1. Introduction
Fusarium head blight (FHB) is a fungal disease caused by several
Fusarium spp. Wheat, barley, oats, corn, and other cereal grains can be affected by FHB, resulting in small lightweight kernels and, thus, loss of yield.
Fusarium spp. produce various amounts and types of trichothecene mycotoxins, which are highly toxic to humans and livestock [
1]. A major mycotoxin produced by
Fusarium spp. is deoxynivalenol (DON). Toxin production occurs during disease development in the field under favorable weather conditions. Contamination of food and feedstuff with DON causes short- and long-term adverse effects on human health and livestock productivity [
2]. In order to limit the mycotoxins in food and feed, regulations specify maximum allowable concentrations, which is 1 mg/kg sample in many countries. According to the regulations, products are monitored, and when mycotoxin concentrations exceed the maximum allowable limits, products are separated from the food chain [
3]. The economic loss from FHB accounts for many millions of dollars in Canada alone.
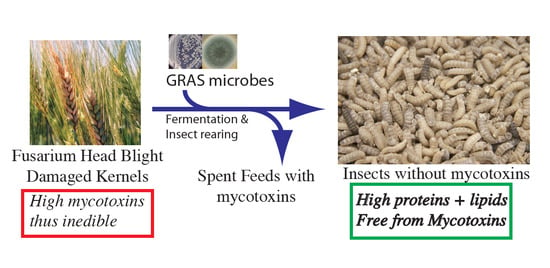
Detoxification methods are expensive, labor-intensive, inefficient, and time-consuming, and there is inadequate capacity for industrial applications. An effective way to prevent FHB in the field is to treat the flowering wheat plants with fungicides, and to develop resistant cultivars to minimize the infection of Fusarium spp. Fungicide, however, has limited effects on the infection, and, every year, a large number of grains are damaged by FHB. Considering that possible approaches to prevent the contamination of grain with mycotoxins are limited before harvest, alternate approaches should be considered to utilize inedible FHB-damaged kernels (FDK). In this study, we aimed to investigate if black soldier fly larvae (BSFL) can grow on FDK without any toxin accumulation in larval body.
World population is increasing, and it is predicted to reach 9.6 billion by 2050, i.e., a 2.3 billion increase in the next 30 years. Food production relies on agriculture, but the current practice in agriculture may not be sufficient to supply enough food for this population increase, without damaging Mother Earth or introducing super-high-yield crops that do not result in environmental damage. Utilization of inedible agriculture products can bypass the above concerns and can yield additional edible products from current practice agricultural production [
4].
There is a considerable interest in the use of insects to recover inedible organic matter because insects can convert carbohydrates into proteins and lipids using organic wastes [
5].
Hermetia illucens (black soldier fly) is one of the most important species, along with other insect species like
Tenebrio molitor (yellow mealworm),
Drosophila melanogaster (common fruit fly),
Amyelois transitella (orange worm),
Helicoverpa zea (corn earworm), and
Trichoplusia ni (cabbage looper) [
3]. While the regulations vary among countries and areas, the nutrient values and ease of utilization of insect nutrients draw huge interests for providing an alternative food source [
6]. BSFL are considered a possibly proteinaceous animal feed or human food source because of the high accumulation of fat (29%) and protein (42%) in their body, and they do not transmit pathogenic microbes to humans and animals [
7]. BSFL have high feed conversion ratios and an ability to convert various organic wastes into body mass [
8,
9].
Solid-state fermentation (SSF) shows great possibilities in the development of high-value products. Fungal and bacterial strains can be used in SSF, utilizing their abilities of enzyme production such as cellulase, pectinase, and xylanases. In this study, we used
Aspergillus oryzae and
Lactobacillus plantarum as microbial strains based on data obtained from our previous study [
4]. The main objective of SSF was increasing the bioavailability of nutrients in FDK in favor of recovering them as BSFL biomass, and changing the nutrient profiles in favor of improved/efficient nutrient recovery in BSFL. Through these microbial modifications, efficiency of nutrient recovery from damaged crops should be enhanced during BSFL digestion.
The BSFL can be utilized to reduce pollution and convert low-value organic resources into a high-quality feed protein. They do not harbor diseases, and their production does not need any special equipment of facilities. BSFL are an extremely resistant species capable of dealing with demanding environmental conditions, such as drought, food shortage, or oxygen deficiency. The BSFL is already used in the waste management of some substrates such as manure, rice straw, food waste, kitchen waste, distillers’ grains, rotting plant tissues, fecal sludge, animal offal, and animal manure [
8]. We showed that BSFL can convert agricultural wastes into biomass with up to 95% recovery of organic matter using SSF [
4,
10]. Our previous research [
4,
10] suggests that FDK could be fed to BSFL to recover nutrients at a high rate with SSF treatment of feedstock. It is, however, unclear if the mycotoxins would be accumulated when BSFL are fed with FDK. We hypothesize that the BSFL are not affected by the mycotoxin content of wheat grain infected with
Fusarium spp., they will not accumulate mycotoxins in their bodies, and a majority of the nutrients in the FDK will be recovered using SSF treatments. Efficient recovery of nutrients from damaged crops can be used to create a high-value product from low-value grain. In this study, the performance of BSFL in converting SSF-treated FDK into insect biomass and the accumulation of DON were investigated.
2. Material and Methods
2.1. Materials
BSFL were purchased from Worm Lady (McGregor, ON, Canada). All chemicals used in this study were commercially available ACS grade and were purchased from Fisher Scientific (Ottawa, ON) and VWR International (Edmonton, AB). Aspergillus oryzae NRRL 32657 (Ao) and Lactobacillus plantarum NRRL B4496 (Lp) were obtained from the ARS Culture Collection (USDA, Peoria, IL, USA).
2.2. Solid-State Fermentation
The initial DON concentration of FDK was 0.63 ± 0.20 µg/g dry matter (dm). The FDKs were soaked in water for 18 h at 21 °C to obtain softer kernel for fermentation. The soaked kernels were shred using a household coffee mill (Cuisinart DBM-8, Woodbridge, ON, Canada). Seed cultures of Ao and Lp were prepared by inoculating Potato Dextrose (PD) and De Man, Rogosa, and Sharpe (MRS) broths. The seed culture of Ao was prepared by inoculating 250 mL of PD broth in an Erlenmeyer flask with two loopfuls of spores, followed by 72-h incubation at 30 °C on a rotary shaker (150 rpm). The seed culture of Lp was prepared by inoculating 1 mL of fully grown preculture in 250 mL of MRS broth in an Erlenmeyer flask and incubating for 24 h at 37 °C on a rotary shaker (150 rpm). After incubation, fungal and bacterial seed cultures were collected by centrifugation at 6000 rpm for 10 min at 10 °C (Sorvall, RC28S, Manasquan, NJ, US). The biomass obtained was re-suspended in 1/10 of the original volume of sterile distilled water.
Approximately 58 g (34 g in dry weight) of shredded kernels were weighed into each glass jar and, from the seed cultures, 1.5 mL of Lp, 2 mL of Ao, and 3.5 mL of a combination of these two strains (Lp + Ao) were inoculated into the crushed kernels. The moisture content was adjusted to 55% (w/w) with sterile water. The control sample was prepared without initial seed culture inoculation. The samples were fermented at 30 °C for four days, and the moisture content was kept constant by adding sterile water and mixing once per day under aseptic conditions.
2.3. BSFL Digestion
Fifty BSFL (second instar) were introduced to each SSF FDK sample in the glass jars that had perforated lids to allow moisture and gas transfer during BSFL digestion. Twelve jars were prepared under the same conditions for each SSF FDK sample. The jars were kept at 30 °C for 12 days. Water was added by weighing the sample jars and mixing each day under aseptic conditions.
2.4. BSFL Separation after Digestion
After interval days (0, 4, 8, and 12 days), the larvae were separated from the residual feed using forceps. The larvae were rinsed with water to remove residual substrate from their surface and dried on paper towel; then, their wet weight was determined. Then, the larvae were frozen at −20 °C for further analysis. The rinsed-off feed residues were placed back in the feed bed to avoid errors in spent feed analyses.
2.5. Larval Weight Gain Determination and Survival Rate of Larvae
For survival analysis, the number of larvae was counted at 0, 4, 8, and 12 days (initially, exactly 50 larvae). To monitor larval growth, the dry weight of each sample was determined. Larval volume was calculated by multiplying the length, width, and thickness of 10 individual larvae before and after digestion.
2.6. Proximate Analysis of BSFL and Spent Feed
Proximate analysis was performed on the BSFL and spent feed before and after larval digestion of the fermented FDK. The dry weight of the samples was measured after drying at 105 °C for 24 h to a constant weight according to AOAC Method #930.15 [
11]. Dried samples were used for ash, crude protein, and crude fat analyses. The ash content of the larval biomass and spent feed was determined by the gravimetric method as described in AOAC Method #942.05 [
11]. Samples were carbonized using a hot plate in the fume hood and, after carbonization, the crucibles were incinerated in a muffle furnace at 550 °C overnight. Crude protein content was measured by the micro-Kjeldahl method as described in AOAC Method #960.52 [
11] with slight modification. Conversion factors of 6.25 and 5.70 were used to calculate total protein of the larval biomass and spent feed, respectively. The crude fat analysis was determined according to the Goldfisch method as described in AACC method #30-20.01 [
12]. Samples were weighed onto Whatman filter paper (No.1) at 0.5 g for larval biomass and 1 g for spent feed and placed in the Goldfisch apparatus (Labconco Corporation, Kansas City, MO, US). Petroleum ether was used as the extraction solvent, and the extraction process lasted 6 h. Crude fat was determined as the weight of fats in the extract after removal of the solvent. The carbohydrate contents of BSFL and spent feed were determined by subtracting the lipid, protein, and ash contents from the total weight.
2.7. Mycotoxin Analysis
Larval biomass and spent feed were finely ground and extracted for mycotoxin analysis to determine the concentration of DON accumulated. Larval samples and spent feed were extracted according to the method developed by Dr. L. Wang, at the Cereal and Flax Pathology program at the University of Saskatchewan (personal communication). Finely ground wheat grain (2 g) and a larval sample (0.1 g) were mixed with acetonitrile/water (84:16, v/v) with a ratio of 1:4 w/v and extracted on a rotary shaker for 2 h at room temperature (250 rpm). The extract was diluted 1:10 with 5 mM ammonium acetate and syringe-filtered. Thirty microliters of this filtrate was injected into the LC–MS/MS.
The LC–MS/MS conditions were developed on a high-performance liquid chromatography system (Agilent 1260 Infinity Quaternary; Agilent Technologies, Mississauga, ON, CA) coupled to an AB Sciex 4000 hybrid triple quadrupole linear ion trap (4000 QTrap) mass spectrometer (Concord, ON, CA) equipped with a TurboionsprayTM interface. Applied Biosystems/MDS Sciex Analyst software (Version 1.6.2, AB Sciex, Foster City, CA, US) was used for system control and quantification. The mobile phase consisted of a mixture of solvent A (5 mM ammonium acetate in water) and solvent B (5 mM ammonium acetate in methanol). Samples were stored in the auto sampler at 4 °C and a 30-µL injection volume with a 3-s flush port wash (to minimize carryover) was used to introduce the sample into the column.
Chromatographic separation was obtained at a flow rate of 300 µL/min through an Agilent ZORBAX Eclipse XDB C18 column (4.6 × 100 mm, 1.8 µm) equipped with an Eclipse XDB C18 (4.6 mm, 1.8 µm) guard column maintained at 30 °C in a column heater. Multiple reaction monitoring (MRM) with electrospray was used to monitor DON with the transitions of m/z 355.0 to m/z 296.1 and m/z 355.0 to m/z 264.9 as quantifier and qualifier ions, respectively.
The DON standard (purity > 99%) was supplied by Romer Labs Inc. (Tulln, Austria), and the stock solution was prepared at a concentration of 1 µg/mL in acetonitrile and kept at −20 °C. Working stock solutions were made by diluting in 5 mM ammonium acetate to the level of 10-fold final working concentration for each standard and quality control (QC) point. Standard and QC samples were prepared by adding 100 µL of each working stock to 900 µL of blank sample and mixing gently. A standard curve of seven points was constructed by determining the best fit of peak area versus the analyte concentration and running a weighed 1/x linear regression analysis.
2.8. Statistical Analysis
Each treatment was analyzed in triplicate, and results are presented as an average with standard deviation. Statistical significance of the results was analyzed by one-way analysis of variance (ANOVA) using MINITAB (MINITAB 18, Minitab Inc., Coventry, UK). Treatment means were declared significantly different from each other using Tukey’s test at p < 0.05.