Production of Anserine-Rich Fish Sauce from Giant Masu Salmon, Oncorhynchus masou masou and γ-Aminobutyric Acid (GABA)-Enrichment by Lactobacillus plantarum Strain N10
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of LAB
2.2. Screening of GABA-Producing LAB by Thin Layer Chromatography (TLC) and Determination of GABA by Liquid Chromatography-Mass Spectrometry (LC-MS)
2.3. Extraction of DNA and Amplification of 16S rRNA Genes by Polymerase Chain Reaction (PCR)
2.4. Basic Local Alightment Search Tool (BLAST) ANALYSIS and Phylogenetic Analysis
2.5. Effect of NaCl, pH and Temperatures on the Growth of GABA-Producing LAB
2.6. Experimental Fish and Proximate Analysis
2.7. Preparation of Crude Enzyme Solution from Giant Masu Salmon
2.8. Protease Assay
2.9. Preparation of Fish Sauce From Giant Masu Salmon
2.10. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.11. Bacteria Plate Count and Histamine Analysis
2.12. Amino Acid Analysis
2.13. Microbial Fermentation of GMSFS by GABA-Producing Strain N10
2.14. Statistical Analysis
3. Results
3.1. Isolation of LAB and Screening of GABA-Producing LAB by TLC
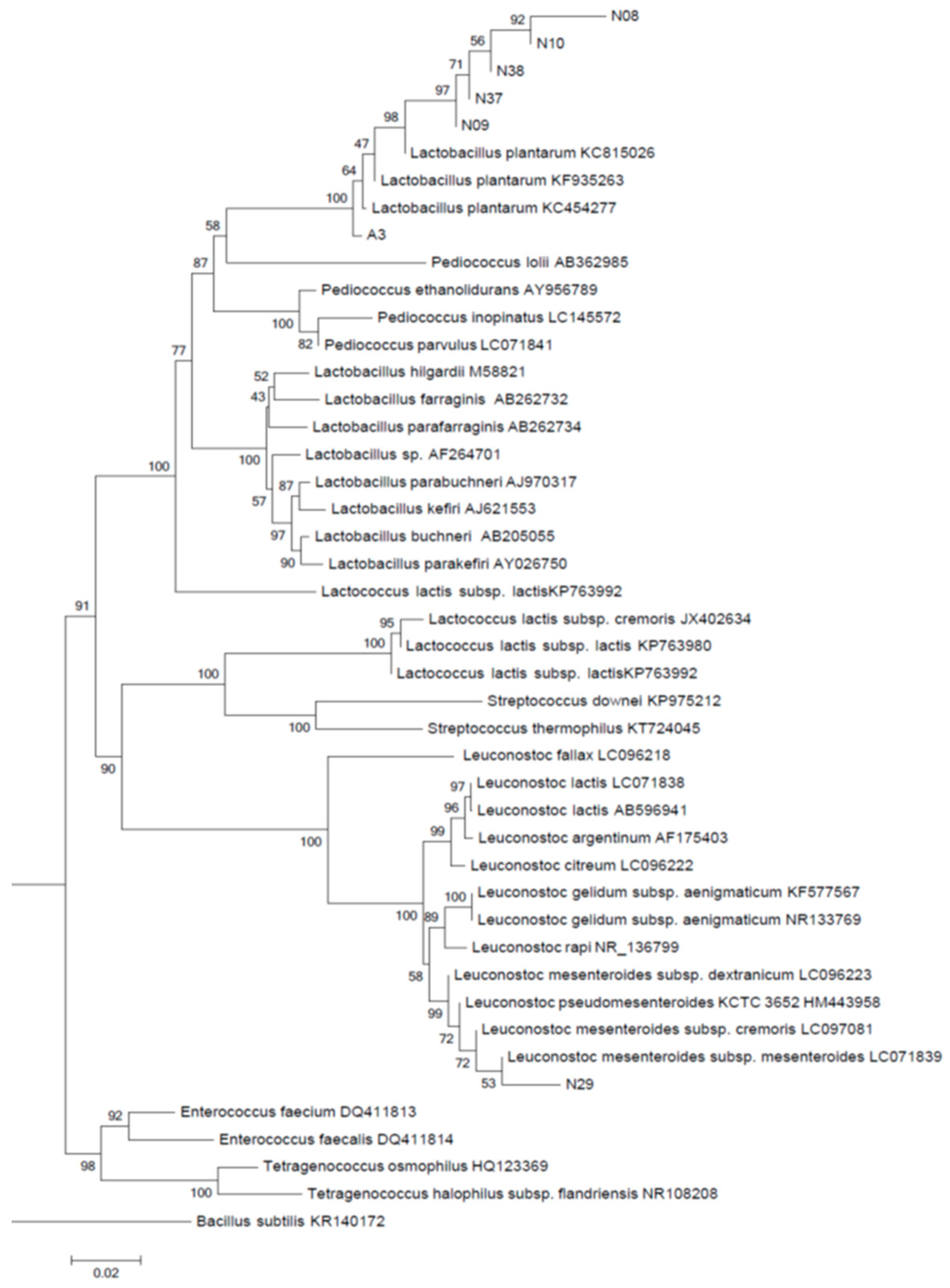
3.2. Identification of Isolates Based on Phylogenetic Analysis
3.3. Growth at Different Salinity, pH, and NaCl
3.4. Proximate Analysis of the Minced Fish Body
3.5. Protease Activity
3.6. Time Course Experiment of Autolysis
3.6.1. Change of Protein Concentration
3.6.2. Distribution of Protein Molecular Size
3.6.3. Bacterial Counts and Histamine Concentration
3.6.4. Total Free Amino Acid Content and Amino Acid Composition
3.7. Microbial Fermentation of GMSFS by Strain N10
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tamate, T.; Maekawa, K. Life cycle of masu salmon (Oncorhynchus masou) in Shumarinai Lake, northern Hokkaido, Japan. Eur. J. For. Res. 2000, 1, 39–42. [Google Scholar]
- Nakajima, T.; Shimura, H.; Yamazaki, M.; Fujioka, Y.; Ura, K.; Hara, A.; Shimizu, M. Lack of hormonal stimulation prevents the landlocked Biwa salmon (Oncorhynchus masou subspecies) from adapting to seawater. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 307, R414–R425. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Ou, J.; Chen, B.W.; Yuan, C.H.; Zhang, X.H.; Cheng, S.S.; Funatsu, Y.; Kawasaki, K.I.; Satomi, M.; Fukuda, Y. Effects of soy sauce koji and lactic acid bacteria on the fermentation of fish sauce from freshwater silver carp Hypophthalmichthys molitrix. Fish. Sci. 2005, 71, 422–430. [Google Scholar] [CrossRef]
- Park, J.; Fukumoto, Y.; Fujita, E.; Tanaka, T.; Washio, T.; Otsuka, S.; Shimizu, T.; Watanabe, K.; Abe, H. Chemical Composition of Fish Sauce Produced in Southeast and East Asian Countries. J. Food Comp. Anal. 2001, 14, 113–125. [Google Scholar] [CrossRef]
- Park, J.; Watanabe, T.; Endo, K.; Watanabe, K.; Abe, H. Tasteactive components in a Vietnamese fish sauce. Fish. Sci. 2002, 68, 913–920. [Google Scholar] [CrossRef]
- Kimura, M.; Furutani, A.; Fukui, Y.; Shibata, Y.; Nei, D.; Yano, Y.; Satomi, M. Isolation and identification of the causative bacterium of histamine accumulation during fish sauce fermentation and the suppression effect of inoculation with starter culture of lactic acid bacterium on the histamine accumulation in fish sauce processing. Nippon Suisan Gakkaishi 2015, 81, 97–106. [Google Scholar] [CrossRef]
- Klomklao, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Simpson, B.K. Effects of the addition of spleen of skipjack tuna (Katsuwonuspelamis) on the liquefaction and characteristics of fish sauce made from sardine (Sardinella gibbosa). Food Chem. 2006, 98, 440–452. [Google Scholar] [CrossRef]
- Utagawa, T. Rapid fermentation of fish sauce and its use. J. Brew. Soc. Jpn. 2012, 107, 477–484. [Google Scholar] [CrossRef][Green Version]
- Kataoka, E.; Tokue, C.; Yamashita, T.; Tanimura, W. Amino Acids, Organic Acids, Fatty Acids, Trimethylamine and Methional in Improved Fish Sauce. Jpn. J. Nutr. Dietet. 1987, 45, 67–76. [Google Scholar] [CrossRef]
- Kimata, M.; Kawai, A. The freshness of fish and the amount of histamine presented in the meat. Mem. Res. Inst. Food Sci. Kyoto Univ. 1953, 5, 25–54. [Google Scholar]
- Steinhoff, M.; Griffiths, C.; Church, M.; Lugar, T.A. Histamine; Blackwell Science: Oxford, UK, 2004; pp. 50–52. [Google Scholar]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Divya, J.; Kulangara Varsha, K.; Madhavan Nampoothiri, K.; Ismail, B.; Pandey, A. Probiotic fermented foods for health benefits. Eng. Life Sci. 2012, 12, 377–390. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Lactic Acid Bacteria in Food Industry: Microorganisms in Sustainable Agriculture and Biotechnology; Satyanarayama, T., Johri, B.M., Prakash, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 757–772. [Google Scholar]
- Fessard, A.; Kapoor, A.; Patche, J.; Assemat, S.; Hoarau, M.; Bourdon, E.; Bahorun, T.; Remize, F. Lactic Fermentation as an Efficient Tool to Enhance the Antioxidant Activity of Tropical Fruit Juices and Teas. Microorganisms 2017, 5, 23. [Google Scholar] [CrossRef]
- Arena, M.P.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V.; Spano, G. Functional Starters for Functional Yogurt. Foods 2015, 4, 15–33. [Google Scholar] [CrossRef]
- Pasini, F.; Soglia, F.; Petracci, M.; Caboni, M.F.; Marziali, S.; Montanari, C.; Gardini, F.; Grazia, L.; Tabanelli, G. Effect of Fermentation with Different Lactic Acid Bacteria Starter Cultures on Biogenic Amine Content and Ripening Patterns in Dry Fermented Sausages. Nutrients 2018, 10, 1497. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.; Merrifield, C.A.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Domingos-Lopes, M.F.P.; Stanton, C.; Paul Ross, R.; Silva, C.C.G. Production of γ-aminobutyric acid (GABA) by Lactobacillus otakiensis and other Lactobacillus sp. isolated from traditional Pico cheese. Int. J. Dairy Technol. 2018, 71, 1012–1017. [Google Scholar] [CrossRef]
- Yoshida, S.; Haramoto, M.; Fukuda, T.; Mizuno, H.; Tanaka, A.; Nishimura, M.; Nishihara, J. Optimization of a γ-aminobutyric acid (GABA) enrichment process for Hokkaido white rice and the effects of GABA-enriched white rice on stress relief in humans. Nippon Shokuhin Kagaku Kogaku Kaishi 2015, 62, 95–103. [Google Scholar] [CrossRef]
- Itoh, T.; Tomita, J.; Motegi, H.; Ebihara, S.; Takahashi, K.; Shimizu, R.; Numata, H.; Watanabe, Y. Antihypertensive and sedative effect of soymilk contained high natural GABA levels in human. Oyo Yakuri 2007, 72, 51–56. [Google Scholar]
- Wang, C.; Fan, L.; Gao, H.; Wu, X.; Li, J.; Lv, G.; Gong, B. Polyamine biosynthesis and degradation are modulated by exogenous gamma-aminobutyric acid in root-zone hypoxia-stressed melon roots. Plant Physiol. Biochem. 2014, 82, 17–26. [Google Scholar] [CrossRef]
- Gordon, F.J.; Sved, A.F. Neutrotransmitters in central cardiovascular regulations: Glutamate and GABA. Clin. Exp. Pharmacol. Physiol. 2002, 29, 522–524. [Google Scholar] [CrossRef]
- Gamel-Didelon, K.; Corsi, C.; Pepeu, G.; Jung, H.; Gratzl, M.; Mayerhofer, A. An autocrine role for pituitary GABA: Activation of GABA-B receptors and regulation of growth hormone levels. Neutroendocrinology 2002, 76, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.; Sanacora, G.; Blumberg, H.; Anand, A.; Charney, D.S.; Marck, G.; Epperson, C.N.; Goddard, A.; Mason, G.F. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol. Psychiatr. 2002, 7, S71–S80. [Google Scholar] [CrossRef]
- Minuk, G.Y. GABA and hepatocellular carcinoma. Mol. Cell. Biochem. 2000, 207, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Hoppu, U.; Hopia, A.; Pohjanheimo, T.; Rotola-Pukkila, M.; Mäkinen, S.; Pihlanto, A.; Sandell, M. Effect of Salt Reduction on Consumer Acceptance and Sensory Quality of Food. Foods 2017, 6, 103. [Google Scholar] [CrossRef]
- Diana, M.; Rafecas, M.; Arco, C.; Quilez, J. Free amino acid profile of Spanish artisanal cheeses: Importance of gamma-aminobutyric acid (GABA) and ornithine content. J. Food Compos. Anal. 2014, 35, 94–100. [Google Scholar] [CrossRef]
- Aryanta, R.W.; Fleet, G.H.; Buckle, K.A. The occurrence and growth of microorganisms during the fermentation of fish sausage. Int. J. Food Microbiol. 1991, 13, 143–155. [Google Scholar] [CrossRef]
- Hill, W.E.; Keasler, S.P.; Trucksess, M.W.; Feng, P.; Kaysner, C.A.; Lampel, K.A. Polymerase Chain Reaction Identification of Vibrio vulnificus in Artificially Contaminated Oysters. Appl. Environ. Microbiol. 1991, 57, 707–711. [Google Scholar]
- Schmizs, A.; Riesner, D. Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Anal. Biochem. 2006, 354, 311–313. [Google Scholar]
- Tompson, J.D.; Gibon, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2006, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Ascoli, I.; Lees, M.; Meath, J.A.; Lebaron, N. Preparation of lipid extracts from brain tissue. J. Biol. Chem. 1951, 195, 833–841. [Google Scholar]
- AOAC International. AOAC International Official Methods of Analysis, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Hirata, K.; Hirokado, M.; Uematsu, Y.; Nakajima, K.; Kazama, M. Investigation on Assay for the Proteolytic Activity of Protease and Papain Preparations for Food Manufacturing. Food Hyg. Saf. Sci. 1987, 45, 67–76. [Google Scholar] [CrossRef]
- Lowly, O.H.; Rosebrough, N.J.; Fan, A.C.; Rondall, R.T. Protein measurement with the folin phenol solution. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zareian, M.; Oskoueian, E.; Forghani, B.; Ebrahimi, M. Production of a wheat-based fermented rice enriched with γ-amino butyric acid using Lactobacillus plantarum MNZ and its antihypertensive effects in spontaneously hypertensive rats. J. Funct. Foods 2015, 16, 194–203. [Google Scholar] [CrossRef]
- Shan, Y.; Man, C.X.; Han, X.; Li, L.; Guo, Y.; Deng, Y.; Li, T.; Zhang, L.W.; Jiang, Y.J. Evaluation of improved γ-aminobutyric acid production in yogurt using Lactobacillus plantarum NDC75017. J. Dairy Sci. 2015, 98, 2138–2149. [Google Scholar] [CrossRef]
- Yoshinaka, R.; Sato, M.; Tsuchiya, N.; Ikeda, S. Production of Fish Sauce from Sardine by Utilization of its Visceral Enzymes. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 463–469. [Google Scholar] [CrossRef]
- Furutani, A.; Satomi, M. Study on proteolytic process of two fish sauce mashes prepared from deepsea smelt and waste from Kamaboko processing using soy sauce Koji mold. J. Brew. Soc. Jpn. 2013, 108, 802–812. [Google Scholar] [CrossRef]
- Auerswald, L.; Morren, C.; Lopata, A.L. Histamine levels in seventeen species of fresh and processed South African seafood. Food Chem. 2006, 98, 231–239. [Google Scholar] [CrossRef]
- Fujii, T. Scombroid Fish Poisoning. Jpn. J. Food Microbiol. 2006, 23, 61–71. [Google Scholar] [CrossRef]
- Katayama, Y.; Uchino, M.; Saro, H.; Takano, K. Chemical Property on Fish Sauce Produced in Thailand, Vietnam, and Japan. Food Preser. Sci. 2008, 34, 307–321. [Google Scholar] [CrossRef][Green Version]
- Ogata, H.; Murai, T. White muscle of masu salmon, Oncorhynchus masou masou, smolts possesses a strong buffering capacity due to a high level of anserine. Fish Phys. Biochem. 1999, 13, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Uchida, K.; Ishimaru, M.; Ito, M.; Matsumoto, N.; Taoka, Y.; Hatate, H. Effect of seawater reared on the nutritional composition and antioxidant activity of edible muscle in smoltified-landlocked masu salmon (Oncorhynchus masou masou). Food Meas. 2018, 12, 200–208. [Google Scholar] [CrossRef]
- Abe, H.; Ohmama, S. Effect of starvation and seawater acclimation on the concentration of free L-histidine and related dipeptides in the muscle of eel, rainbow trout and Japanese dace. Comp. Biochem. Physiol. 1987, 88B, 507–511. [Google Scholar]
- Ogata, H.Y.; Konno, S.; Silverstein, J. Muscular buffering capacity of the parr and smolts in Oncorhynchus masou. Aquaculture 1998, 168, 303–310. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirano, T.; Suyama, M. Free imidazole compounds in white and dark muscles of migratory marine fish. Comp. Biochem. Physiol. 1987, 87B, 615–619. [Google Scholar] [CrossRef]
- Abe, H. Distribution of free L-histidine and related dipeptides in the muscle of fresh-water fishes. Comp. Biochem. Physiol. 1983, 76B, 35–39. [Google Scholar] [CrossRef]
- Abe, H. Effect of growth on the concentration of L-histidine and anserine in the white muscle of carp and rainbow trout. Nippon Suisan Gakkaishi 1987, 53, 1657–1661. [Google Scholar] [CrossRef]
- Peng, H.C.; Lin, S.H. Effects of chicken extract on antioxidative status and liver protection under oxidative stress. J. Nut. Sci. Vitaminol. 2004, 50, 325–329. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, F.; Mizokawa, S.; Matsumura, A.; Nozaki, S.; Watanabe, Y. Establishment and assessment of a rat model of fatigue. Neurosci. Lett. 2003, 352, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, S.; Koeners, M.P.; Joles, J.A. Taurine red bull or red herring? Hypertension 2009, 53, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.P.; Davis, D.A. Taurine: Critical nutrients for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Schuller Levis, G.B.; Park, E. Taurine: New implications for an old amino acid. FEMS Microbiol. Lett. 2003, 226, 195–202. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taoka, Y.; Nakamura, M.; Nagai, S.; Nagasaka, N.; Tanaka, R.; Uchida, K. Production of Anserine-Rich Fish Sauce from Giant Masu Salmon, Oncorhynchus masou masou and γ-Aminobutyric Acid (GABA)-Enrichment by Lactobacillus plantarum Strain N10. Fermentation 2019, 5, 45. https://doi.org/10.3390/fermentation5020045
Taoka Y, Nakamura M, Nagai S, Nagasaka N, Tanaka R, Uchida K. Production of Anserine-Rich Fish Sauce from Giant Masu Salmon, Oncorhynchus masou masou and γ-Aminobutyric Acid (GABA)-Enrichment by Lactobacillus plantarum Strain N10. Fermentation. 2019; 5(2):45. https://doi.org/10.3390/fermentation5020045
Chicago/Turabian StyleTaoka, Yousuke, Miho Nakamura, Setsuko Nagai, Noriko Nagasaka, Ryusuke Tanaka, and Katsuhisa Uchida. 2019. "Production of Anserine-Rich Fish Sauce from Giant Masu Salmon, Oncorhynchus masou masou and γ-Aminobutyric Acid (GABA)-Enrichment by Lactobacillus plantarum Strain N10" Fermentation 5, no. 2: 45. https://doi.org/10.3390/fermentation5020045
APA StyleTaoka, Y., Nakamura, M., Nagai, S., Nagasaka, N., Tanaka, R., & Uchida, K. (2019). Production of Anserine-Rich Fish Sauce from Giant Masu Salmon, Oncorhynchus masou masou and γ-Aminobutyric Acid (GABA)-Enrichment by Lactobacillus plantarum Strain N10. Fermentation, 5(2), 45. https://doi.org/10.3390/fermentation5020045




