica, A.; Schückel, J.; Zietsman, A.J.J.; Willats, W.G.T.; Moore, J.P.; Du Toit, W.J. Investigating the relationship between grape cell wall polysaccharide composition and the extractability of phenolic compounds into Shiraz wines. Part I: Vintage and ripeness effects. Food Chem. 2019, 278, 36–46. [Google Scholar] [CrossRef]
- de Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic Reactions during Winemaking and Aging. Am. J. Enol. Vitic. 2006, 57, 289–297. [Google Scholar]


| Harvest Treatment | Harvest Date | Brix | pH | Titratable Acidity (g/L) |
|---|---|---|---|---|
| First harvest | 3-Feb | 21.16 ± 0.08 a a | 3.24 ± 0.18 a | 8.18 ± 0.15 c |
| Second harvest | 27-Feb | 23.14 ± 0.09 b | 3.48 ± 0.01 b | 5.05 ± 0.04 b |
| Third harvest | 29-Mar | 25.16 ± 0.08 c | 3.75 ± 0.01 c | 4.61 ± 0.04 a |
| Harvest Treatment | Microwave Treatment | Alcohol % (v/v) | pH | Titratable Acidity (g/L) | Tartaric Acid (g/L) | Citric Acid (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) | Acetic Acid (g/L) | Glucose + Fructose (g/L) | Residual Sugars (g/L) | Glycerol (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First harvest | Control | 12.41 ± 0.06 a a | 3.43 ± 0.09 a | 5.57 ± 0.03 b | 2.32 ± 0.03 d | 0.31 ± 0.05 b | 0.50 ± 0.06 a | 1.53 ± 0.03 bc | 0.92 ± 0.01 b | 0.60 ± 0.28 a | 0.87 ± 0.21 a | 9.50 ± 0.01 a |
| MW | 12.42 ± 0.08 a | 3.46 ± 0.07 a | 5.68 ± 0.12 b | 2.22 ± 0.05 cd | 0.27 ± 0.08 b | 0.57 ± 0.03 a | 1.51 ± 0.07 bc | 0.93 ± 0.03 b | 0.98 ± 0.41 a | 1.26 ± 0.66 a | 9.87 ± 0.26 ab | |
| Second harvest | Control | 13.50 ± 0.06 b | 3.60 ± 0.07 cd | 5.53 ± 0.17 b | 1.73 ± 0.10 a | 0.37 ± 0.05 b | 0.93 ± 0.06 b | 1.49 ± 0.06 bc | 0.93 ± 0.03 b | 0.27 ± 0.14 a | 0.73 ± 0.35 a | 10.43 ± 0.07 bc |
| MW | 13.76 ± 0.09 c | 3.62 ± 0.02 d | 5.50 ± 0.06 b | 1.60 ± 0.05 a | 0.41 ± 0.08 b | 0.90 ± 0.06 b | 1.54 ± 0.11 c | 0.92 ± 0.01 b | 0.47 ± 0.06 a | 0.53 ± 0.27 a | 10.41 ± 0.15 bc | |
| Third harvest | Control | 15.11 ± 0.06 d | 3.58 ± 0.09 bc | 5.08 ± 0.12 a | 2.11 ± 0.06 bc | 0.05 ± 0.02 a | 0.50 ± 0.11 a | 1.33 ± 0.03 ab | 0.81 ± 0.01 a | 1.93 ± 0.35 b | 2.81 ± 0.11 b | 10.56 ± 0.17 c |
| MW | 15.27 ± 0.09 d | 3.54 ± 0.01 b | 5.13 ± 0.07 a | 2.03 ± 0.04 b | 0.06 ± 0.03 a | 0.43 ± 0.03 a | 1.18 ± 0.06 b | 0.89 ± 0.02 b | 2.01 ± 0.32 b | 3.16 ± 0.33 b | 10.83 ± 0.32 c | |
| ANOVA factors and interactions | ||||||||||||
| Harvest treatment (H) | <0.0001 b | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | 0.002 | 0.001 | <0.0001 | <0.0001 | 0.001 | |
| Microwave treatment (MW) | 0.025 | 0.830 | 0.612 | 0.034 | 0.827 | 0.831 | 0.476 | 0.116 | 0.387 | 0.535 | 0.229 | |
| H × M interaction | 0.194 | 0.056 | 0.805 | 0.842 | 0.745 | 0.555 | 0.317 | 0.092 | 0.874 | 0.658 | 0.575 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casassa, L.F.; Sari, S.E.; Bolcato, E.A.; Fanzone, M.L. Microwave-Assisted Extraction Applied to Merlot Grapes with Contrasting Maturity Levels: Effects on Phenolic Chemistry and Wine Color. Fermentation 2019, 5, 15. https://doi.org/10.3390/fermentation5010015
Casassa LF, Sari SE, Bolcato EA, Fanzone ML. Microwave-Assisted Extraction Applied to Merlot Grapes with Contrasting Maturity Levels: Effects on Phenolic Chemistry and Wine Color. Fermentation. 2019; 5(1):15. https://doi.org/10.3390/fermentation5010015
Chicago/Turabian StyleCasassa, L. Federico, Santiago E. Sari, Esteban A. Bolcato, and Martin L. Fanzone. 2019. "Microwave-Assisted Extraction Applied to Merlot Grapes with Contrasting Maturity Levels: Effects on Phenolic Chemistry and Wine Color" Fermentation 5, no. 1: 15. https://doi.org/10.3390/fermentation5010015
APA StyleCasassa, L. F., Sari, S. E., Bolcato, E. A., & Fanzone, M. L. (2019). Microwave-Assisted Extraction Applied to Merlot Grapes with Contrasting Maturity Levels: Effects on Phenolic Chemistry and Wine Color. Fermentation, 5(1), 15. https://doi.org/10.3390/fermentation5010015







