Ginger Beer: An Overview of Health Benefits and Recent Developments
Abstract
1. Introduction
2. Global Market Trends of Ginger Beer (Ale)
3. Proximate Analysis of Ginger
4. Phytochemical Composition of Ginger
5. Biological Activities
5.1. Antioxidant Activity
5.2. Anticancer Activity
5.3. Antidiabetic Activity
- Ginger inhibits prominent enzymes associated with hyperglycemia in the carbohydrate metabolism (that is α-amylase and α-glucosidase).
- Additionally, ginger enhances insulin release by β cells and sensitivity by promoting glucose clearances in insulin responsive peripheral tissues to aid in maintaining blood glucose homeostasis.
- Finally, ginger improves lipid profile with its prominent lipid lowering effect [61].
5.4. Anti-Hypercholesteremic Effect
5.5. Anti-Inflammatory Effect
6. Toxicological Aspect and Health Concern
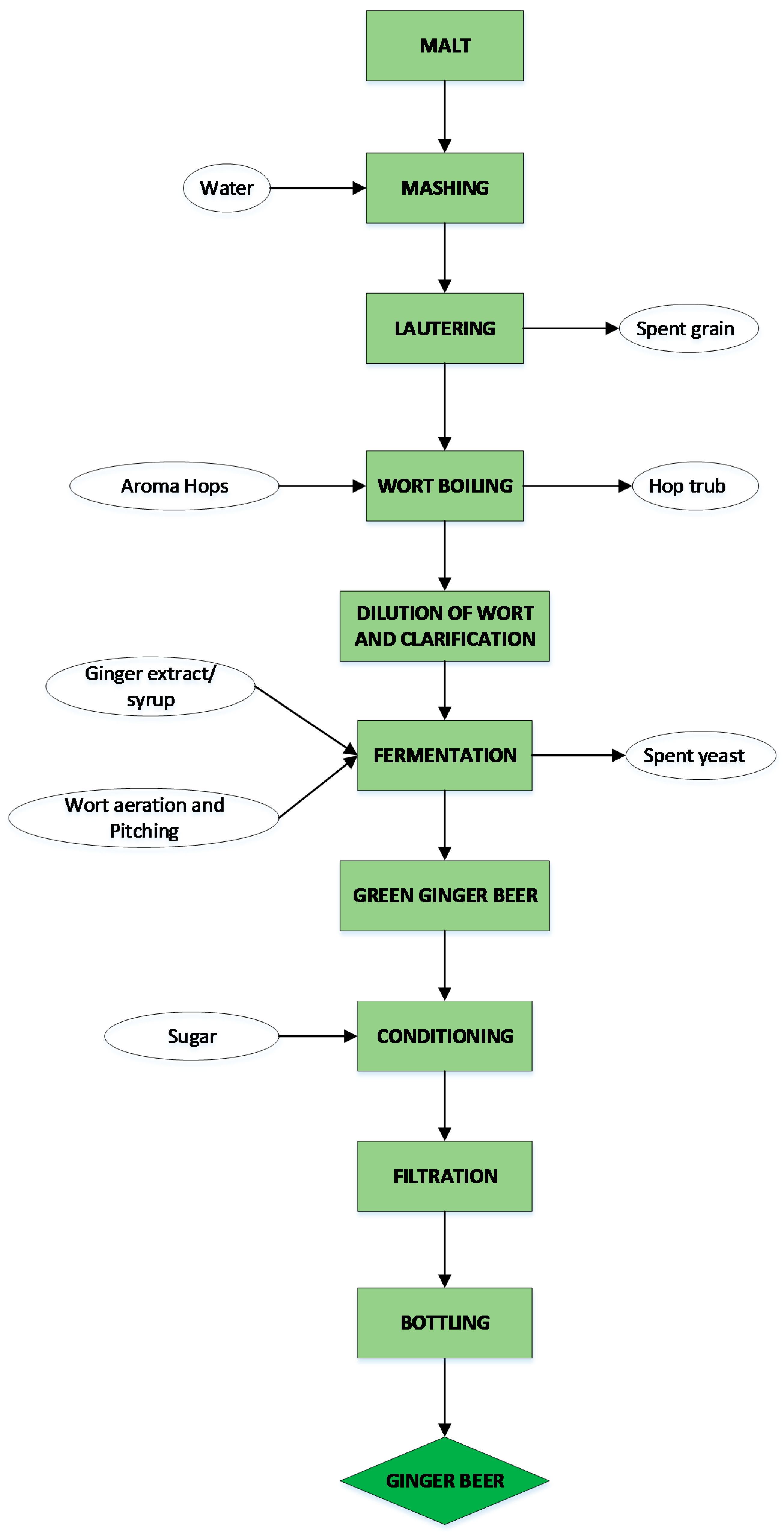
7. Production Technology of Ginger Beer
8. Composition of Volatile Organic Compounds (VOCs) in Ginger
Volatile Characteristics of Ginger Beverages
9. Other Ginger Species: Shell Ginger (Alpinia zerumbet)
10. Study Limitations
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.B. Ginger processing in India (Zingiber officinale): A review. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1639–1651. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Luo, D.; Ma, Y.; Zhang, J.; Li, M.; Yao, L.; Shi, X.; Liu, X.; Yang, K. Ginger for health care: An overview of systematic reviews. Complement. Ther. Med. 2019, 45, 114–123. [Google Scholar] [CrossRef]
- Srinivasan, K. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Madden, D. Ginger beer: A traditional fermented lowalcohol drink. Eur. J. Sci. Teach. 2008, 8, 29–33. [Google Scholar]
- Bellut, K.; Arendt, E.K. Chance and challenge: Non-saccharomyces yeasts in nonalcoholic and low alcohol beer brewing—A review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Adadi, P.; Kovaleva, E.; Glukhareva, T.; Shatunova, S.; Petrov, A. Production and analysis of non-traditional beer supplemented with sea buckthorn. Agron. Res. 2017, 15, 1831–1845. [Google Scholar] [CrossRef]
- Ducruet, J.; Rébénaque, P.; Diserens, S.; Kosińska-Cagnazzo, A.; Héritier, I.; Andlauer, W. Amber ale beer enriched with goji berries—The effect on bioactive compound content and sensorial properties. Food Chem. 2017, 226, 109–118. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Adamenko, K.; Kucharska, A.Z.; Prorok, P.; Piórecki, N. Physicochemical and antioxidative properties of Cornelian cherry beer. Food Chem. 2019, 281, 147–153. [Google Scholar] [CrossRef]
- Dookeran, M.M.; Baccus-Taylor, G.S.; Akingbala, J.O. Laboratory manufacture and comparison of ginger (Zingiber officinale Roscoe) beer quality. J. Food Agric. Environ. 2004, 2, 29–33. [Google Scholar] [CrossRef]
- Tozetto, L.M.; Nascimento, R.F.d.; Oliveira, M.H.d.; Van Beik, J.; Canteri, M.H.G. Production and physicochemical characterization of craft beer with ginger (Zingiber officinale). Food Sci. Technol. 2019, 39, 962–970. [Google Scholar] [CrossRef]
- Zion Market Research. Ginger Beer Market: Global Industry Perspective, Comprehensive Analysis, and forecast, 2018–2025. 2019. Available online: https://www.zionmarketresearch.com/report/ginger-beer-market (accessed on 30 August 2020).
- Gaikwad, K.K.; Singh, S.; Shakya, B. Studies on the development and shelf life of low calorie herbal aonla-ginger RTS beverage by using artificial sweeteners. J. Food Process. Technol. 2013, 4, 2. [Google Scholar] [CrossRef]
- OpenPR. Ginger Beer Market to Witness Huge Growth by 2025. Affinity Beverages, Fever-Tree, Q Mixers, Goslings Rum. 2019. Available online: https://www.openpr.com/news/1829148/ginger-beer-market-to-witness-huge-growth-by-2025-affinity (accessed on 30 August 2020).
- Singh, A. Nutritional benefits and pharmacological effects of ginger: An overview. Indian J. Basic Appl. Med. Res. 2015, 4, 377–383. [Google Scholar]
- Langner, E.; Greifenberg, S.; Gruenwald, J. Ginger: History and use. Adv. Ther. 1998, 15, 25–44. [Google Scholar]
- Al-Achi, A. A current look at ginger use. US Pharm. 2001, 26, HS13. [Google Scholar]
- Ajayi, O.B.; Akomolafe, S.F.; Akinyemi, F.T. Food value of two varieties of ginger (Zingiber officinale) commonly consumed in Nigeria. ISRN Nutr. 2013, 2013, 359727. [Google Scholar] [CrossRef]
- Bhatt, N.; Waly, M.I.; Essa, M.M.; Ali, A. Ginger: A functional herb. In Food as Medicine; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 51–72. [Google Scholar]
- Latona, D.; Oyeleke, G.; Olayiwola, O. Chemical analysis of ginger root. J. Appl. Chem. 2012, 1, 47–49. [Google Scholar]
- Yeh, H.-Y.; Chuang, C.-H.; Chen, H.-C.; Wan, C.-J.; Chen, T.-L.; Lin, L.-Y. Bioactive components analysis of two various gingers (Zingiber officinale Roscoe) and antioxidant effect of ginger extracts. LWT-Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Onimawo, I.A.; Esekheigbe, A.; Okoh, J.E. Determination of Proximate and Mineral Composition of Three Traditional Spices. ASNH 2019, 3, 111–114. [Google Scholar] [CrossRef]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; Thilakchand, K.R.; Rao, S.; Arora, R. Radioprotective effects of Zingiber officinale Roscoe (ginger): Past, present and future. Food Funct. 2012, 3, 714–723. [Google Scholar] [CrossRef]
- Tohma, H.; Gülçin, İ.; Bursal, E.; Gören, A.C.; Alwasel, S.H.; Köksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2016, 11, 556–566. [Google Scholar] [CrossRef]
- McKay, D. Can hibiscus tea lower blood pressure. Agro. Food Ind. Hi-Tech. 2009, 20, 40–42. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Synthesis of phenolics and flavonoids in ginger (Zingiber officinale Roscoe) and their effects on photosynthesis rate. Int. J. Mol. Sci. 2010, 11, 4539–4555. [Google Scholar] [CrossRef] [PubMed]
- Mošovská, S.; Nováková, D.; Kaliňák, M. Antioxidant activity of ginger extract and identification of its active components. Acta Chim. Slov. 2015, 8, 115–119. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Tanweer, S.; Mehmood, T.; Zainab, S.; Ahmad, Z.; Shehzad, A. Comparison and HPLC quantification of antioxidant profiling of ginger rhizome, leaves and flower extracts. Clin. Phytosci. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef]
- Osabor, V.; Bassey, F.; Umoh, U. Phytochemical screening and quantitative evaluation of nutritional values of Zingiber officinale (Ginger). Chem. Sci. Int. J. 2015, 1–6. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Ogbuewu, I.; Jiwuba, P.; Ezeokeke, C.; Uchegbu, M.; Okoli, I.; Iloeje, M. Evaluation of phytochemical and nutritional composition of ginger rhizome powder. Int. J. Agric. Rural Develop. 2014, 17, 1663–1670. [Google Scholar]
- Cao, S.-Y.; Li, Y.; Meng, X.; Zhao, C.-N.; Li, S.; Gan, R.-Y.; Li, H.-B. Dietary natural products and lung cancer: Effects and mechanisms of action. J. Funct. Foods 2019, 52, 316–331. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Al Shabrmi, F.M.; Aly, S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int. J. Physiol. Pathophysiol. Pharmacol. 2014, 6, 125–136. [Google Scholar]
- Aeschbach, R.; Loliger, J.; Scott, B.C.; Murcia, A.; Butler, J.; Halliwell, B.; Aruoma, O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994, 32, 31–36. [Google Scholar] [CrossRef]
- Chang, W.S.; Chang, Y.H.; Lu, F.J.; Chiang, H.C. Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res. 1994, 14, 501–506. [Google Scholar] [PubMed]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef]
- Bak, M.J.; Ok, S.; Jun, M.; Jeong, W.S. 6-shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules 2012, 17, 8037–8055. [Google Scholar] [CrossRef]
- Abd El-Kaream, S.A. Biochemical and biophysical study of chemopreventive and chemotherapeutic anti-tumor potential of some Egyptian plant extracts. Biochem. Biophys. Rep. 2019, 18, 100637. [Google Scholar] [CrossRef]
- Habib, S.H.; Makpol, S.; Abdul Hamid, N.A.; Das, S.; Ngah, W.Z.; Yusof, Y.A. Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 2008, 63, 807–813. [Google Scholar] [CrossRef]
- Manju, V.; Nalini, N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin. Chim. Acta 2005, 358, 60–67. [Google Scholar] [CrossRef]
- Ramakrishnan, R. Anticancer properties of Zingiber officinale–Ginger: A review. Int. J. Med. Pharm. Sci. 2013, 3, 11–20. [Google Scholar]
- Rhode, J.; Fogoros, S.; Zick, S.; Wahl, H.; Griffith, K.A.; Huang, J.; Liu, J.R. Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells. BMC Complement. Altern. Med. 2007, 7, 44. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, S.; Pei, Z.; Drozda, M.; Stavrovskaya, I.G.; Del Signore, S.J.; Cormier, K.; Shimony, E.M.; Wang, H.; Ferrante, R.J.; et al. Inhibitors of cytochrome c release with therapeutic potential for Huntington’s disease. J. Neurosci. 2008, 28, 9473–9485. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Jeong, C.H.; Bode, A.M.; Pugliese, A.; Cho, Y.Y.; Kim, H.G.; Shim, J.H.; Jeon, Y.J.; Li, H.; Jiang, H.; Dong, Z. [6]-Gingerol suppresses colon cancer growth by targeting leukotriene A4 hydrolase. Cancer Res. 2009, 69, 5584–5591. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Chun, K.S.; Kundu, J.K.; Surh, Y.J. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors 2004, 21, 27–31. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Sung, B.; Limtrakul, P.; Aggarwal, B.B. Zerumbone enhances TRAIL-induced apoptosis through the induction of death receptors in human colon cancer cells: Evidence for an essential role of reactive oxygen species. Cancer Res. 2009, 69, 6581–6589. [Google Scholar] [CrossRef]
- Sung, M.-H.; Salvatore, L.; De Lorenzi, R.; Indrawan, A.; Pasparakis, M.; Hager, G.L.; Bianchi, M.E.; Agresti, A. Sustained oscillations of NF-κB produce distinct genome scanning and gene expression profiles. PLoS ONE 2009, 4, e7163. [Google Scholar] [CrossRef]
- Huang, W.C.; Hung, M.C. Induction of Akt activity by chemotherapy confers acquired resistance. J. Formos Med. Assoc. 2009, 108, 180–194. [Google Scholar] [CrossRef]
- Ling, H.; Yang, H.; Tan, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-kappaB activation. Br. J. Pharmacol. 2010, 161, 1763–1777. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tao, Q.; Wu, X.; Dou, H.; Spencer, S.; Mang, C.; Xu, L.; Sun, L.; Zhao, Y.; Li, H.; et al. Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger. Fitoterapia 2012, 83, 568–585. [Google Scholar] [CrossRef]
- Liu, Y.; Whelan, R.J.; Pattnaik, B.R.; Ludwig, K.; Subudhi, E.; Rowland, H.; Claussen, N.; Zucker, N.; Uppal, S.; Kushner, D.M.; et al. Terpenoids from Zingiber officinale (Ginger) induce apoptosis in endometrial cancer cells through the activation of p53. PLoS ONE 2012, 7, e53178. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Warin, R.F.; Soroka, D.N.; Chen, H.; Sang, S. Metabolites of ginger component [6]-shogaol remain bioactive in cancer cells and have low toxicity in normal cells: Chemical synthesis and biological evaluation. PLoS ONE 2013, 8, e54677. [Google Scholar] [CrossRef] [PubMed]
- Kanwugu, O.N.; Helegbe, G.K.; Aryee, P.A.; Akontatiba, N.A.; Ankrah, J.; Anabire, N.G.; Anaba, F.; Ahenkora, B. A comparative assessment of the glucose monitor (SD Codefree) and auto analyzer (BT-3000) in measuring blood glucose concentration among diabetic patients. BMC Res. Notes 2017, 10, 453. [Google Scholar] [CrossRef]
- Jafri, S.A.; Abass, S.; Qasim, M. Hypoglycemic effect of ginger (Zingiber officinale) in alloxan induced diabetic rats (Rattus norvagicus). Pak. Vet. J. 2011, 31, 160–162. [Google Scholar]
- American Diabetes Association (ADA). Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Preventive and protective properties of Zingiber officinale (Ginger) in diabetes mellitus, diabetic complications, and associated lipid and other metabolic disorders: A brief review. Evid Based Complement. Altern. Med. 2012, 2012, 516870. [Google Scholar] [CrossRef]
- Sharma, M.; Shukla, S. Hypoglycaemic effect of ginger. J. Res. Indian Med. Yoga Homeopat. 1977, 12, 127–130. [Google Scholar]
- Oboh, G.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Inhibitory effects of aqueous extract of two varieties of ginger on some key enzymes linked to type-2 diabetes in vitro. J. Food. Nutr. Res. 2010, 49, 14–20. [Google Scholar]
- Akhani, S.P.; Vishwakarma, S.L.; Goyal, R.K. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J. Pharm. Pharmacol. 2004, 56, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, U.; Kanojia, R.; Pillai, K.K. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J. Ethnopharmacol. 2005, 97, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Chukwudike, A.M.M.; Mercy, B.O. Dose-dependent antidiabetic and antiobesity potentials of aqueous extract of Zingiber officinale Linn (Ginger) rhizomes in experimental diabetic rats: Need for precaution? EC Nutr. 2019, 14, 468–473. [Google Scholar]
- Salim, K.S. The Effect of oral administration of green tea and ginger extracts on blood glucose in diabetic rats. Int. J. Drug Deliv. Technol. 2019, 9, 41–46. [Google Scholar] [CrossRef]
- Hajimoosayi, F.; Jahanian Sadatmahalleh, S.; Kazemnejad, A.; Pirjani, R. Effect of ginger on the blood glucose level of women with gestational diabetes mellitus (GDM) with impaired glucose tolerance test (GTT): A randomized double-blind placebo-controlled trial. BMC Complement. Med. Ther 2020, 20, 116. [Google Scholar] [CrossRef] [PubMed]
- Rafie, R.; Hosseini, S.A.; Hajiani, E.; Saki Malehi, A.; Mard, S.A. Effect of ginger powder supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Clin. Exp. Gastroenterol. 2020, 13, 35–45. [Google Scholar] [CrossRef]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef]
- Abifadel, M.; Varret, M.; Rabes, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef]
- Goldstein, J.L. Familial Hypercholesterolemia; Parkland Memorial Hospital: Dallas, TX, USA, 1974. [Google Scholar]
- ElRokh el, S.M.; Yassin, N.A.; El-Shenawy, S.M.; Ibrahim, B.M. Antihypercholesterolaemic effect of ginger rhizome (Zingiber officinale) in rats. Inflammopharmacology 2010, 18, 309–315. [Google Scholar] [CrossRef]
- Bhandari, U.; Sharma, J.N.; Zafar, R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J. Ethnopharmacol. 1998, 61, 167–171. [Google Scholar] [CrossRef]
- Thomson, M.; Al-Qattan, K.; Al-Sawan, S.; Alnaqeeb, M.; Khan, I.; Ali, M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Srivastava, K.C.; Gan, E.K. Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology 1994, 49, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-inflammatory effects of the essential oils of ginger (Zingiber officinale Roscoe) in experimental rheumatoid arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Zhao, J.; Zhang, L.; Sun, B.; Bao, M.; Li, F.; Shen, J.; Shen, H.; Zhao, Y.; Xie, Q. Analgesic and anti-inflammatory effects of ginger oil. Chin. Herb. Med. 2011, 3, 150–155. [Google Scholar] [CrossRef]
- Luettig, J.; Rosenthal, R.; Lee, I.M.; Krug, S.M.; Schulzke, J.D. The ginger component 6-shogaol prevents TNF-alpha-induced barrier loss via inhibition of PI3K/Akt and NF-kappaB signaling. Mol. Nutr. Food Res. 2016, 60, 2576–2586. [Google Scholar] [CrossRef]
- Zhang, G.; Nitteranon, V.; Chan, L.Y.; Parkin, K.L. Glutathione conjugation attenuates biological activities of 6-dehydroshogaol from ginger. Food Chem. 2013, 140, 1–8. [Google Scholar] [CrossRef]
- Mohammadi, F.; Nikzad, H.; Taghizadeh, M.; Taherian, A.; Azami-Tameh, A.; Hosseini, S.M.; Moravveji, A. Protective effect of Zingiber officinale extract on rat testis after cyclophosphamide treatment. Andrologia 2014, 46, 680–686. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Hsiang, C.-Y.; Lo, H.-Y.; Huang, H.-C.; Li, C.-C.; Wu, S.-L.; Ho, T.-Y. Ginger extract and zingerone ameliorated trinitrobenzene sulphonic acid-induced colitis in mice via modulation of nuclear factor-κB activity and interleukin-1β signalling pathway. Food Chem. 2013, 136, 170–177. [Google Scholar] [CrossRef]
- Kaneko, M.; Kishimoto, Y.; Suzuki, R.; Kawai, Y.; Tateya, I.; Hirano, S. Protective effect of astaxanthin on vocal fold injury and inflammation due to vocal loading: A clinical trial. J. Voice 2017, 31, 352–358. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Essential Oils, Oleoresins (Solvent Free), and Natural Extractives Including Distillates; Code of Federal Regulations; U.S. Government Publishing Office: Washington, DC, USA, 2003.
- Wilkinson, J.M. Effect of ginger tea on the fetal development of Sprague-Dawley rats. Reprod. Toxicol. 2000, 14, 507–512. [Google Scholar] [CrossRef]
- Weidner, M.S.; Sigwart, K. Investigation of the teratogenic potential of a Zingiber officinale extract in the rat. Reprod. Toxicol. 2000, 15, 75–80. [Google Scholar] [CrossRef]
- Rong, X.; Peng, G.; Suzuki, T.; Yang, Q.; Yamahara, J.; Li, Y. A 35-day gavage safety assessment of ginger in rats. Regul. Toxicol. Pharmacol. 2009, 54, 118–123. [Google Scholar] [CrossRef]
- Idang, E.O.; Yemitan, O.K.; Mbagwu, H.O.; Udom, G.J.; Ogbuagu, E.O.; Udobang, J.A. Toxicological assessment of Zingiber officinale Roscoe (Ginger) root oil extracts in Albino rats. Toxicol. Digest 2019, 4, 108–119. [Google Scholar]
- Jeena, K.; Liju, V.B.; Kuttan, R. A preliminary 13-week oral toxicity study of ginger oil in male and female Wistar rats. Int. J. Toxicol. 2011, 30, 662–670. [Google Scholar] [CrossRef]
- Nwaopara, A.O.; Odike, M.A.C.; Inegbenebor, U.; Adoye, M.I. The Combined effects of excessive consumption of ginger, clove, red pepper and black pepper on the histology of the liver. Pak. J. Nutr. 2007, 6, 524–527. [Google Scholar] [CrossRef]
- Dookeran, M.M. Investigation into the Laboratory-Scale Production of Ginger (Zingiber officinale Roscoe) Beer by Natural and Controlled Fermentation. Master’s Thesis, University of the West Indies, Trinidad and Tobago, 2003. [Google Scholar]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Barakova, N.V. Production and investigations of antioxidant rich beverage: Utilizing monascus purpureus IHEM LY2014-0696 and various malts. Agron. Res. 2018, 16, 1312–1321. [Google Scholar] [CrossRef]
- Adadi, P.; Kovaleva, E.G.; Glukhareva, T.V.; Shatunova, S.A. Biotechnological production of non-traditional beer. AIP Conf. Proc. 2017, 1886, 1–13. [Google Scholar] [CrossRef]
- Adadi, P.; Kanwugu, O.N. Potential application of tetrapleura tetraptera and hibiscus sabdariffa (malvaceae) in designing highly flavoured and bioactive pito with functional properties. Beverages 2020, 6, 22. [Google Scholar] [CrossRef]
- Nsengumuremyi, D.; Adadi, P.; Ukolova, M.V.; Barakova, N.V. Effects of ultradisperse humic sapropel suspension on microbial growth and fermentation parameters of barley distillate. Fermentation 2019, 5, 24. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Shen, H.-J.; Zhang, S.-J. Development of ginger beer. Liquor-Making Sci. Technol. 1999, 6. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-NJKJ199906028.htm?fbclid=IwAR2P8K5cxMbjPUxQ-h5kaHXbuu2SQosanxgu4qJRpgWzVQa7-1wxoJbPHjg (accessed on 30 August 2020).
- Liu, F.; Song, S.; Zhang, X.; Tan, C.; Karangwa, E. Effect of sterilization methods on ginger flavor beverage assessed by partial least squares regression of descriptive sensory analysis and gas chromatography–mass spectrometry. Eur. Food Res. Technol. 2013, 238, 247–257. [Google Scholar] [CrossRef]
- AECOM Services Pty Ltd. Development of a Ginger Product to Add Value to the Fijian Ginger Industry; AECOM Services: Adelaide, SA, Australia, 2016. [Google Scholar]
- Yang, Z.-N.; Yang, W.; Peng, Q.; He, Q.; Feng, Y.; Luo, S.; Yu, Z. Volatile phytochemical composition of rhizome of ginger after extraction by headspace solid-phase microextraction, petrol ether extraction and steam distillation extraction. Bangladesh J. Pharmacol. 2009, 4, 136–143. [Google Scholar] [CrossRef]
- Gong, F.; Fung, Y.-S.; Liang, Y.-Z. Determination of volatile components in ginger using gas chromatography–mass spectrometry with resolution improved by data processing techniques. J. Agric. Food Chem. 2004, 52, 6378–6383. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Stoilova, I.; Wanner, J.; Schmidt, E.; Jirovetz, L.; Trifonova, D.; Stanchev, V.; Krastanov, A. Composition and Comprehensive Antioxidant Activity of Ginger (Zingiber officinale) Essential Oil from Ecuador. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef]
- Ding, S.H.; An, K.J.; Zhao, C.P.; Li, Y.; Guo, Y.H.; Wang, Z.F. Effect of drying methods on volatiles of Chinese ginger (Zingiber officinale Roscoe). Food Bioprod. Process. 2012, 90, 515–524. [Google Scholar] [CrossRef]
- Saerens, S.M.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Blanco, C.A.; Andres-Iglesias, C.; Montero, O. Low-alcohol beers: Flavor compounds, defects, and improvement strategies. Crit Rev. Food Sci. Nutr. 2016, 56, 1379–1388. [Google Scholar] [CrossRef]
- Pinho, O.; Ferreira, I.M.; Santos, L.H. Method optimization by solid-phase micro-extraction in combination with gas chromatography-mass spectrometry for analysis of beer volatile fraction. J. Chrom. A 2006, 1121, 145–153. [Google Scholar] [CrossRef]
- Eyres, G.; Dufour, J.P. Hop Essential Oil: Analysis, chemical composition and odor characteristics. In Beer in Health and Disease Prevention; Elsevier: Cambridge, MA, USA, 2008; pp. 239–254. [Google Scholar]
- Eyres, G.T.; Marriott, P.J.; Dufour, J.P. Comparison of odor-active compounds in the spicy fraction of hop (Humulus lupulus L.) essential oil from four different varieties. J. Agric. Food Chem. 2007, 55, 6252–6261. [Google Scholar] [CrossRef] [PubMed]
- Rettberg, N.; Biendl, M.; Garbe, L.-A. Hop aroma and hoppy beer flavor: Chemical backgrounds and analytical tools—A review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- Richter, T.M.; Silcock, P.; Algarra, A.; Eyres, G.T.; Capozzi, V.; Bremer, P.J.; Biasioli, F. Evaluation of PTR-ToF-MS as a tool to track the behavior of hop-derived compounds during the fermentation of beer. Food Res. Int. 2018, 111, 582–589. [Google Scholar] [CrossRef]
- Richter, T.M.; Eyres, G.T.; Silcock, P.; Bremer, P.J. Comparison of four extraction methods for analysis of volatile hop-derived aroma compounds in beer. J. Sep. Sci. 2017, 40, 4366–4376. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Scientific approaches to improving artisan methods of producing local food condiments in Ghana. Food Control. 2019, 106, 106682. [Google Scholar] [CrossRef]
- Saison, D.; De Schutter, D.P.; Delvaux, F.; Delvaux, F.R. Optimisation of a complete method for the analysis of volatiles involved in the flavor stability of beer by solid-phase microextraction in combination with gas chromatography and mass spectrometry. J. Chromatogr. A 2008, 1190, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Halimah, H.; Maddeppungeng, N.R. Instant Ginger Beer the Traditional Health Drinks from Buginese-Makassar; Trans Tech Publications Ltd.: Kapellweg, Switzerland, 2019; Volume 967 MSF, pp. 107–112. [Google Scholar]
- Teschke, R.; Xuan, T.D. A contributory role of shell ginger (Alpinia zerumbet) for human longevity in Okinawa, Japan? Nutrients 2018, 10, 166. [Google Scholar] [CrossRef]
- Ma, X.-N.; Xie, C.-L.; Miao, Z.; Yang, Q.; Yang, X.-W. An overview of chemical constituents from Alpinia species in the last six decades. RSC Adv. 2017, 7, 14114–14144. [Google Scholar] [CrossRef]
- Tao, L.; Hu, H.S.; Shen, X.C. Endothelium-dependent vasodilatation effects of the essential oil from Fructus alpiniae zerumbet (EOFAZ) on rat thoracic aortic rings in vitro. Phytomedicine 2013, 20, 387–393. [Google Scholar] [CrossRef]
- Tawata, S.; Fukuta, M.; Xuan, T.D.; Deba, F. Total utilization of tropical plants Leucaena leucocephala and Alpinia zerumbet. J. Pestic. Sci 2008, 33, 40–43. [Google Scholar] [CrossRef]
- Zahra, M.H.; Salem, T.A.; El-Aarag, B.; Yosri, N.; El-Ghlban, S.; Zaki, K.; Marei, A.H.; El-Wahed, A.; Saeed, A.; Khatib, A. Alpinia zerumbet (Pers.): Food and medicinal plant with potential in vitro and in vivo anti-cancer activities. Molecules 2019, 24, 2495. [Google Scholar] [CrossRef] [PubMed]
- Tawata, S.; Taira, S.; Kobamoto, N.; Ishihara, M.; Toyama, S. Syntheses and biological activities of dihydro-5, 6-dehydrokawain derivatives. Biosci. Biotechnol. Biochem. 1996, 60, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Chompoo, J.; Taira, N.; Fukuta, M.; Tawata, S. Significant longevity-extending effects of Alpinia zerumbet leaf extract on the life span of Caenorhabditis elegans. Biosci. Biotechnol. Biochem. 2013, 77, 217–223. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Sobeh, M.; Rezq, S.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. HPLC-ESI-MS/MS profiling of polyphenolics of a leaf extract from Alpinia zerumbet (Zingiberaceae) and its anti-inflammatory, anti-nociceptive, and antipyretic activities in vivo. Molecules 2018, 23, 3238. [Google Scholar] [CrossRef]
- Kumagai, M.; Mishima, T.; Watanabe, A.; Harada, T.; Yoshida, I.; Fujita, K.; Watai, M.; Tawata, S.; Nishikawa, K.; Morimoto, Y. 5, 6-Dehydrokawain from Alpinia zerumbet promotes osteoblastic MC3T3-E1 cell differentiation. Biosci. Biotechnol. Biochem. 2016, 80, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Burnett, C.; Valentini, S.; Cabreiro, F.; Goss, M.; Somogyvári, M.; Piper, M.D.; Hoddinott, M.; Sutphin, G.L.; Leko, V.; McElwee, J.J. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 2011, 477, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Satoh, A.; Stein, L.; Imai, S. The role of Mammalian Sirtuins in the Regulation of Metabolism, Aging, and Longevity. In Histone Deacetylases: The Biology and Clinical Implication; Springer: Berlin/Heidelberg, Germany, 2011; pp. 125–162. [Google Scholar]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- Xuan, T.; Quan, N.; Quan, N.; Rayee, R.; Khanh, T.; Tran, H.; Trung, N. Allelopathic plants: 26. Alpinia zerumbet (Pers.) BL Burtt & RM Sm.(Zingiberaceae). Allelopathy J. 2019, 48, 1–13. [Google Scholar] [CrossRef]
- Kuraya, E.; Yamashiro, R.; Touyama, A.; Nakada, S.; Watanabe, K.; Iguchi, A.; Itoh, S. Aroma profile and antioxidant activity of essential oil from Alpinia zerumbet. Nat. Prod. Commun. 2017, 12, 1322–1325. [Google Scholar] [CrossRef]
- Dias, D.M.; Costa, N.M.B.; Nutti, M.R.; Tako, E.; Martino, H.S.D. Advantages and limitations of in vitro and in vivo methods of iron and zinc bioavailability evaluation in the assessment of biofortification program effectiveness. Crit. Rev. Food Sci. Nutr. 2018, 58, 2136–2146. [Google Scholar] [CrossRef]
- Roberts, A.E.; Kragh, K.N.; Bjarnsholt, T.; Diggle, S.P. The limitations of in vitro experimentation in understanding biofilms and chronic infection. J. Mol. Biol. 2015, 427, 3646–3661. [Google Scholar] [CrossRef] [PubMed]

| Nutrients | [15] | [20] | [21] | [21] | [22] |
|---|---|---|---|---|---|
| Moisture | 15.02 ± 0.04% | 13.75% | 84.16 ± 0.97% | 89.14 ± 0.72% | 75.20 ± 0.53% |
| Carbohydrate | 38.35 ± 0.1% | 7.64% | 15.39 a% | 15.41 a% | 2.01 ± 0.23% |
| Crude ash | 3.85 ± 0.61 (4.53)% | NR | 0.02 a% | 0.04 a% | 0.81 ± 0.01% |
| Crude fat | 3.72 ± 0.03 (4.37)% | 4.02% | 0.09 a% | 0.08 a% | 11.71 ± 0.19% |
| Crude fiber | 25.5 ± 0.04 (30.0)% | 13.75% | 0.17 a% | 0.16 a% | 1.38 ± 0.50% |
| Crude protein | 5.087 ± 0.09 (5.98)% | 34.13% | 0.17 a% | 0.15 a% | 8.91 ± 0.04% |
| Sodium | NR | NR | NR | NR | 7.32 ± 0.02 mg |
| Zinc | 0.92 ± 0 (1.08) mg | 64.0 mg | NR | NR | 4.99 ± 0.04 mg |
| Calcium | 88.4 ± 0.97 (104.02) mg | 280.0 mg | NR | NR | 182.67 ± 0.04 mg |
| Iron | 8.0 ± 0.2 (9.41) mg | 279.7 mg | NR | NR | 9.68 ± 0.02 mg |
| Manganese | 9.13 ± 001 (10.74) mg | 5.90 mg | NR | NR | NR |
| Copper | 0.545 ± 0.002 (0.641) mg | 8.80 mg | NR | NR | NR |
| Phosphorus | 174 ± 1.2 (204.75) mg | 8068.0 mg | NR | NR | NR |
| Chromium | 70 ± 0 (83.37) µg | NR | NR | NR | NR |
| Vitamin C | 9.33 ± 0.08 (10.97) mg | 1.036 | NR | NR | NR |
| Essential Amino Acids | White Type | Yellow Type | Non-Essential Amino Acids | White Type | Yellow Type |
|---|---|---|---|---|---|
| Lysine (Lys) | 2.70 | 15.90 | Alanine (Ala) | 10.60 | 9.90 |
| Arginine (Arg) | 41.40 | 26.80 | Aspartic acid (Asp) | 29.80 | 31.60 |
| Threonine (Thre) | 9.10 | 23.20 | Glutamic acid (Glu) | 56.80 | 35.80 |
| Phenylalanine (Phe) | 10.00 | 27.40 | Serine (Ser) | 23.10 | 10.20 |
| Valine (Val) | 22.00 | 23.70 | Proline (Pro) | 15.00 | 8.10 |
| Methionine (Met) | 5.70 | 4.70 | Glycine (Gly) | 22.60 | 17.10 |
| Isoleucine (Ile) | 10.70 | 10.40 | Cystine (Cys) | 4.60 | 4.60 |
| Leucine (Leu) | 42.00 | 56.00 | Tyrosine (Tyr) | 11.10 | 14.20 |
| Histidine (His) | 10.40 | 5.00 |
| Phenolic Compounds | WEG [25] | EEG [25] |
|---|---|---|
| Pyrogallol | 142.4 | 264.3 |
| p-Hydroxybenzoic acid | 321.1 | 29.4 |
| Ferulic acid | 88.8 | 224.7 |
| Vanillin | 101.2 | 89.4 |
| p-Coumaric acid | 291.4 | 170.2 |
| Gallic acid | 29.8 | 39.6 |
| Ascorbic acid | BDL | 31.3 |
| Caffeic acid | 9.8 | 91.2 |
| Syringic acid | BDL | BDL |
| Ellagic acid | BDL | BDL |
| Quercetin | BDL | BDL |
| α-Tocopherol | BDL | BDL |
| Catechol | BDL | BDL |
| Compound Name | Cancer | Mechanism | Cell Lines/System | References |
|---|---|---|---|---|
| Ginger extract | Liver cancer | Reduced the elevated expression of TNF-α and NF-κB | Rats | [43] |
| Ginger—whole and [6]-gingerol | Ovarian cancer | Inhibition NF-κB and tumor growth | In vitro | [46] |
| β-Elemene | non-small-cell lung cancer cells | Release of cytochrome c | In vitro | [47] |
| 6-gingerol | Breast cancer | Inhibits cell adhesion invasion motility | In vitro | [48] |
| Skin cancer | Enhances apoptosis | Mouse | [47] | |
| Colon cancer | Inhibition of leukotriene activity | Mice | [49] | |
| Zerumbone | Lung and colon cancer | Suppresses modulatory mechanisms of growth and induces apoptosis. Reduces expression of NF-κB | Mouse | [50] |
| Colon cancer | Activation of extracellular signal-regulated kinase 1/2 p38 mitogen-activated protein kinase | In vitro | [51] | |
| Osteoclastogenesis. | Blocks NF-kappa B expression. | Mouse monocyte | [52] | |
| 6-Shogaol | Lungs cancer | Inhibition of AKT | In vitro | [53] |
| 6-Shogaol | Breast cancer | Anti-metastasis | In vitro | [54] |
| Enone-diaryl heptanoid, 6-Shogaol, [10]-gingerol, | Liver/against nine human tumor cell (lines) | Inhibition of lipid peroxidation, Antioxidant activity, cytotoxic | In vitro | [55] |
| Terpenoids | Endometrial Cancer Cells | Induces apoptosis by activation of p53 | In vitro | [56] |
| 6-Shogaol | Cancer cell | Anticancer | In vitro | [57] |
| Constituent | Study Type | Subjects | Dose | Potential Mechanisms | Ref. |
|---|---|---|---|---|---|
| 6-shogaol | In vitro | HT-29/B6 and Caco-2 human intestinal epithelial cells | 100 μM | Inhibiting the PI3K/Akt and NF-κB signaling pathways | [79] |
| 6-shogaol and 6-gingerol, 6-dehydroshogaol | In vitro | RAW 264.7 mouse macrophage cells | 2.5, 5, and 10 μM | Inhibiting the production of NO and PGE2 | [80] |
| 6-gingerol-rich fraction | In vivo | Female Wistar rats | 50 and 100 mg/kg | Increasing the levels of myeloperoxidase, NO, and TNF-α | [81] |
| GDNPs 2 | In vivo | Female C57BL/6 FVB/NJ mice | 0.3 mg | Increasing the levels of IL-10 and IL-22; decreasing the levels of TNF-α, IL-6, and IL-1β | [82] |
| Ginger extract and zingerone | In vivo | Female BALB/c mice | 0.1, 1, 10, and 100 mg/kg | Inhibiting NF-κB activation and decreasing the level of IL-1β | [83] |
| Ginger extract | In vivo | C57BL6/J mice | 50 mg/mL | Inhibiting the production of TNF-α; Activating Akt and NF-κB | [84] |
| Code a | RI b | Compound | Area Ratio of the Compounds to the IS Compound c | |||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | |||
| Terpene hydrocarbons | ||||||
| 1 | 958 | Camphene | ND | ND | ND | 0.373 ± 0.007 |
| 2 | 974 | Sabinene | 0.182 ± 0.003 | 0.175 ± 0.003 | 0.231 ± 0.003 | 0.838 ± 0.002 |
| 3 | 987 | β-Myrcene | ND | 0.104 ± 0.003 | ND | 0.334 ± 0.007 |
| 4 | 998 | α-Phellandrene | ND | ND | ND | 0.222 ± 0.003 |
| 5 | 1040 | (E)-β-Ocimene | 0.490 ± 0.104 | 0.263 ± 0.033 | 0.513 ± 0.024 | 0.348 ± 0.044 |
| 6 | 1052 | α-Terpinene | ND | 0.127 ± 0.007 | 0.220 ± 0.004 | 0.479 ± 0.001 |
| 7 | 1340 | δ-Elemene | 0.321 ± 0.004 | 0.659 ± 0.001 | 0.458 ± 0.003 | 0.303 ± 0.001 |
| 8 | 1475 | (Z)-2,6-dimethyl-2,6-octadiene | 0.610 ± 0.008 | 0.659 ± 0.003 | 0.743 ± 0.004 | 0.903 ± 0.001 |
| 9 | 1482 | α-Curcumene | 1.064 ± 0.008 | 0.714 ± 0.003 | 0.570 ± 0.006 | 0.948 ± 0.014 |
| 10 | 1493 | Valencene | ND | ND | 0.389 ± 0.002 | ND |
| 11 | 1496 | β-Selinene | ND | 0.385 ± 0.007 | 0.208 ± 0.003 | 0.382 ± 0.002 |
| 12 | 1498 | Zingiberene | 12.519 ± 0.083 | 4.886 ± 0.003 | 7.081 ± 0.006 | 4.406 ± 0.014 |
| 13 | 1510 | (E,E)-α-farnesene | 1.664 ± 0.002 | 0.198 ± 0.003 | 1.058 ± 0.002 | 0.376 ± 0.002 |
| 14 | 1526 | β-Sesquiphellandrene | 7.667 ± 0.170 | 4.855 ± 0.081 | 4.970 ± 0.038 | 3.635 ± 0.036 |
| 15 | 1540 | α-Cadinene | 0.227 ± 0.002 | 0.384 ± 0.003 | 0.273 ± 0.002 | 0.419 ± 0.001 |
| 16 | 1557 | Epoxyalloaromadendrene | 0.206 ± 0.001 | 0.340 ± 0.002 | 0.263 ± 0.002 | 0.286 ± 0.003 |
| Alcohols | ||||||
| 17 | 902 | 2-Heptanol | ND | 0.185 ± 0.003 | 0.214 ± 0.001 | ND |
| 18 | 1017 | (E)-2-Caren-4-ol | 0.340 ± 0.003 | 0.340 ± 0.002 | 0.295 ± 0.001 | 0.321 ± 0.003 |
| 19 | 1088 | L-Linalool | 2.089 ± 0.024 | 1.963 ± 0.019 | 2.301 ± 0.021 | 1.970 ± 0.011 |
| 20 | 1105 | 2-Nonanol | 0.706 ± 0.008 | 0.404 ± 0.003 | 0.637 ± 0.001 | 0.537 ± 0.006 |
| 21 | 1147 | Eucalyptol | 3.580 ± 0.010 | 2.794 ± 0.019 | 4.684 ± 0.008 | 4.231 ± 0.045 |
| 22 | 1167 | Borneol | 3.260 ± 0.022 | 2.634 ± 0.041 | 3.564 ± 0.019 | 3.801 ± 0.014 |
| 23 | 1177 | α-Terpineol | 5.811 ± 0.043 | 5.860 ± 0.034 | 4.983 ± 0.029 | 4.178 ± 0.024 |
| 24 | 1198 | Myrtenol | 0.246 ± 0.002 | 0.239 ± 0.003 | 0.253 ± 0.003 | 0.263 ± 0.007 |
| 25 | 1203 | Isopulegol | 0.263 ± 0.003 | 0.477 ± 0.004 | 0.312 ± 0.001 | ND |
| 26 | 1230 | Nerol | ND | 0.201 ± 0.008 | ND | ND |
| 27 | 1250 | Geraniol | 0.674 ± 0.012 | 1.453 ± 0.102 | 0.533 ± 0.003 | 0.576 ± 0.012 |
| 28 | 1359 | α-Farnesol | 1.430 ± 0.007 | 1.079 ± 0.021 | 0.971 ± 0.017 | 0.981 ± 0.008 |
| 29 | 1429 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl) | 1.156 ± 0.015 | 1.019 ± 0.008 | 1.294 ± 0.007 | 1.108 ± 0.018 |
| 30 | 1522 | Agarospirol | 1.281 ± 0.011 | 2.394 ± 0.007 | 2.686 ± 0.012 | 2.808 ± 0.009 |
| 31 | 1529 | β-Bisabolol | 2.442 ± 0.026 | 2.325 ± 0.021 | 2.645 ± 0.025 | 2.782 ± 0.035 |
| 32 | 1551 | Elemol | 1.393 ± 0.005 | 1.193 ± 0.013 | 0.939 ± 0.025 | 1.138 ± 0.011 |
| 33 | 1557 | Citronellol | 2.214 ± 0.029 | 1.154 ± 0.016 | 1.974 ± 0.016 | 1.743 ± 0.025 |
| 34 | 1560 | (E)-Nerolidol | 2.257 ± 0.005 | 1.928 ± 0.003 | 1.634 ± 0.003 | 1.401 ± 0.012 |
| 35 | 1573 | 6-Camphenol | ND | 0.321 ± 0.028 | 0.210 ± 0.012 | 0.294 ± 0.003 |
| 36 | 1583 | Zingiberenol | 0.475 ± 0.004 | 0.943 ± 0.001 | 0.558 ± 0.003 | 1.446 ± 0.002 |
| 37 | 1584 | Spathulenol | ND | 0.198 ± 0.005 | 0.263 ± 0.009 | 0.994 ± 0.006 |
| 38 | 1614 | β-Eudesmol | 5.479 ± 0.029 | 4.802 ± 0.135 | 4.517 ± 0.081 | 5.544 ± 0.031 |
| 39 | 1625 | Cedren-9-ol | 0.669 ± 0.004 | 0.745 ± 0.001 | 0.384 ± 0.003 | 0.570 ± 0.002 |
| 40 | 1631 | α-Acorenol | 0.199 ± 0.002 | ND | ND | 0.224 ± 0.007 |
| 41 | 1650 | Cubenol | 1.787 ± 0.008 | 1.471 ± 0.013 | 1.490 ± 0.007 | 1.393 ± 0.010 |
| Ketones | ||||||
| 42 | 1144 | Camphor | 0.325 ± 0.002 | 0.942 ± 0.003 | 0.436 ± 0.008 | 0.384 ± 0.003 |
| 43 | 1236 | 5-Hepten-2-one,6-methyl- | 1.965 ± 0.028 | 1.864 ± 0.054 | 2.518 ± 0.022 | 2.508 ± 0.029 |
| 44 | 1421 | 2-Undecanone | 0.377 ± 0.022 | 0.373 ± 0.016 | 0.271 ± 0.008 | 0.376 ± 0.017 |
| 45 | 1425 | 7-Decen-2-one | 0.433 ± 0.003 | 0.113 ± 0.001 | 0.392 ± 0.012 | ND |
| 46 | 1541 | β-Ionone | 0.327 ± 0.005 | 0.455 ± 0.027 | 0.277 ± 0.019 | 0.260 ± 0.013 |
| Aldehydes | ||||||
| 47 | 1001 | Octanal | 0.185 ± 0.007 | 0.140 ± 0.003 | 0.204 ± 0.003 | 0.277 ± 0.001 |
| 48 | 1208 | 2-Thujenal | ND | ND | ND | 0.411 ± 0.017 |
| 49 | 1244 | Neral | 0.181 ± 0.007 | 0.330 ± 0.001 | 0.204 ± 0.003 | ND |
| 50 | 1273 | Geranial | 19.574 ± 0.167 | 18.836 ± 0.141 | 20.502 ± 0.109 | 21.145 ± 0.177 |
| 51 | 1295 | (E)-2-Octenal | 0.486 ± 0.019 | 0.138 ± 0.017 | 0.457 ± 0.010 | 0.417 ± 0.004 |
| 52 | 1322 | Furfural | 0.309 ± 0.058 | 0.330 ± 0.027 | 0.244 ± 0.021 | 0.365 ± 0.011 |
| 53 | 1332 | Citronella | 0.454 ± 0.002 | 0.256 ± 0.002 | ND | ND |
| 54 | 1452 | Myrtenal | 0.435 ± 0.004 | 0.242 ± 0.005 | 0.442 ± 0.002 | 0.440 ± 0.004 |
| 55 | 1460 | (Z)-2-Decenal | ND | 0.306 ± 0.008 | ND | ND |
| Acids | ||||||
| 56 | 1876 | Hexadecanoic acid | 0.247 ± 0.002 | 0.173 ± 0.003 | 0.212 ± 0.003 | 0.187 ± 0.002 |
| Esters | ||||||
| 57 | 1410 | Bornyl acetate | 0.587 ± 0.007 | 0.246 ± 0.008 | 0.546 ± 0.013 | 0.550 ± 0.004 |
| 58 | 1550 | Neryl acetate | 0.943 ± 0.014 | 0.178 ± 0.009 | 0.425 ± 0.003 | 0.554 ± 0.006 |
| 59 | 1805 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl)ester | ND | ND | ND | 0.263 ± 0.008 |
| Total | 89.529 | 74.771 | 81.449 | 80.692 | ||
| Samples | Aroma of Ginger Flavored Beverages (GFB) | Taste of GFBs | |||||
|---|---|---|---|---|---|---|---|
| Fruity | Floral | Gingery | Cooked | Piquant | Sweet | Sour | |
| G1 | 43.23 ± 2.01 a | 28.40 ± 4.73 a | 85.36 ± 3.44 b | 27.68 ± 4.55 a | 90.60 ± 2.35 b | 91.29 ± 0.87 a | 71.83 ± 3.71 a |
| G2 | 37.74 ± 2.11 b | 27.20 ± 7.37 a | 72.91 ± 4.31 a | 32.93 ± 4.39 b | 89.24 ± 1.79 b | 91.25 ± 1.02 a | 69.54 ± 3.2 a |
| G3 | 35.75 ± 2.95 b | 30.75 ± 5.19 a | 85.70 ± 2.97 b | 46.24 ± 3.81 c | 80.71 ± 2.34 a | 90.90 ± 0.60 a | 72.00 ± 3.50 a |
| G4 | 34.89 ± 3.59 b | 28.88 ± 4.12 a | 85.39 ± 3.19 b | 67.01 ± 3.99 d | 79.36 ± 2.27 a | 91.48 ± 0.69 a | 70.91 ± 3.57 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nutakor, C.; Essiedu, J.A.; Adadi, P.; Kanwugu, O.N. Ginger Beer: An Overview of Health Benefits and Recent Developments. Fermentation 2020, 6, 102. https://doi.org/10.3390/fermentation6040102
Nutakor C, Essiedu JA, Adadi P, Kanwugu ON. Ginger Beer: An Overview of Health Benefits and Recent Developments. Fermentation. 2020; 6(4):102. https://doi.org/10.3390/fermentation6040102
Chicago/Turabian StyleNutakor, Christabel, Justice A. Essiedu, Parise Adadi, and Osman N. Kanwugu. 2020. "Ginger Beer: An Overview of Health Benefits and Recent Developments" Fermentation 6, no. 4: 102. https://doi.org/10.3390/fermentation6040102
APA StyleNutakor, C., Essiedu, J. A., Adadi, P., & Kanwugu, O. N. (2020). Ginger Beer: An Overview of Health Benefits and Recent Developments. Fermentation, 6(4), 102. https://doi.org/10.3390/fermentation6040102





