Production and Purification of l-lactic Acid in Lab and Pilot Scales Using Sweet Sorghum Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Microorganism
2.3. Fermentation
2.3.1. Laboratory Scale Fermentations
2.3.2. Pilot Scale Fermentation
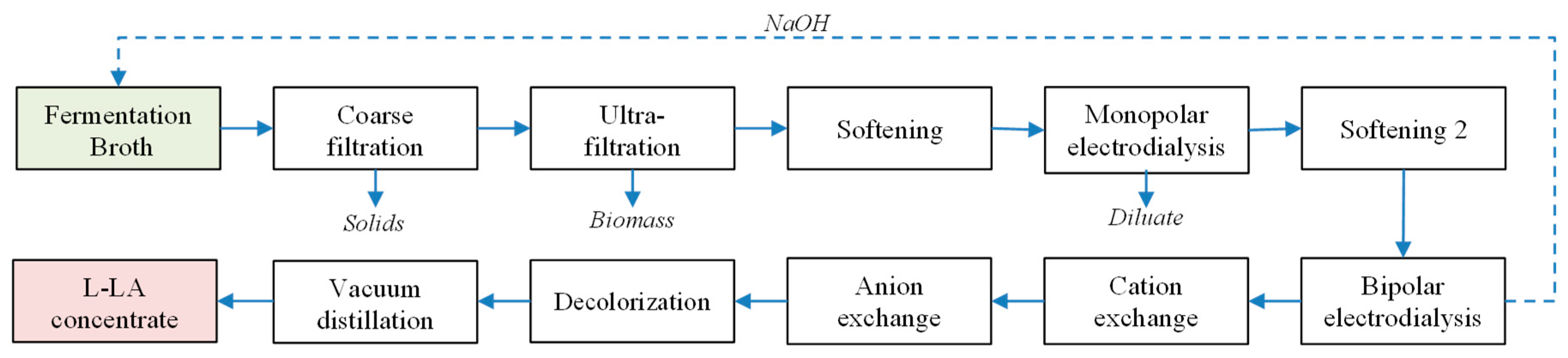
2.4. Downstream Process
2.4.1. Filtration and Softening
2.4.2. Electrodialysis
2.4.3. Decolorization and Chromatography
2.5. Analytical Assays
3. Results and Discussion
3.1. Optimization of the Fermentation Process
3.2. Lab Scale Fermentations Using SSJ as Sole Nutrient Source
3.3. Pilot Scale Fermentation using SSJ
3.4. Downstream Process of the Lactic Acid Produced in Pilot Scale
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rodrigues, C.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; de Oliveira, J.; Letti, L.A.J.; Soccol, C.R. Production and application of lactic Acid. Curr. Dev. Biotechnol. Bioeng. Prod. Isol. Purif. Ind. Prod. 2016, 543–556. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Brock, S.; Kuenz, A.; Prüße, U. Impact of hydrolysis methods on the utilization of agricultural residues as nutrient source for D-lactic acid production by Sporolactobacillus inulinus. Fermentation 2019, 5, 12. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Vaz Rossell, C.E.; Venus, J.; Cândida Rabelo, S.; Maciel Filho, R. Detoxification of sugarcane-derived hemicellulosic hydrolysate using a lactic acid producing strain. J. Biotechnol. 2018, 278, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pleissner, D.; Lau, K.Y.; Schneider, R.; Venus, J.; Lin, C.S.K. Fatty acid feedstock preparation and lactic acid production as integrated processes in mixed restaurant food and bakery wastes treatment. Food Res. Int. 2015, 73, 52–61. [Google Scholar] [CrossRef]
- Neu, A.K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure l(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Tarraran, L.; Mazzoli, R. Alternative strategies for lignocellulose fermentation through lactic acid bacteria: the state of the art and perspectives. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernandez, C.; Bellesteros, M.; Tomas-Pejo, E. Biotechnological advances in lactic acid production by lactic acid bacteria: lignocellulose as novel substrate. Biofuels Bioprod. Biorefining 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Johnston, D.; Nghiem, N. Evaluation of sweet sorghum juice for the production of lysine using Corynebacterium glutamicum. Fermentation 2018, 4, 29. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, H.; Zheng, J.; Fu, C.; Chen, C.; Qin, P.; Wang, Z. A combination of evaporation and chemical preservation for long-term storage of fresh sweet sorghum juice and subsequent bioethanol production. J. Food Process. Preserv. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Takaki, M.; Tan, L.; Murakami, T.; Tang, Y.Q.; Sun, Z.Y.; Morimura, S.; Kida, K. Production of biofuels from sweet sorghum juice via ethanol-methane two-stage fermentation. Ind. Crops Prod. 2015, 63, 329–336. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, S. Direct fermentation of sweet sorghum juice by Clostridium acetobutylicum and Clostridium tetanomorphum to produce bio-butanol and organic acids. Biofuel Res. J. 2015, 6, 248–252. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, J.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. Repeated-batch fermentation of L-lactic acid from acid hydrolysate of sweet sorghum juice using mixed neutralizing agent under unsterilized conditions. J. Chem. Technol. Biotechnol. 2011, 61, 66–68. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.; Cai, D.; Wang, B.; Qin, P.; Wang, Z.; Tan, T. Improvement of L-lactic acid productivity from sweet sorghum juice by repeated batch fermentation coupled with membrane separation. Bioresour. Technol. 2016, 211, 291–297. [Google Scholar] [CrossRef]
- Glaser, R.; Venus, J. Co-fermentation of the main sugar types from a beechwood organosolv hydrolysate by several strains of Bacillus coagulans results in effective lactic acid production. Biotechnol. Rep. 2018, 18, 22–27. [Google Scholar] [CrossRef]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative assessment of downstream processing options for lactic acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Sasiradee, J.; Kienberger, M.; Nuttakul, M.; Siebenhofer, M. Potential and assessment of lactic acid production and isolation—A review. J. Chem. Technol. Biotechnol. 2017, 92, 2885–2893. [Google Scholar]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane technologies for lactic acid separation from fermentation broths derived from renewable resources. Membranes (Basel) 2018, 8, 94. [Google Scholar] [CrossRef]
- Oonkhanond, B.; Jonglertjunya, W.; Srimarut, N.; Bunpachart, P.; Tantinukul, S.; Nasongkla, N.; Sakdaronnarong, C. Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J.Environ. Chem. Eng. 2017, 5, 2533–2541. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Ngo, H.H.; Li, Y. Dynamic membrane-assisted fermentation of food wastes for enhancing lactic acid production. Bioresour. Technol. 2017, 234, 40–47. [Google Scholar] [CrossRef]
- Tong, W.Y.; Fu, X.Y.; Lee, S.M.; Yu, J.; Liu, J.W.; Wei, D.Z.; Koo, Y.M. Purification of L(+)-lactic acid from fermentation broth with paper sludge as a cellulosic feedstock using weak anion exchanger Amberlite IRA-92. Biochem. Eng. J. 2004, 18, 89–96. [Google Scholar] [CrossRef]
- DIN-EN-25663 Total Kjeldahl Nitrogen in Water and Biosolids by Automated Colorimetry with Preliminary Distillation/Digestion. Available online: https://www.epa.gov/sites/production/files/2015-10/documents/method_1687_draft_2001.pdf (accessed on 1 January 2019).
- ISO 15681-1 Water quality—Determination of orthophosphate and total phosphorus contents by flow analysis (FIA and CFA)—Part 1: Method by flow injection analysis (FIA) Qualité. 2003. Available online: https://www.sis.se/api/document/preview/904242/ (accessed on 1 January 2019).
- Cai, D.; Wang, Y.; Chen, C.; Qin, P.; Miao, Q.; Zhang, C.; Li, P.; Tan, T. Acetone-butanol-ethanol from sweet sorghum juice by an immobilized fermentation-gas stripping integration process. Bioresour. Technol. 2016, 211, 704–710. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. The optimization of L-lactic acid production from sweet sorghum juice by mixed fermentation of Bacillus coagulans and Lactobacillus rhamnosus under unsterile conditions. Bioresour. Technol. 2016, 218, 1098–1105. [Google Scholar] [CrossRef]
- Hoffmann, E.; Ye, J.; Hahn, H.H. Recent advances in application of electrodialysis with bipolar membranes for organic acid recovery from fermentation broth. Curr. Org. Chem. 2016, 20, 2753–2761. [Google Scholar] [CrossRef]
- Lech, M.; Trusek, A. Batch Electrodialysis of Lactic Acid Obtained from Lab Fermentation. Polish J. Chem. Technol. 2018, 20, 81–86. [Google Scholar] [CrossRef]
- Moldes, A.B.; Alonso, J.L.; Parajó, J.C. Recovery of lactic acid from simultaneous saccharification and fermentation media using anion exchange resins. Bioprocess Biosyst. Eng. 2003, 25, 357–363. [Google Scholar] [CrossRef]
- Garrett, B.G.; Srinivas, K.; Ahring, B.K. Performance and stability of AmberliteTM IRA-67 ion exchange resin for product extraction and pH control during homolactic fermentation of corn stover sugars. Biochem. Eng. J. 2015, 94, 1–8. [Google Scholar] [CrossRef]


| Component | Batch A | Batch B |
|---|---|---|
| Sucrose (g∙L−1) | 66.78 | 68.87 |
| Glucose (g∙L−1) | 24.87 | 23.43 |
| Fructose (g∙L−1) | 13.46 | 17.51 |
| Total nitrogen (mg∙L−1) | 1013.15 | 957.00 |
| Total Phosphorus (mg∙L−1) | 422.40 | 355.00 |
| Cl− (mg∙L−1) | 150.9 | 143.00 |
| SO42− (mg∙L−1) | 189.67 | 253.00 |
| Na+ (mg∙L−1) | 3.82 | 11.1 |
| K+ (mg∙L−1) | 5183.8 | 4347.00 |
| Mg2+ (mg∙L−1) | 333.33 | 295.00 |
| Ca2+ (mg∙L−1) | 668.71 | 565.00 |
| Product | Fermentation type | Nitrogen source | Microorganism | Volume (L) | Yield (g∙g−1) | Productivity (g∙L−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|
| d-lactic acid | Fed-batch | YE* | Sporolactobacillus inulinus | 0.3 | 0.96 | 1.55 | [4] |
| l-lactic acid | Repeated batch | YE & Soya peptone | Bacillus coagulans | 3 | 0.93 | 2.45 | [15] |
| Batch and purification | YE/Corn steep powder | Bacillus coagulans/Lactobacillus rhamnosus | 2 | 0.92 | 1.84 | [27] | |
| Batch and purification | YE | Lactobacillus plantarum | 1 | 0.93 | 0.6 | [5] | |
| Pilot scale and downstream | - | Bacillus coagulans | 50 | 0.70 | 1.47 | This study | |
| Lysine | Batch | YE & soya peptone | Corynebacterium glutamicum | 1 | 0.22 | - | [11] |
| Ethanol-methane | Batch | (NH4)2 SO4/(NH2)2 CO/Urea | Saccharomyces cerevisiae | 0.3/0.8 | 0.89 | 24.7 | [13] |
| Acetone-butanol-ethanol | Batch | Ammonium acetate | Clostridium acetobutylicum | 1.5 | 0.41 | 0.53 | [26] |
| DSP Step | V (L) | LA (g∙L−1) | Ntotal (mg∙L−1) | Ptotal (mg∙L−1) | Cl− (mg∙L−1) | SO42− (mg∙L−1) | Na+ (mg∙L−1) | K+ (mg∙L−1) | Mg2+ (mg∙L−1) | Ca2+ (mg∙L−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| End of fermentation | 57.6 | 73.4 | 780 | 316 | 141.3 | 349 | 17033 | 3793 | 263 | 499 |
| Coarse Filtration | 57.5 | 72.5 | 844 | 298 | 104 | 259 | 16831 | 3665 | 263 | 494 |
| Permeate UF | 54.4 | 70.6 | 560 | 260 | 102 | 231 | 16427 | 3514 | 251 | 474 |
| Softening 1 | 61.7 | 62.5 | 449 | 211 | 87.4 | 190 | 15837 | 2694 | 0.48 | 6.5 |
| Concentrate MED | 20.6 | 180.1 | 415 | 492 | 281 | 536 | 44111 | 7363 | 7.05 | 26.7 |
| Softening 2 | 21.1 | 166.8 | 323 | 465 | 277 | 555 | 41349 | 6989 | 1.53 | 12.0 |
| Acid stream BED | 18.9 | 159.7 | 221 | 435 | 265 | 556 | 492 | 73.4 | 2.74 | 39.0 |
| Salt stream BED | 8.5 | n.d. | 139 | 67.0 | 1.66 | 17.6 | 1161 | 86.2 | 2.19 | 49.8 |
| Base stream BED | 32.0 | 13.2 | 73.5 | 20.3 | 39.2 | 111 | 27779 | 41.36 | 0.98 | 24.1 |
| Cation exchange | 19.5 | 133.4 | 125 | 367 | 248 | 494 | 10.9 | 0.87 | 0.13 | 6.12 |
| Anion exchange | 21.0 | 128.1 | 109 | <8 | 2.36 | 169 | 10.4 | 0.97 | 0.21 | 8.86 |
| Decolorization | 23.0 | 111.6 | 70.1 | <9 | 2.63 | 147 | 10.7 | 0.63 | 0.17 | 1.07 |
| Concentrate LA stream | 2.9 | 905.8 | 415.7 | 24.9 | <0.05 | 966 | 81.0 | 2.16 | <0.025 | <0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Mandl, M.; Venus, J. Production and Purification of l-lactic Acid in Lab and Pilot Scales Using Sweet Sorghum Juice. Fermentation 2019, 5, 36. https://doi.org/10.3390/fermentation5020036
Olszewska-Widdrat A, Alexandri M, López-Gómez JP, Schneider R, Mandl M, Venus J. Production and Purification of l-lactic Acid in Lab and Pilot Scales Using Sweet Sorghum Juice. Fermentation. 2019; 5(2):36. https://doi.org/10.3390/fermentation5020036
Chicago/Turabian StyleOlszewska-Widdrat, Agata, Maria Alexandri, José Pablo López-Gómez, Roland Schneider, Michael Mandl, and Joachim Venus. 2019. "Production and Purification of l-lactic Acid in Lab and Pilot Scales Using Sweet Sorghum Juice" Fermentation 5, no. 2: 36. https://doi.org/10.3390/fermentation5020036
APA StyleOlszewska-Widdrat, A., Alexandri, M., López-Gómez, J. P., Schneider, R., Mandl, M., & Venus, J. (2019). Production and Purification of l-lactic Acid in Lab and Pilot Scales Using Sweet Sorghum Juice. Fermentation, 5(2), 36. https://doi.org/10.3390/fermentation5020036








