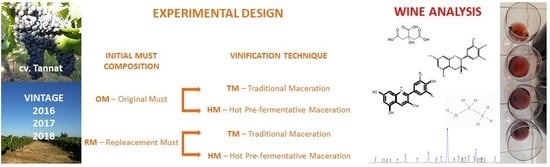
Impact of Must Replacement and Hot Pre-Fermentative Maceration on the Color of Uruguayan Tannat Red Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment
2.2. Grapes and Wines
2.3. Standard Grape Juice and Wine Analysis
2.4. Color Parameters
2.5. Spectrophotometric Analysis of Anthocyanins and Related Parameters
2.6. HPLC Anthocyanidin Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fermentation Kinetics
3.2. General Composition of Wines
3.3. Spectrophotometrical Phenolic Composition and Related Parameters
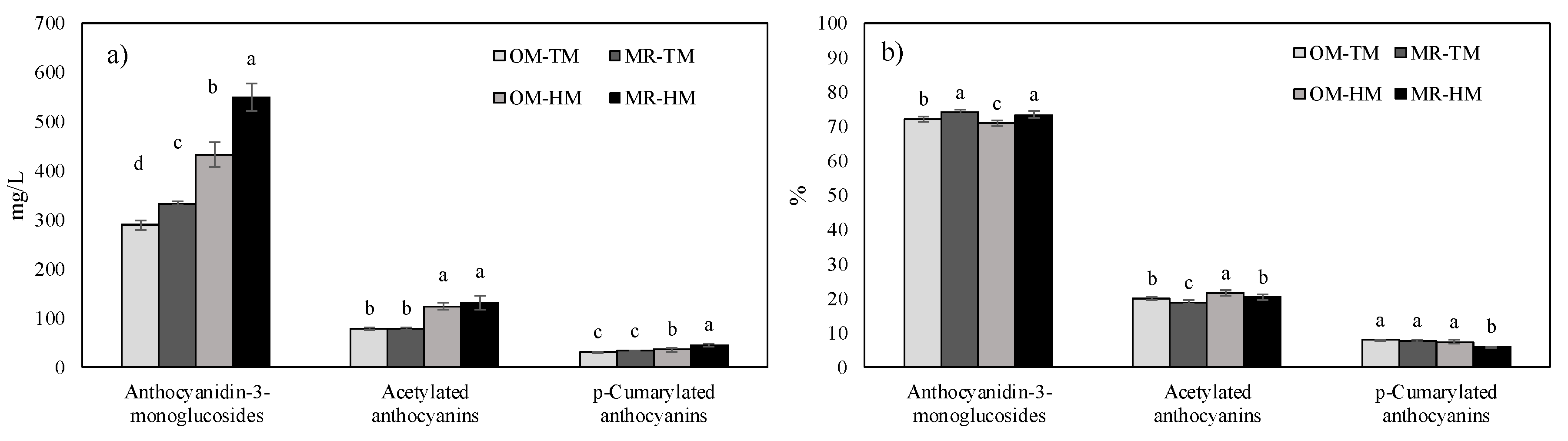
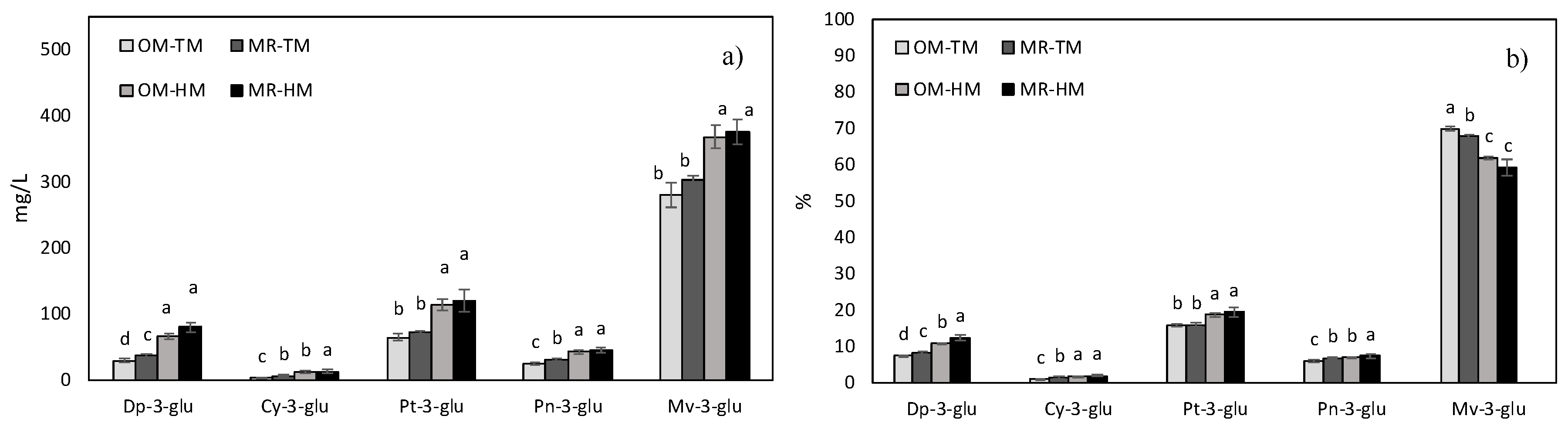
3.4. Wine Anthocyanin Composition
3.5. Wine Color
3.6. Multifactorial Analysis of Variance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- González-Neves, G.; Favre, G.; Gil, G. Effect of fining on the colour and pigment composition of young red wines. Food. Chem. 2014, 157, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Österbauer, R.A.; Matthews, P.M.; Jenkinson, M.; Beckmann, C.F.; Hansen, P.C.; Calvert, G.A. Color of scents: Chromatic stimuli modulate odor responses in the human brain. J. Neurophysiol. 2005, 93, 3434–3441. [Google Scholar] [CrossRef] [PubMed]
- Alcalde-Eon, C.; Escribano-Bailón, M.; Santos-Buelga, C.; Rivas-Gonzalo, J. Changes in the detailed pigment composition of red wine during maturity and ageing. A comprehensive study. Anal. Chim. Acta. 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Llaudy, M.C.; Canals, R.; Canals, J.M.; Zamora, F. Influence of ripening stage and maceration length on the contribution of grape skins, seeds and stems to phenolic composition and astringency in wine-simulated macerations. Eur. Food Res. Technol. 2008, 226, 337–344. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; Ros-García, J.M.; López-Roca, J.M.; Gómez-Plaza, E. A first approach towards the relationship between grape skin cell-wall composition and anthocyanin extractability. Anal. Chim. Acta 2006, 563, 26–32. [Google Scholar] [CrossRef]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2009, 15, 27–35. [Google Scholar] [CrossRef]
- González-Neves, G.; Franco, J.; Barreiro, L.; Gil, G.; Moutounet, M.; Carbonneau, A. Varietal differentiation of Tannat, Cabernet-Sauvignon and Merlot grapes and wines according to their anthocyanin composition. Eur. Food Res. Technol. 2007, 225, 111–117. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Botany 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Rolle, L.; Gerbi, V.; Schneider, A.; Spanna, F.; Río Segade, S. Varietal Relationship between Instrumental Skin Hardness and Climate for Grapevines (Vitis vinifera L.). J. Agric. Food Chem. 2011, 59, 10624–10634. [Google Scholar] [CrossRef]
- Sacchi, K.; Bisson, L.; Adams, D. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Cheynier, V.; Dueñas-Paton, M.; Souquet, J.M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar]
- González-Neves, G.; Favre, G.; Piccardo, D.; Gil, G. Anthocyanin profile of young red wines of Tannat, Syrah and Merlot made using maceration enzymes and cold soak. Int. J. Food Sci. Technol. 2016, 51, 260–267. [Google Scholar] [CrossRef]
- Castellarin, S.; Matthews, M.; Di Gaspero, G.; Gambetta, G. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef] [PubMed]
- González-Neves, G.; Gil, G.; Favre, G.; Ferrer, M. Influence of grape composition and winemaking on the anthocyanin composition of red wines of Tannat. Int. J. Food Sci. Technol. 2012, 47, 900–909. [Google Scholar] [CrossRef]
- Cerpa-Calderón, F.; Kennedy, J. Effect of berry crushing on skin and seed tannin extraction during fermentation and maceration. Am. J. Enol. Vitic. 2008, 59, 350. [Google Scholar]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Updated knowledge about the presence of phenolic compounds in wine. Crit. Rev. Food Sci. 2005, 45, 85–118. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Rolle, L.; Torchio, F.; Lorrain-Lorette, B.; Giacosa, S.; Río-Segade, S.; Cagnasso, E.; Gerbi, V.; Teissedre, P.L. Rapid methods for the evaluation of total phenol content and extractability in intact grape seeds of cabernet sauvignon: Instrumental mechanical properties and FT-NIR spectrum. J. Int. Sci. Vigne Vin. 2012, 46, 29–40. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.; Busse-Valverde, N.; Fernández-Fernández, J.; Gómez-Plaza, E.; Gil-Muñoz, R. The extraction kinetics of anthocyanins and proanthocyanidins from grape to wine in three different varieties. J. Int. Sci. Vigne Vin. 2016, 50, 91–100. [Google Scholar] [CrossRef]
- Amrani-Joutei, K.; Glories, Y. Tanins et anthocyanes: Localization dans la baie de raisin et mode d’extraction. Rev. Franç. Oenol. 1995, 153, 28–31. [Google Scholar]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Fulcrand, H.; Dueñas, M.; Salas, E.; Cheynier, V. Phenolic reactions during winemaking and aging. Am. J. Enol. Vitic. 2006, 57, 289–297. [Google Scholar]
- Cheynier, V.; Souquet, J.; Kontek, A.; Moutounet, M. Anthocyanin degradation in oxidizing grape musts. J. Sci. Food Agric. 1994, 66, 283–288. [Google Scholar] [CrossRef]
- Dallas, C.; Ricardo-Da-Silva, J.; Laureano, O. Degradation of oligomeric procyanidins and anthocyanins in a Tinta Roriz red wine during maturation. Vitis 1995, 34, 51–56. [Google Scholar]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar] [CrossRef]
- McRae, J.; Kennedy, J. Wine and Grape Tannin Interactions with Salivary Proteins and Their Impact on Astringency: A Review of Current Research. Molecules 2011, 16, 2348–2364. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, N.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Studies on the acetaldehyde-induced condensation of (-)-epicatechin and malvidin 3-O-glucoside in a model solution system. J. Agric. Food Chem. 1999, 47, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colombo, B.; Suárez-Lepe, J. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef]
- Geffroy, O.; Lopez, R.; Feilhes, C.; Violleau, F.; Kleiber, D.; Favarel, J.; Ferreira, V. Modulating analytical characteristics of thermovinified Carignan musts and the volatile composition of the resulting wines through the heating temperature. Food Chem. 2018, 257, 7–14. [Google Scholar] [CrossRef] [PubMed]
- El Darra, N.; Grimi, N.; Maroun, R.; Louka, N.; Vorobiev, E. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur. Food Res. Technol. 2013, 236, 47–56. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.; Zamora, F. Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and pH of wine. Aust. J. Grape Wine Res. 2011, 17, 203–208. [Google Scholar] [CrossRef]
- Rolle, L.; Englezos, V.; Torchio, F.; Cravero, F.; Río Segade, S.; Rantsiou, K.; Giacosa, S.; Gambuti, A.; Gerbi, V.; Cocolin, L. Alcohol reduction in red wines by technological and microbiological approaches: A comparative study. Aust. J. Grape Wine Res. 2017, 24, 1–13. [Google Scholar] [CrossRef]
- Piccardo, D.; Gombau, J.; Pascual, O.; Vignault, A.; Pons, P.; Canals, J.M.; González-Neves, G.; Zamora, F. Influence of two prefermentative treatments to reduce the ethanol content and pH of red wines obtained from overripe grapes. Vitis 2019. In Press. [Google Scholar]
- Piccardo, D.; Favre, G.; Pascual, O.; Canals, J.M.; Zamora, F.; González-Neves, G. Influence of the use of unripe grapes to reduce ethanol content and pH on the color, polyphenol and polysaccharide composition of conventional and hot macerated Pinot Noir and Tannat wines. Eur. Food Res. Technol. 2019, 245, 1321–1335. [Google Scholar] [CrossRef]
- Sadras, V.; Petrie, P.; Moran, M. Effects of elevated temperature in grapevine. II Juice pH, titratable acidity and wine sensory attributes. Aust. J. Grape Wine Res. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Jackson, D.I.; Lombard, P.B. Environmental and management practices affecting grape composition and wine quality: A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Nicholas, K.A.; Matthews, M.A.; Lobell, D.B.; Willits, N.H.; Field, C.B. Effect of vineyard-scale climate variability on Pinot noir phenolic composition. Agric. For. Meteorol. 2011, 151, 1556–1567. [Google Scholar] [CrossRef]
- Atanackovic, M.; Petrovic, A.; Jovic, S.; Gojkovic-Bukarica, L. Influence of winemaking techniques on the resveratrol content, total phenolic content and antioxidant potential of red wines. Food Chem. 2012, 131, 513–518. [Google Scholar] [CrossRef]
- Vitis International Variety Catalogue. 2018. Available online: http://www.vivc.de/OIV (accessed on 1 September 2019).
- Organización Internationale de la Vigne et du Vin. International Oenological Codex; OIV: Paris, France, 2018; 772p. [Google Scholar]
- Glories, Y. La couleur des vins rouges. 2e. Partie: Mesure, origine et interpretation. Conn. Vigne Vin. 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. A new simplified method for measuring the color of wines. I. Red and Rosé Wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. MSCVes.zip. 2001. Available online: http://www.unizar.es/negueruela/MSCV.es (accessed on 1 September 2019).
- González-Neves, G.; Charamelo, D.; Balado, J.; Barreiro, L.; Bochicchio, R.; Gatto, G.; Gil, G.; Tessore, A.; Carbonneau, A.; Moutounet, M. Phenolic potential of Tannat, Cabernet-Sauvignon and Merlot grapes and their correspondence with wine composition. Anal. Chim. Acta. 2004, 513, 191–196. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic and phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Ribéreau-Gayon, P.; Stonestreet, E. Le dosage des anthocyanes dans les vins rouges. Bull. Soc. Chim. 1965, 9, 2649–2653. [Google Scholar]
- Swain, T.; Hillis, W. The phenolic constituents of Prunus domestica: I. The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Stonestreet, E. Dosage des tanins dans du vin rouge et détermination de leur structure. Chim. Anal. 1966, 48, 188–196. [Google Scholar]
- Valls, J. Composició Fenòlica en Varietats Negres de Vitis vinifera. Influència de Diferents Factors. Ph.D. Thesis, Universitat Rovira i Virgili, Tarragona, Spain, 2004. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2015. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available online: http://www.infostat.com.ar (accessed on 1 September 2019).
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Tratado de Enología: Química del Vino Estabilización y Tratamientos, 1st ed.; Hemisferio Sur: Buenos Aires, Argentina, 2003; p. 537. [Google Scholar]
- Geffroy, O.; Lopez, R.; Serrano, E.; Dufourcq, T.; Gracia-Moreno, E.; Cacho, J.; Ferreira, V. Changes in analytical and volatile compositions of red wines induced by pre-fermentation heat treatment of grapes. Food Chem. 2015, 187, 243–253. [Google Scholar] [CrossRef]
- Lukic’, I.; Budic´-Leto, I.; Bubola, M.; Damijanic´, K.; Staver, M. Pre-fermentative cold maceration, saignée, and various thermal treatments as options for modulating volatile aroma and phenol profiles of red wine. Food Chem. 2017, 224, 251–261. [Google Scholar] [CrossRef]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar]
- Baiano, A.; Terracone, G.; Gambacorta, G.; La Notte, E. Phenolic content and antioxidant activity of Primitivo wine: Comparison among winemaking technologies. J. Food Sci. 2009, 74, 258–267. [Google Scholar] [CrossRef]
- Fourment, M.; Ferrer, M.; González-Neves, G.; Barbeau, G.; Bonnardot, V.; Quénol, H. Tannat grape composition responses to spatial variability of temperature in a Uruguay’s coastal wine region. Int. J. Biomet. 2017, 61, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Andrade Neves, N.; Pantoja, L.; Soares dos Santos, A. Thermovinification of grapes from the Cabernet sauvignon and Pinot noir varieties using immobilized yeasts. Eur. Food Res. Technol. 2014, 238, 79–84. [Google Scholar] [CrossRef]
- Casassa, L.F.; Beaver, C.W.; Mireles, M.S.; Harbertson, J.F. Effect of extended maceration and ethanol concentration on the extraction and evolution of phenolics, color components and sensory attributes of Merlot wines. Aust. J. Grape Wine Res. 2013, 19, 25–39. [Google Scholar] [CrossRef]


| Factor Analyzed | Ethanol (% v/v) | Titratable Acidity (gH2SO4/L) | pH | Residual Sugars (g/L) | Volatile Acidity (gH2SO4/L) | |
|---|---|---|---|---|---|---|
| Year of vintage (*) | 2016 | 14.0 ± 0.1 b | 4.30 ± 0.27 a | 3.92 ± 0.16 a | 1.47 ± 0.41 c | 0.36 ± 0.07 b |
| 2017 | 11.2 ± 0.2 c | 2.93 ± 0.05 c | 3.86 ± 0.04 c | 1.85 ± 0.21 b | 0.43 ± 0.09 a | |
| 2018 | 15.4 ± 0.2 a | 3.85 ± 0.03 b | 3.89 ± 0.09 b | 2.44 ± 0.44 a | 0.44 ± 0.07 a | |
| Must composition (**) | OM | 14.0 ± 0.1 a | 3.51 ± 0.17 b | 3.95 ± 0.09 a | 2.07 ± 0.59 a | 0.43 ± 0.09 a |
| MR | 13.0 ± 0.1 b | 3.88 ± 0.06 a | 3.83 ± 0.09 b | 1.83 ± 0.39 a | 0.39 ± 0.08 b | |
| Maceration technique (***) | TM | 13.3 ± 0.2 b | 3.74 ± 0.19 a | 3.87 ± 0.09 a | 2.01 ± 0.55 a | 0.47 ± 0.06 a |
| HM | 13.7 ± 0.1 a | 3.64 ± 0.04 a | 3.92 ± 0.09 a | 1.89 ± 0.46 a | 0.35 ± 0.05 b | |
| Must composition - Maceration techinque (****) | OM-TM | 14.0 ± 0.2 a | 3.61 ± 0.30 b | 3.92 ± 0.09 b | 2.30 ± 0.56 a | 0.50 ± 0.06 a |
| MR-TM | 12.6 ± 0.2 c | 3.87 ± 0.09 a | 3.81 ± 0.12 d | 1.72 ± 0.37 c | 0.45 ± 0.06 b | |
| OM-HM | 14.0 ± 0.1 a | 3.40 ± 0.03 c | 3.98 ± 0.08 a | 1.84 ± 0.53 bc | 0.36 ± 0.05 c | |
| MR-HM | 13.4 ± 0.1 b | 3.88 ± 0.04 a | 3.85 ± 0.09 c | 1.95 ± 0.39 b | 0.33 ± 0.05 c |
| Factor Analyzed | Total Polyphenol (mg/L) | Anthocyanins (mg/L) | Catechins (mg/L) | Proanthocyanidins (mg/L) | |
|---|---|---|---|---|---|
| Year of vintage (*) | 2016 | 2479 ± 252 a | 1052 ± 156 a | 1769 ± 455 b | 4172 ± 714 a |
| 2017 | 1624 ± 68 c | 614 ± 68 b | 1420 ± 58 c | 2690 ± 60 c | |
| 2018 | 2140 ± 43 b | 1165 ± 43 a | 1883 ± 86 a | 3260 ± 80 b | |
| Must composition (**) | OM | 2045 ± 140 a | 960 ± 67 a | 1667 ± 239 a | 3397 ± 372 a |
| MR | 2117 ± 102 a | 994 ± 73 a | 1714 ± 160 a | 3352 ± 197 a | |
| Maceration technique (***) | TM | 1784 ± 112 b | 838 ± 69 b | 1281 ± 215 b | 2764 ± 261 b |
| HM | 2379 ± 129 a | 1117 ± 71 a | 2100 ± 184 a | 3985 ± 308 a | |
| Must composition- Maceration techinque (****) | OM-TM | 1821 ± 131 c | 832 ± 69 c | 1273 ± 268 b | 2792 ± 352 b |
| MR-TM | 1747 ± 94 d | 843 ± 69 c | 1289 ± 161 b | 2735 ± 170 b | |
| OM-HM | 2345 ± 149 b | 1088 ± 66 b | 2061 ± 209 a | 4001 ± 390 a | |
| MR-HM | 2413 ± 109 a | 1146 ± 77 a | 2141 ± 159 a | 3968 ± 225 a |
| Factor Analyzed | Ionization Index (%) | Copigmentation Index (%) | PVPP Index (%) | |
|---|---|---|---|---|
| Year of vintage (*) | 2016 | 33.9 ± 2.3 a | 16.5 ± 3.7 c | 45.2 ± 0.8 a |
| 2017 | 15.7 ± 2.4 c | 17.8 ± 4.2 b | 35.9 ± 0.8 c | |
| 2018 | 17.7 ± 0.6 b | 31.7 ± 3.1 a | 40.0 ± 1.2 b | |
| Must composition (**) | OM | 20.1 ± 1.8 b | 20.9 ± 3.2 b | 38.2 ± 0.9 b |
| MR | 24.8 ± 1.7 a | 23.1 ± 4.0 a | 42.4 ± 0.9 a | |
| Maceration technique (***) | TM | 18.0 ± 2.1 b | 18.4 ± 3.4 b | 35.9 ± 0.9 b |
| HM | 26.9 ± 1.4 a | 26.6 ± 3.9 a | 44.7 ± 0.9 a | |
| Must composition - Maceration techinque (****) | OM-TM | 16.0 ± 2.6 d | 15.7 ± 3.0 c | 35.2 ± 0.9 c |
| MR-TM | 20.0 ± 1.7 c | 21.0 ± 3.9 b | 36.6 ± 1.0 c | |
| OM-HM | 24.2 ± 1.1 b | 26.0 ± 3.4 a | 41.3 ± 0.9 b | |
| MR-HM | 29.6 ± 1.7 a | 25.3 ± 4.3 a | 48.2 ± 0.9 a |
| Factor Analyzed | Color Intensity | Lightness (L*) | Chroma (C*) | Hue (hab) | |
|---|---|---|---|---|---|
| Year of vintage (*) | 2016 | 32.5 ± 1.4 a | 31.5 ± 1.2 b | 45.0 ± 1.0 b | 348.1 ± 1.6 a |
| 2017 | 16.0 ± 0.5 c | 60.5 ± 1.5 a | 28.1 ± 1.5 c | 10.6 ± 1.3 c | |
| 2018 | 24.2 ± 0.5 b | 25.5 ± 0.9 c | 53.1 ± 0.8 a | 11.8 ± 0.5 b | |
| Must composition (**) | OM | 23.2 ± 0.9 b | 40.2 ± 1.3 a | 41.0 ± 1.2 b | 3.27 ± 1.0 a |
| MR | 25.1 ± 0.8 a | 37.9 ± 1.2 b | 43.1 ± 1.4 a | 3.74 ± 1.2 a | |
| Maceration technique (***) | TM | 20.4 ± 0.7 b | 44.8 ± 1.2 a | 41.4 ± 1.3 b | 4.66 ± 0.8 a |
| HM | 27.9 ± 0.9 a | 33.3 ± 1.3 b | 42.7 ± 1.3 a | 2.35 ± 1.4 a | |
| Must composition - Maceration techinque (****) | OM-TM | 19.6 ± 1.0 d | 45.9 ± 1.4 a | 40.4 ± 1.0 c | 5.11 ± 0.6 a |
| MR-TM | 21.2 ± 0.4 c | 43.8 ± 0.9 b | 42.6 ± 1.7 b | 4.21 ± 0.9 a | |
| OM-HM | 26.8 ± 0.8 b | 34.6 ± 1.2 c | 41.6 ± 1.5 bc | 1.43 ± 1.4 c | |
| MR-HM | 29.0 ± 1.1 a | 32.0 ± 1.4 d | 43.7 ± 1.0 a | 3.27 ± 1.5 b |
| Year of Vintage (Y) | Must Composition (M) | Vinification Technique (V) | Y × M | Y × V | M × V | Y × M × V | |
|---|---|---|---|---|---|---|---|
| Ethanol | 5152.9 *** | 939.6 *** | 137.5 *** | 61.8 *** | 52.5 *** | 131.1 *** | 120.2 *** |
| Titratable acidity | 185.5 *** | 38.8 *** | 2.93 * | 25.1 *** | 6.9 *** | 3.3 * | 3.9 ** |
| pH | 10.6 *** | 101.0 *** | 18.3 *** | 41.9 *** | 10.8 *** | 0.4 | 21.9 *** |
| Reducing sugars | 80.9 *** | 9.1 ** | 2.2 | 8.0 ** | 4.6 ** | 43.4 *** | 9.4 *** |
| Volatile acidity | 21.5 *** | 11.7 *** | 193.1 *** | 7.2 ** | 10.1 *** | 0.2 | 17.8 *** |
| Total polyphenols | 574.8 *** | 11.7 *** | 824.7 *** | 11.9 *** | 25.8 *** | 0.1 | 2.9 |
| Anthocyanins | 1232.6 *** | 10.8 *** | 728.2 *** | 14.4 *** | 89.5 *** | 5.11 ** | 10.1 *** |
| Catechins | 92.4 *** | 2.7 | 800.3 *** | 9.5 *** | 12.0 *** | 1.2 | 3.0 * |
| Proanthocyanidins | 193.6 *** | 0.5 | 387.0 *** | 2.2 | 0.2 | 0.1 | 0.4 |
| Ionization index | 248.7 *** | 41.6 *** | 149.9 *** | 4.1 ** | 28.2 *** | 0.9 | 3.6 ** |
| Copigmentation index | 690.4 *** | 36.8 *** | 385.1 *** | 3.8 ** | 12.9 *** | 66.1 *** | 3.6 ** |
| PVPP index | 15.4 *** | 9.33 *** | 41.4 *** | 1.6 | 28.8 *** | 4.0 * | 6.6 *** |
| Color intensity | 1526.4 *** | 60.9 *** | 966.5 *** | 7.6 * | 29.5 *** | 2.6 | 2.7 |
| Lightness (L*) | 5272.7 *** | 65.0 *** | 1519.0 *** | 10.8 *** | 9.6 *** | 0.8 | 5.5 *** |
| Chroma (C*) | 1180.2 *** | 25.3 *** | 8.0 *** | 6.4 *** | 94.7 *** | 0.1 | 5.8 *** |
| Hue (hab) | 2160.5 *** | 2.0 | 48.3 *** | 5.8 *** | 37.0 *** | 17.0 *** | 7.4 *** |
| Anthocyanidin-3-monoglucosides | 566.1 *** | 25.3 *** | 364.3 *** | 15.5 *** | 53.6 *** | 1.4 | 4.6 ** |
| Acetylated anthocyanins | 138.1 *** | 1.2 | 231.5 *** | 1.2 | 25.1 *** | 1.3 | 0.1 |
| p-Coumarylated anthocyanins | 439.0 *** | 16.3 *** | 91.2 *** | 22.7 *** | 1.7 | 41.9 *** | 3.8 ** |
| Delphinidin-3-glucoside | 219.3 *** | 31.1 *** | 531.8 *** | 4.9 * | 85.2 *** | 3.6 * | 11.3 *** |
| Cyanidin-3-glucoside | 58.1 *** | 2.4 | 71.2 *** | 1.5 | 20.0 *** | 3.7 * | 2.6 * |
| Petunidin-3-glucoside | 117.1 *** | 3.3 * | 158.0 *** | 4.9 ** | 16.6 *** | 0.1 | 0.6 |
| Peonidin-3-glucoside | 208.5 *** | 12.9 *** | 209.2 *** | 0.5 | 12.3 *** | 3.2 * | 1.6 |
| Malvidin-3-glucoside | 623.8 *** | 1.6 | 207.5 *** | 8.4 *** | 33.6 *** | 7.7 *** | 1.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccardo, D.; González-Neves, G.; Favre, G.; Pascual, O.; Canals, J.M.; Zamora, F. Impact of Must Replacement and Hot Pre-Fermentative Maceration on the Color of Uruguayan Tannat Red Wines. Fermentation 2019, 5, 80. https://doi.org/10.3390/fermentation5030080
Piccardo D, González-Neves G, Favre G, Pascual O, Canals JM, Zamora F. Impact of Must Replacement and Hot Pre-Fermentative Maceration on the Color of Uruguayan Tannat Red Wines. Fermentation. 2019; 5(3):80. https://doi.org/10.3390/fermentation5030080
Chicago/Turabian StylePiccardo, Diego, Gustavo González-Neves, Guzman Favre, Olga Pascual, Joan Miquel Canals, and Fernando Zamora. 2019. "Impact of Must Replacement and Hot Pre-Fermentative Maceration on the Color of Uruguayan Tannat Red Wines" Fermentation 5, no. 3: 80. https://doi.org/10.3390/fermentation5030080
APA StylePiccardo, D., González-Neves, G., Favre, G., Pascual, O., Canals, J. M., & Zamora, F. (2019). Impact of Must Replacement and Hot Pre-Fermentative Maceration on the Color of Uruguayan Tannat Red Wines. Fermentation, 5(3), 80. https://doi.org/10.3390/fermentation5030080