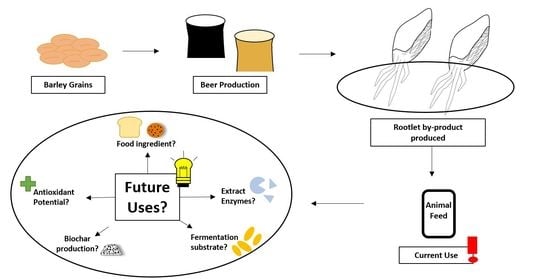
Rootlets, a Malting By-Product with Great Potential
Abstract
1. Introduction
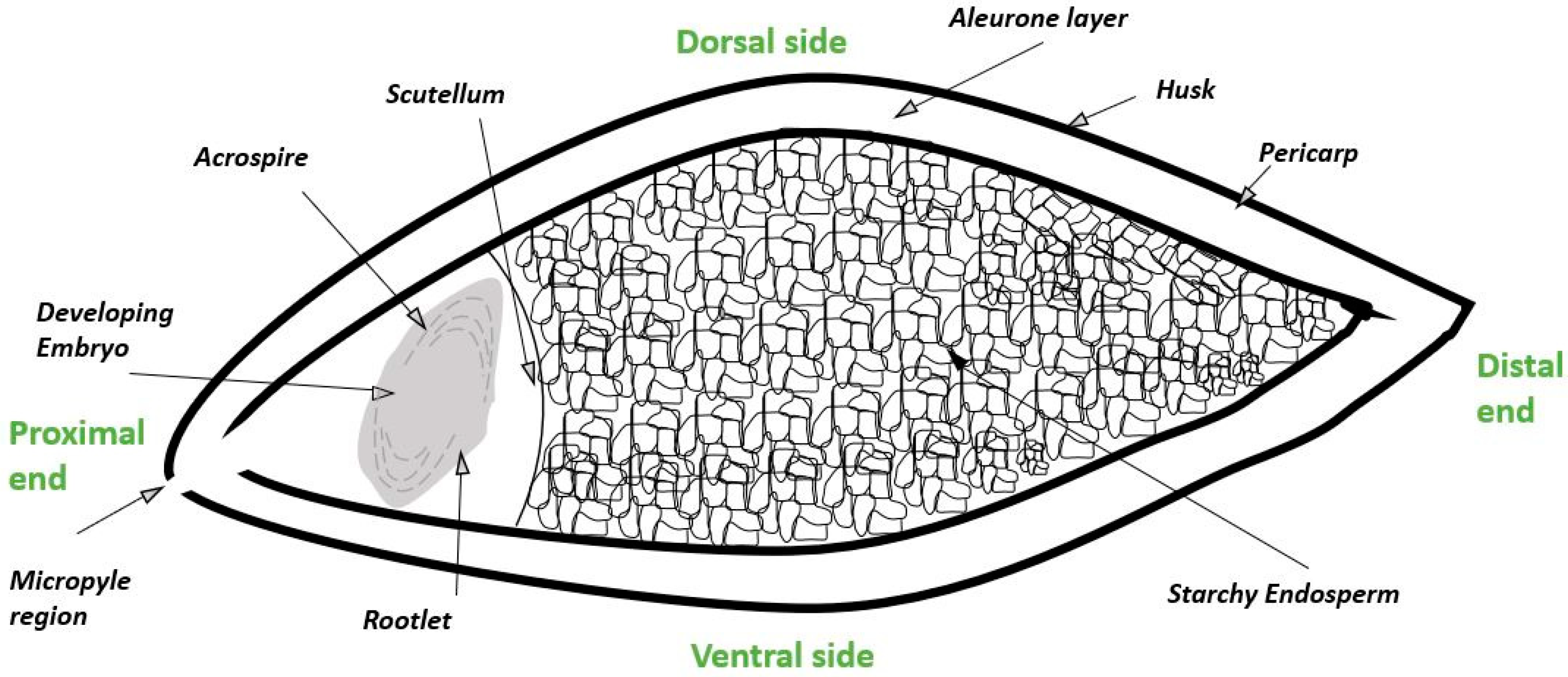
2. Biological Steps in Grain Germination and Relationship to the Malting Process
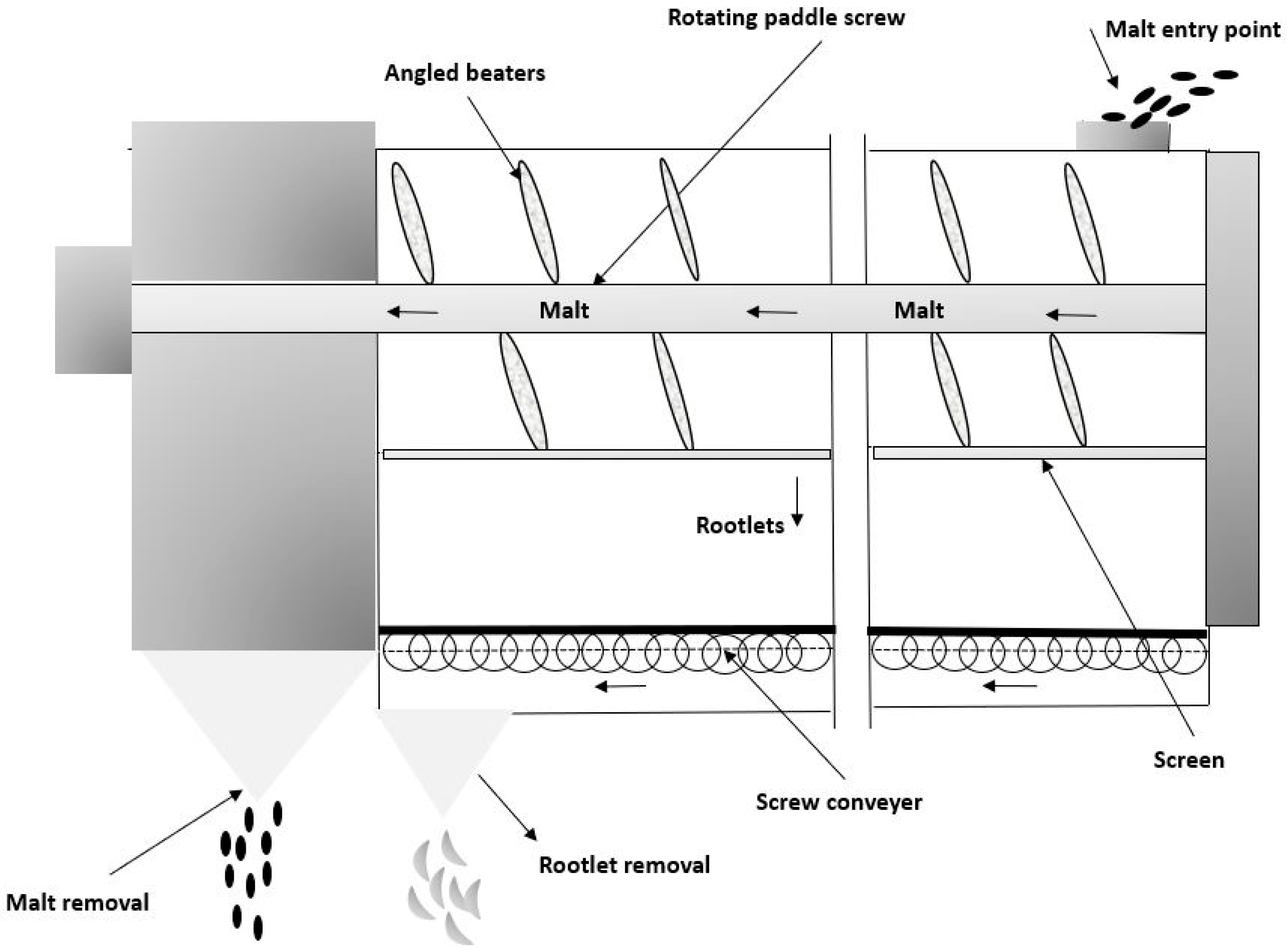
3. Formation and Processing of Barley Rootlets
4. Rootlet Composition and Quality
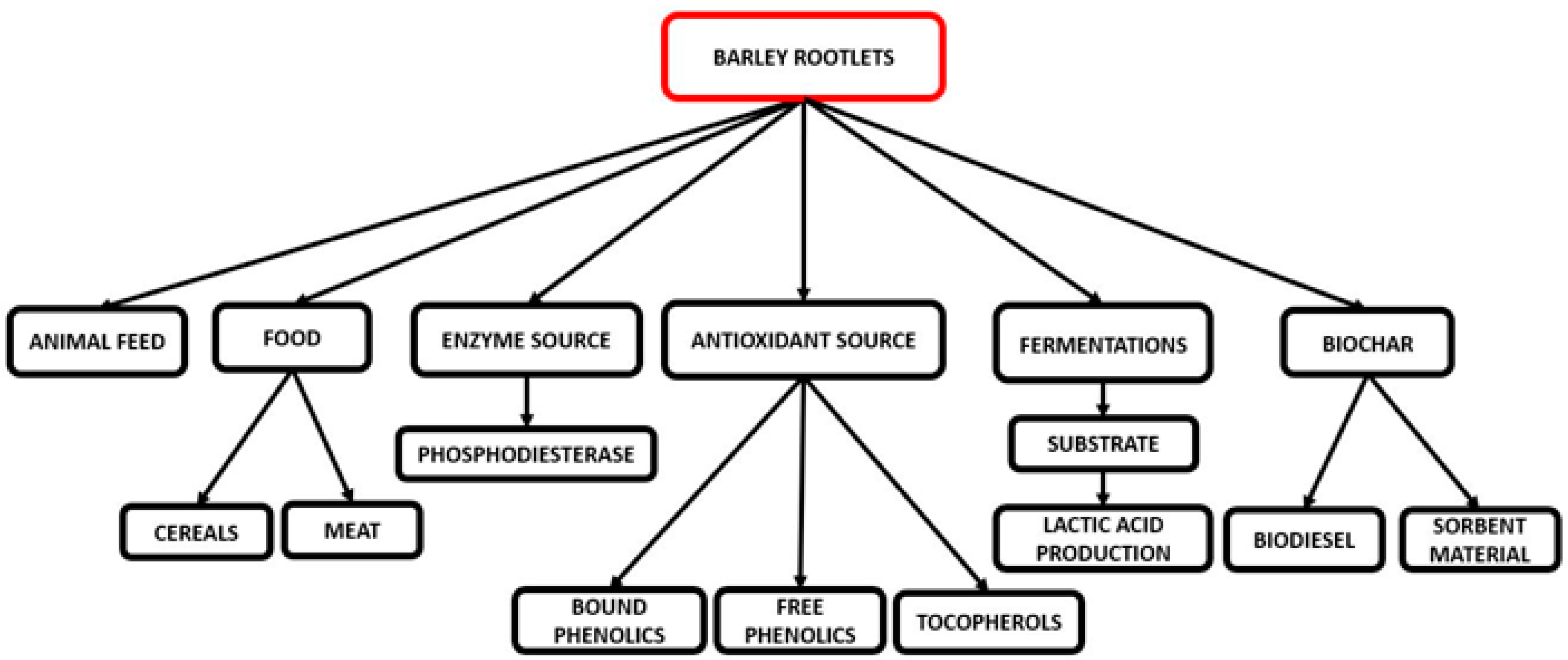
5. Rootlet Applications
5.1. Animal Feed
5.2. Food Applications
5.3. Enzyme Applications
5.4. Antioxidant Source
5.5. Growth Medium for Fermentation
5.6. Biochar Production
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arendt, E.K.; Zannini, E. Barley. In Cereal Grains for the Food and Beverage Industries; Woodhead Publishing: Cambridge, UK, 2013; pp. 155–201. ISBN 9780857098924. [Google Scholar]
- Stewart, G.G.; Russell, I.; Anstruther, A. Handbook of Brewing, 3rd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2017; ISBN 9781498751919. [Google Scholar]
- Mussatto, S.I. Biotechnological Potential of Brewing Industry By-Products. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh Nee Nigam, P., Pandey, A., Eds.; 2009; pp. 1–466. ISBN 9781402099410. [Google Scholar]
- Kusch-Brandt, S.; Mumme, J.; Nashalian, O.; Girotto, F.; Lavagnolo, M.C.; Udenigwe, C. Valorization of Residues From Beverage Production. In Processing and Sustainability of Beverages; Elsevier Inc.: Philadelphia, PA, USA, 2019; pp. 451–494. ISBN 9780128152591. [Google Scholar]
- Briggs, D.E. Malts and Malting, 1st ed.; Blackie Academic & Professional: London, UK, 1998. [Google Scholar]
- Kunze, W. Technology Brewing and Malting, 3rd ed.; VLB Berlin: Berlin, Germany, 2004. [Google Scholar]
- Waters, D.M.; Kingston, W.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Wheat bread biofortification with rootlets, a malting by-product. J. Sci. Food Agric. 2013, 93, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, R. Analysis of the barley malt rootlet proteome. Int. J. Mol. Sci. 2020, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Beluhan, S.; Karmelić, I.; Novak, S.; Marić, V. Partial purification and biochemical characterization of alkaline 5′-phosphodiesterase from barley malt sprouts. Biotechnol. Lett. 2003, 25, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Budaraju, S.; Mallikarjunan, K.; Annor, G.; Schoenfuss, T.; Raun, R. Effect of pre-treatments on the antioxidant potential of phenolic extracts from barley malt rootlets. Food Chem. 2018, 266, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mastanjević, K.; Lukinac, J.; Jukić, M.; Šarkanj, B.; Krstanović, V.; Mastanjević, K. Multi-(Myco)toxins in malting and brewing by-products. Toxins (Basel) 2019, 11, 30. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-products in the malting and brewing industries-re-usage possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Robbins, G.S.; Pomeranz, Y. Amino Acid Composition of Malted Cereals and Malt Sprouts. Proceedings. Annu. Meet. Am. Soc. Brew. Chem. 1971, 29, 15–21. [Google Scholar] [CrossRef]
- Salama, A.R.A.; El-Sahn, M.A.; Mesallam, A.S.; El-Tabey Shehata, A.M. The chemical composition, the nutritive value and the functional properties of malt sprout and its components (acrospires, rootlets and husks). J. Sci. Food Agric. 1997, 75, 50–56. [Google Scholar] [CrossRef]
- Palmer, G.H. Structure of Ancient Cereal Grains. J. Inst. Brew. 1995, 101, 103–112. [Google Scholar] [CrossRef]
- Lewis, M.J.; Young, T.W. Brewing, 2nd ed.; Springer Science+Business Media: New York, NY, USA, 2002; ISBN 978-1-4615-0729-1. [Google Scholar]
- Bamforth, C.W. Brewing: New Technologies; Elsevier Inc.: Philadelphia, PA, USA, 2006; ISBN 9781845690038. [Google Scholar]
- Gupta, M.; Abu-Ghannam, N.; Gallaghar, E. Barley for brewing: Characteristic changes during malting, brewing and applications of its by-products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 318–328. [Google Scholar] [CrossRef]
- Betts, N.S.; Dockter, C.; Berkowitz, O.; Collins, H.M.; Hooi, M.; Burton, R.A.; Bulone, V.; Skadhauge, B.; Whelan, J.; Fincher, G.B. Transcriptional and biochemical analyses of gibberellin expression and content in germinated barley grain. J. Exp. Bot. 2019, 71, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- Jeantet, R.; Croguennec, T.; Schuck, P.; Brulé, G. (Eds.) Handbook of Food Science and Technology 3: Food Chemistry and Technology; Wiley-ISTE: Great Britain, UK; Hoboken, NJ, USA, 2016; ISBN 9781848219342. [Google Scholar]
- Bamforth, C. Beer: Tap into the Art and Science of Brewing, 2nd ed.; Oxford University Press: Oxford, UK, 2003; ISBN 0195154797. [Google Scholar]
- Mosher, M.; Trantham, K. The “Food” for the Brew. In Brewing Science: A Multidisciplinary Approach; Springer International Publishing: Cham, Switzerland, 2017; pp. 125–156. ISBN 978-3-319-46393-3. [Google Scholar]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing Science and Practice; Woodhead Publishing Limited: Cambridge, UK, 2004. [Google Scholar]
- Jamar, C.; Jardin, P.; Fauconnier, M. Cell wall polysaccharides hydrolysis of malting barley. Biotechnol. Agron. Soc. Environ. 2011, 15, 301–313. [Google Scholar]
- Ogushi, K.; Lim, P.; Barr, A.R.; Takahashi, S.; Asakura, T.; Ito, K. Japanese barley meets Australia: Quality performance of malting barley grown in different countries. J. Inst. Brew. 2002, 108, 303–309. [Google Scholar] [CrossRef]
- Karababa, E.; Schwarz, P.B.; Horsley, R.D.; Karababa, E.; Crops, F.; Affairs, R.; Schwarz, P.B. Effect of Kiln Schedule on Micromalt Quality Parameters. J. Am. Soc. Brew. Chem. 1993, 51, 163–197. [Google Scholar] [CrossRef]
- Douglas, P.E.; Flannigan, B. A Microbiological evaluation of barley malt production. J. Inst. Brew. 1988, 94, 85–88. [Google Scholar] [CrossRef]
- Rennie, H.; Ball, K. The Influence of Malt Storage on Wort Separation. J. Inst. Brew. 1979, 85, 247–249. [Google Scholar] [CrossRef]
- The Maltsters Association of Great Britain Malting Co-Products-Valuable Nutritional Ingredients for the Feed Industry. Available online: http://www.ukmalt.com/malting-co-products (accessed on 14 May 2020).
- Hackett, C. A Study of the Root System of Barley: I. Effects of Nutrition on Two Varieties. New Phytol. 1968, 67, 287–299. [Google Scholar] [CrossRef]
- Rathjen, J.R.; Strounina, E.V.; Mares, D.J. Water movement into dormant and non-dormant wheat (Triticum aestivum L.) grains. J. Exp. Bot. 2009, 60, 1619–1631. [Google Scholar] [CrossRef]
- Boulton, C.M. Encyclopaedia of Brewing; John Wiley and Sons Ltd.: Chicester, UK, 2013; ISBN 9781405167444. [Google Scholar]
- Ahmad, A.; Khalid, N. Dietary Fibers in Modern Food Production: A Special Perspective With β-Glucans; Elsevier Inc.: Philadelphia, PA, USA, 2018; ISBN 9780128115015. [Google Scholar]
- KW alternative feeds Malt Culms Pellets- Typical Analysis. Available online: https://www.kwalternativefeeds.co.uk/products/view-products/malt-culms-pellets/ (accessed on 20 May 2020).
- World Health Organisation. Mycotoxin Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 15 June 2020).
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative analysis of plant-based high-protein ingredients and their impact on quality of high-protein bread. J. Cereal Sci. 2019, 89. [Google Scholar] [CrossRef]
- Hashitani, Y. On the chemical constituents of malt-rootlets with special reference to hordenine. J. Coll. Agric. 1925, 14, 1–56. [Google Scholar]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition. WHO Tech. Rep. Ser. 2007, 35, 150. [Google Scholar]
- Hoehnel, A.; Bez, J.; Petersen, I.L.; Amarowicz, R.; Juśkiewicz, J.; Arendt, E.K.; Zannini, E. Enhancing the nutritional profile of regular wheat bread while maintaining technological quality and adequate sensory attributes. Food Funct. 2020, 11, 4732–4751. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, S.M.; Ghali, Y.; Foda, M.S.; Youssef, A. Nutritive value of barley rootlets, a by-product of malting. J. Sci. Food Agric. 1975, 26, 1077–1081. [Google Scholar] [CrossRef]
- De-Oliveira, L.D.; Takakura, F.S.; Kienzle, E.; Brunetto, M.A.; Teshima, E.; Pereira, G.T.; Vasconcellos, R.S.; Carciofi, A.C. Fibre analysis and fibre digestibility in pet foods - a comparison of total dietary fibre, neutral and acid detergent fibre and crude fibre. J. Anim. Physiol. Anim. Nutr. (Berl). 2012, 96, 895–906. [Google Scholar] [CrossRef]
- Spiller, G.A.; Amen, R.J. Dietary fiber in human nutrition. CRC Crit. Rev. Food Sci. Nutr. 1975, 7, 39–70. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W.; Furda, I.; Lee, S.C. Determination of soluble dietary fiber in foods and food products: Collaborative study. J. AOAC Int. 1992, 75. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Courtin, C.M.; Delcour, J.A. Arabinoxylans and endoxylanases in wheat flour bread-making. J. Cereal Sci. 2002, 35, 225–243. [Google Scholar] [CrossRef]
- Begea, M.; Sirbu, A.; Pacala, M.-L.; Ciric, A.; Moisac, A. Pilot Technology to Obtain a Bio-based Product from Barley Malt Rootlets. In Proceedings of the I.C FaBE 2013-International Conference on Food and Biosystems Engineering, Skiathos Island, GRE, 30 May–2 June 2013; Volume 2. [Google Scholar]
- INRA-CIRAD-AFZ Feed Tables. Available online: https://www.feedtables.com/content/table-dry-matter (accessed on 13 November 2020).
- Kim, Y.; Je, Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: A meta-analysis of prospective cohort studies. Arch. Cardiovasc. Dis. 2015. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Coulibaly, A.; Kouakou, B.; Chen, J. Phytic Acid in Cereal Grains: Structure, Healthy or Harmful Ways to Reduce Phytic Acid in Cereal Grains and Their Effects on Nutritional Quality. Am. J. Plant Nutr. Fertil. Technol. 2011, 1, 1–22. [Google Scholar] [CrossRef]
- Cavaglieri, L.R.; Keller, K.M.; Pereyra, C.M.; González Pereyra, M.L.; Alonso, V.A.; Rojo, F.G.; Dalcero, A.M.; Rosa, C.A.R. Fungi and natural incidence of selected mycotoxins in barley rootlets. J. Stored Prod. Res. 2009, 45, 147–150. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Bĕláková, S.; Benešová, K.; Pernica, M.; Savi, G.D.; Rocha, L.O.; Hartman, I.; Čáslavský, J.; Corrêa, B. Fusarium mycotoxins stability during the malting and brewing processes. Toxins (Basel) 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.M.; Cavaglieri, L.R.; Fraga, M.E.; Direito, G.M.; Dalcero, A.M.; Rosa, C.A.R. Influence of water activity, temperature and time on mycotoxins production on barley rootlets. Lett. Appl. Microbiol. 2006, 42, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Mastanjević, K.; Šarkanj, B.; Mastanjević, K.; Šantek, B.; Krstanović, V. Fusarium culmorum mycotoxin transfer from wheat to malting and brewing products and by-products. World Mycotoxin J. 2019, 12, 55–66. [Google Scholar] [CrossRef]
- Krstanović, V.; Mastanjević, K.; Velić, N.; Pleadin, J.; Perši, N.; Španić, V. The influence of Fusarium culmorum contamination level on deoxynivalenol content in wheat, malt and beer. Rom. Biotechnol. Lett. 2015, 20, 10901–10910. [Google Scholar]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. Toxicol. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Mulder, C.J. Malts and Malting. Kirk-Othmer Encycl. Chem. Technol. 2005. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/0471238961.1301122004151403.a01.pub2 (accessed on 24 November 2020).
- Aggelopoulos, T.; Bekatorou, A.; Pandey, A.; Kanellaki, M.; Koutinas, A.A. Discarded oranges and brewer’s spent grains as promoting ingredients for microbial growth by submerged and solid state fermentation of agro-industrial waste mixtures. Appl. Biochem. Biotechnol. 2013, 170, 1885–1895. [Google Scholar] [CrossRef]
- Chiş, M.S.; Pop, A.; Păucean, A.; Socaci, S.A.; Alexa, E.; Man, S.M.; Bota, M.; Muste, S. Fatty acids, volatile and sensory profile of multigrain biscuits enriched with spent malt rootles. Molecules 2020, 25, 442. [Google Scholar] [CrossRef]
- Pereira, J.C.; Carro, M.D.; González, J.; Alvir, M.R.; Rodríguez, C.A. Rumen degradability and intestinal digestibility of brewers’ grains as affected by origin and heat treatment and of barley rootlets. Anim. Feed Sci. Technol. 1998, 74, 107–121. [Google Scholar] [CrossRef]
- Hashish, S.M.; Abd El-Samee, L. Egg Yolk Cholesterol of Hens fed Barley Malt Rootlets. Iran. J. Appl. Anim. Sci. 2012, 2, 83–88. [Google Scholar]
- Lewis, L.D. Feeding and Care of the Horse, 2nd ed.; Blackwell Publishing Ltd.: Oxford, UK, 1996; ISBN 978-0-683-04967-1. [Google Scholar]
- Frank, M.; Weckman, T.J.; Wood, T.; Woods, W.E.; Tai, C.L.; Chang, S.; Ewing, A.; Toblnt, T. Hordenine: Pharmacology, pharmaco kinetics and behavioural effects in the horse. Equine Vet. J. 1991, 22, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Salama, A.A.; El-Sahn, M.A.; Mesallam, A.S.; Shehata, A.M.E. Evaluation of the quality of bread, biscuit and butcher’s sausage supplemented with rootlets of malt sprouts. Nahrung 1997, 41, 228–231. [Google Scholar] [CrossRef]
- Bamforth, C.W. Superoxide Dismutase in Barley. J. Inst. Brew. 1983, 89, 420–423. [Google Scholar] [CrossRef]
- Billich, A.; Stockhowe, U.; Witzel, H. Nudeoside Phosphotransferase from Malt Sprouts-Isolation, Characterisation and Specificity of the enzyme. Biol. Chem. 1986, 367, 267–278. [Google Scholar]
- Friers, W.; Vandendriessche, L. The ribonuclease-activity of barley. Arch. Physiol. Biochem. 1961, 69, 339–369. [Google Scholar] [CrossRef]
- Prentice, N. Characterization of a nuclease from malted barley roots. J. Cereal Sci. 1987, 5, 175–187. [Google Scholar] [CrossRef]
- Prentice, N. Invertase of Germinated Barley. J. Agric. Food Chem. 1972, 20, 764–768. [Google Scholar] [CrossRef]
- Laufer, L.; Gutcho, S. Hydrolysis of RNA to 5′-nucleotide by seed sprouts, particularly malt sprouts. Biotechnol. Bioeng. 1968, 10, 257–275. [Google Scholar] [CrossRef]
- Deoda, A.J.; Singhal, R.S. 5′-Phosphodiesterase (5′-PDE) from germinated barley for hydrolysis of RNA to produce flavour nucleotides. Bioresour. Technol. 2003, 88, 245–250. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Kuninaka, A. Production of 5’Nucleotides. U.S. Patent No. 3223592A, 14 December 1965. [Google Scholar]
- Sakaguchi, K.; Kibi, M.; Kuminaka, A. Process for Improving the Flavour of Foods by the Addition of 5’Nucleotides. U.S. Patent No. 3104171A, 17 September 1963. [Google Scholar]
- Rehm, H.-J.; Reed, G.; Puhle, A.; Stardler, P. Biotechnology-Products of Primary Metabolism, 2nd ed.; Roehr, M., Rehm, H.-J., Reed, G., Puhler, A., Stadler, P.J.W., Eds.; VCH: Weinheim, Germany; New York, NY, USA, 1996; Volume 6, ISBN 3-527-28316-1. [Google Scholar]
- Kuninaka, A. Studies on taste of ribonucleic acid derivatives. J. Agric. Chem. Soc. Jpn. 1960, 34, 489–492. [Google Scholar]
- Yamaguchi, S. Basic properties of umami and its effects on food flavor. Food Rev. Int. 1998, 14, 139–176. [Google Scholar] [CrossRef]
- Hua, J.; Huang, K.-L. Preparation and Characterisation of 5’-Phosphodiesterase from Barley Malt Rootlets. Nat. Prod. Commun. 2010, 5, 265–268. [Google Scholar]
- Beluhan, S.; Maric, V. Comparative biochemical characterization of 5′-phosphodiesterase and phosphomonoesterase from barley malt sprouts. Nat. Prod. Commun. 2011, 6, 73–78. [Google Scholar] [CrossRef]
- Benaiges, M.D.; López-Santín, J.; Solà, C. Partial purification of 5′-phosphodiesterase activity from barley rootlets. Enzyme Microb. Technol. 1989, 11, 444–451. [Google Scholar] [CrossRef]
- Benaiges, M.D.; López-Santin, J.; Solà, C. Production of 5′-ribonucleotides by enzymatic hydrolysis of RNA. Enzyme Microb. Technol. 1990, 12, 86–89. [Google Scholar] [CrossRef]
- Sombutyanuchit, P.; Suphantharika, M.; Verduyn, C. Preparation of 5′-GMP-rich yeast extracts from spent brewer’s yeast. World J. Microbiol. Biotechnol. 2001, 17, 163–168. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; D’Souza, C.J.M. An overview on nucleases (DNase, RNase, and phosphodiesterase) in snake venoms. Biochemistry 2010, 75, 1–6. [Google Scholar] [CrossRef]
- Valério, A.A.; Corradini, A.C.; Panunto, P.C.; Mello, S.M.; Hyslop, S. Purification and characterization of a phosphodiesterase from Bothrops alternatus snake venom. J. Protein Chem. 2002, 21, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Landt, M.; Butler, L.G. 5ʹ-Nucleotide Phosphodiesterase: Isolation of Covalently Bound 5ʹ-Adenosine Monophosphate, an Intermediate in the Catalytic Mechanism. Biochemistry 1978, 17, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar]
- Dudjak, J.; Lachman, J.; Miholová, D.; Kolihová, D.; Pivec, V. Effect of cadmium on flavonoid content in young barley (Hordeum sativum L.) plants. Plant Soil Environ. 2004, 50, 471–477. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants (Basel) 2019, 8, 297. [Google Scholar] [CrossRef]
- Bonnely, S.; Peyrat-Maillard, M.N.; Rondini, L.; Masy, D.; Berset, C. Antioxidant activity of malt rootlet extracts. J. Agric. Food Chem. 2000, 48, 2785–2792. [Google Scholar] [CrossRef]
- Constantinou, C.; Papas, A.; Constantinou, A.I. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer 2008, 123, 739–752. [Google Scholar] [CrossRef]
- Peyrat-Maillard, M.N.; Bonnely, S.; Rondini, L.; Berset, C. Effect of Vitamin E and Vitamin C on the Antioxidant Activity of Malt Rootlets Extracts. LWT-Food Sci. Technol. 2001, 34, 176–182. [Google Scholar] [CrossRef]
- Meng, D.-J.; Lu, J.; Fan, W.; Dong, J.-J.; Zhang, J.; Kong, W.; Lin, Y.; Shan, L. Optimization of Extraction Conditions for Antioxidant Phenolic Compounds From Malt Rootlets using Response Surface Methodology. J. Food Biochem. 2009, 33, 291–305. [Google Scholar] [CrossRef]
- Cheng, H.-J.; Zhang, L.-M.; Duan, H.-X.; Li, J.; Dai, Y.-J. Optimization of Extraction Technology of the Alkali-soluble Components of Barley Malt Roots and its Composition Analysis. Med. Biopharm. 2016, 1497–1505. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Harden, L.A. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J. Agric. Food Chem. 2000, 48, 5702–5709. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Peterson, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas. Available online: https://www.osti.gov/biblio/15008859-top-value-added-chemicals-from-biomass-volume-results-screening-potential-candidates-from-sugars-synthesis-gas (accessed on 24 November 2020).
- Cejas, L.; Romano, N.; Moretti, A.; Mobili, P.; Golowczyc, M.; Gómez-Zavaglia, A. Malt sprout, an underused beer by-product with promising potential for the growth and dehydration of lactobacilli strains. J. Food Sci. Technol. 2017, 54, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- Laitila, A.; Saarela, M.; Kirk, L.; Siika-Aho, M.; Haikara, A.; Mattila-Sandholm, T.; Virkajärvi, I. Malt sprout extract medium for cultivation of Lactobacillus plantarum protective cultures. Lett. Appl. Microbiol. 2004, 39, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Radosavljević, M.; Pejin, J.; Pribić, M.; Kocić-Tanackov, S.; Romanić, R.; Mladenović, D.; Djukić-Vuković, A.; Mojović, L. Utilization of brewing and malting by-products as carrier and raw materials in l-(+)-lactic acid production and feed application. Appl. Microbiol. Biotechnol. 2019, 103, 3001–3013. [Google Scholar] [CrossRef]
- Radosavljević, M.; Pejin, J.; Pribić, M.; Kocić-Tanackov, S.; Mladenović, D.; Djukić-Vuković, A.; Mojović, L. Brewing and malting technology by-products as raw materials in L-(+)-lactic acid fermentation. J. Chem. Technol. Biotechnol. 2020, 95, 339–347. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Qi, B.; Chen, X.; Su, Z.; Wan, Y. Optimizing L-(+)-lactic acid production by thermophile Lactobacillus plantarum As.1.3 using alternative nitrogen sources with response surface method. Biochem. Eng. J. 2010, 52, 212–219. [Google Scholar] [CrossRef]
- Hujanen, M.; Linko, Y.Y. Effect of temperature and various nitrogen sources on L (+)-lactic acid production by Lactobacillus casei. Appl. Microbiol. Biotechnol. 1996, 45, 307–313. [Google Scholar] [CrossRef]
- Göksungur, Y.; Güvenç, U. Batch and continuous production of lactic acid from beet molasses by Lactobacillus delbrueckii IFO 3202. J. Chem. Technol. Biotechnol. 1997, 69, 399–404. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochar from spent malt rootlets for the degradation of trimethoprim in the presence of inorganic ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]
- Anagnostopoulos, V.; Symeopoulos, B.; Bourikas, K.; Bekatorou, A. Biosorption of U(VI) from aqueous systems by malt spent rootlets. Kinetic, equilibrium and speciation studies. Int. J. Environ. Sci. Technol. 2016, 13. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fakas, D.; Tzanakis, N.; Karapanagioti, H.K. Magnetic Biochar from Different Feedstocks for the Removal of Chromium (VI) from Aqueous Solutions. Available online: https://meetingorganizer.copernicus.org/EGU2019/EGU2019-12480.pdf (accessed on 24 November 2020).
- Orfanos, A.; Manariotis, I.D.; Karapanagioti, H.K. Methylene Blue Removal by Biochars from Food Industry By-Products. Available online: https://meetingorganizer.copernicus.org/EGU2016/EGU2016-921.pdf (accessed on 21 November 2020).
- Paschalidou, P.; Pashalidis, I.; Manariotis, I.D.; Karapanagioti, H.K. Hyper sorption capacity of raw and oxidized biochars from various feedstocks for U(VI). J. Environ. Chem. Eng. 2020, 8, 103932. [Google Scholar] [CrossRef]
- Sklivaniotis, L.; Manariotis, I.; Karapanagioti, H. Removal of Chlorine from Tap Water in Biochar Packed Columns. Available online: https://ui.adsabs.harvard.edu/abs/2018EGUGA..20.6312S/abstract (accessed on 24 November 2020).
- Tzachristas, A.; Papanikolaou, P.; Tzepkinli, V.; Manariotis, I.D.; Karapanagioti, H.K. Biochars Made from Agro-Industrial by-Products Remove Chlorine and Chlorination by-Product (Chloroform) from Water and Wastewater. Available online: https://meetingorganizer.copernicus.org/EGU2017/EGU2017-494.pdf (accessed on 24 November 2020).
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Manariotis, I.D.; Fotopoulou, K.N.; Karapanagioti, H.K. Preparation and Characterization of Biochar Sorbents Produced from Malt Spent Rootlets. Ind. Eng. Chem. Res. 2015, 54, 9577–9584. [Google Scholar] [CrossRef]
- Valili, S.; Siavalas, G.; Karapanagioti, H.K.; Manariotis, I.D.; Christanis, K. Phenanthrene removal from aqueous solutions using well-characterized, raw, chemically treated, and charred malt spent rootlets, a food industry by-product. J. Environ. Manage. 2013, 128, 252–258. [Google Scholar] [CrossRef]
- Boutsika, L.G.; Karapanagioti, H.K.; Manariotis, I.D. Effect of chloride and nitrate salts on Hg(II) sorption by raw and pyrolyzed malt spent rootlets. J. Chem. Technol. Biotechnol. 2017, 92, 1912–1918. [Google Scholar] [CrossRef]
- Anagnostopoulos, V.A.; Manariotis, I.D.; Karapanagioti, H.K.; Chrysikopoulos, C.V. Removal of mercury from aqueous solutions by malt spent rootlets. Chem. Eng. J. 2012, 213, 135–141. [Google Scholar] [CrossRef]
- Boutsika, L.G.; Karapanagioti, H.K.; Manariotis, I.D. Aqueous mercury sorption by biochar from malt spent rootlets. Water. Air. Soil Pollut. 2013, 225. [Google Scholar] [CrossRef]
- Tsouloufa, A.; Dailianis, S.; Karapanagioti, H.K.; Manariotis, I.D. Physicochemical and Toxicological Assay of Leachate from Malt Spent Rootlets Biochar. Bull. Environ. Contam. Toxicol. 2020, 104, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Pantiora, D.; Karapanagioti, H.K.; Manariotis, I.D.; Lycourghiotis, A.; Kordulis, C. Evaluation of Malt Spent Rootlets Biochar as Catalyst for Biodiesel. Available online: https://meetingorganizer.copernicus.org/EGU2014/EGU2014-6508.pdf (accessed on 24 November 2020).
- Ntaflou, M.; Vakros, J. Transesterification activity of modified biochars from spent malt rootlets using triacetin. J. Clean. Prod. 2020, 259. [Google Scholar] [CrossRef]
- Tsavatopoulou, V.D.; Vakros, J.; Manariotis, I.D. Lipid conversion of Scenedesmus rubescens biomass into biodiesel using biochar catalysts from malt spent rootlets. J. Chem. Technol. Biotechnol. 2020, 95, 2421–2429. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Production of biodiesel using palm oil. In Biofuels; Elsevier Inc.: Philadelphia, PA, USA, 2011; pp. 353–374. ISBN 9780123850997. [Google Scholar]
- Kihara, M.; Okada, Y.; Ito, K. Functional Component-Enriched Barley Malt Rootlets and Process for Producing the Same. U.S. Patent No. 20070148317A1, 28 June 2007. [Google Scholar]
- Kondo, K.; Nagao, K.; Yokoo, Y. Process for Producing Food and Beverage Products from Malt Sprouts. U.S. Patent No. 9326542B2, 3 May 2016. [Google Scholar]
- Bowles, L.K. 5’Phosphodiesterase Enzyme Preparation and Method for Its Production. U.S. Patent No. 5034325A, 23 July 1991. [Google Scholar]
- Laufer, L.; Gutcho, S. Enzymatic Material and Method of Preparing Same. U.S. Patent No. 3304238A, 14 February 1967. [Google Scholar]
- Laufer, L.; Gutcho, S. Enzyme Digestion of Nucleic Acids. U.S. Patent No. 3459637A, 5 August 1969. [Google Scholar]
- Baker, D.L.; Dockstader, W.B. Preparation of an Antioxidant from Rootlets. U.S. Patent No. 2925345A, 16 February 1960. [Google Scholar]
- Frieden, A.; Hoogerheide, J.C.; Stern, R.M. Poultry and Swine Feeds Containing Rootlets of Germinated Barley. U.S. Patent No. 2694011A, 9 November 1954. [Google Scholar]
- Fulger, C.V.; Haas, G.J.; Herman, E.B.; Lazarus, C.R. Malt-like Flavour from Cereal Grain Root Cultures. U.S. Patent No. 4613507A, 23 September 1986. [Google Scholar]
- Bazzoli, P.; Gomes Da Silva, G. Process for Preparing a Cereal Based Beverage with Malt and Malt Rootlets. WO Patent No. 2019238928A1, 19 December 2019. [Google Scholar]
- Fallourd, M.-J.; Chafil, S.; Baret, J.-L. Malt Sprouts Extracts and Their Uses. U.S. Patent No. 20200178580A1, 11 June 2020. [Google Scholar]
- Fallourd, M.-J.; Chafil, S.; Baret, J.-L. Compositions and Methods for Stimulating Plant Growth. WO Patent No. 2018104531A1, 14 June 2016. [Google Scholar]

| Component | Hashitani Y. [37] | Hegazi et al. [40] | Salama A.R.A et al. [14] | INRA-CIRAD-AFZ [47] | Aggelopoulos et al. [58] | Waters et al. [7] | Begea et al. [46] | Chiş et al. [59] |
|---|---|---|---|---|---|---|---|---|
| Protein | 23.9 | 25.0 | 31.9 | 22.6 | 31.1 | 36.75 | 20.34 | 38.7 |
| Fat | 3.6 | 1.8 | n.m. | 1.8 | 4.4 | 1.7 | 1.9 | 2.1 |
| Ash | 3.4 | 8.0 | 8.7 | 5.9 | 6.8 | 2.8 | 3.78 | 8.4 |
| Moisture (%) | 10.2 | 8.5 | 12.6 | 10.2 | 12.9 | n.m. | 8.6 | 8.2 |
| Carbohydrates | n.m. | n.m. | n.m. | n.m. | n.m. | 60 | n.m. | 50.9 |
| Total Fibre | n.d. | n.m. | n.m. | n.m. | n.m. | 43.0 | n.m. | n.m. |
| Crude fibre | 20.5 | 9.7 | 10.7 | 13.9 | n.m. | n.m. | n.m. | n.m. |
| Starch | n.m. | 7.0 | 26.5 | 16.5 | n.m. | 2.6 | n.m. | n.m. |
| Arabinoxylans (% T.F.) | n.m. | n.m. | n.m. | n.m. | n.m. | 14.4 | n.m. | n.m. |
| Polyphenols | n.m. | n.m. | 0.35 | n.m. | n.m. | 0.0102 | n.m. | n.m. |
| Phytic Acid | n.m. | n.m. | 0.018 | n.m. | n.m. | n.m. | n.m. | n.m. |
| Robbins et al. [13] (g/100g AA Rec) | Hegazi et al. [40] (mg/g N) | Salama A.R.A et al. [14] (g/16g N) | Waters et al. [7] (g/100g Protein) | |
|---|---|---|---|---|
| Essential Amino Acids | ||||
| Threonine | 3.9 | 298 | 3.82 | 0.055 |
| Methionine | 2.0 | 101 | n.m. | 0.107 |
| Tryptophan | n.m. | 122 | 2.51 | 0.022 |
| Phenylalanine | 3.6 | 101 | 3.84 | 0.875 |
| Isoleucine | 3.9 | n.m. | 3.40 | 1.055 |
| Leucine | 5.8 | n.m. | 5.43 | 1.455 |
| Lysine | 5.5 | 244 | 5.29 | n.m. |
| Non-Essential Amino Acids | ||||
| Aspartic Acid | 6.3 | 382 | 12.62 | 2.617 |
| Glutamic Acid | 13.1 | 596 | 11.32 | 3.025 |
| Asparagine | n.m. | n.m. | n.m. | 0.430 |
| Serine | n.m. | 306 | 3.9 | 0.882 |
| Glutamine | n.m. | n.m. | n.m. | n.d. |
| Histidine | 2.2 | 260 | 6.16 | 7.589 |
| Glycine | 4.3 | 216 | 4.05 | 0.470 |
| Arginine | 5.2 | 493 | 4.78 | 1.117 |
| Alanine | 5.2 | 200 | 11.31 | 1.198 |
| γ-Aminobutryic Acid | n.m. | n.m. | n.m. | 7.302 |
| Tyrosine | 2.3 | 295 | 1.21 | 0.617 |
| Valine | 5.5 | 268 | 6.09 | 1.334 |
| Proline | 5.9 | 110 | 6.72 | n.m. |
| Cystine | 0.4 | 112 | n.m. | n.m. |
| Components | Waters et al. [6] | Chiş et al. [59] |
|---|---|---|
| Fat % | 1.7 | 1.9 |
| Saturates | 24.12 | 33.40 |
| Monounsaturated fatty acids | 8.39 | 14.15 |
| Polyunsaturated fatty acids | 69.47 | 70.20 |
| Fatty Acids Present | ||
| Caproic | 0.02 | n.m. |
| Caprylic | 0.03 | n.m. |
| Capric | 0.15 | 0.31 |
| Lauric | 0.11 | 0.69 |
| Myristic | 0.65 | n.m. |
| Pentadecanoic | 0.30 | 0.42 |
| Palmitic | 14.81 | 30.50 |
| Palmitoleic | 0.26 | 0.26 |
| Heptadecanoic | 0.10 | 0.03 |
| Stearic | 1.40 | 1.45 |
| Elaidic | 0.09 | 0.09 |
| Oleic | 4.95 | 12.13 |
| Cis-Vaccenic | 1.15 | n.m. |
| Linoleic | 34.63 | 35.61 |
| Linolenic | 32.60 | 32.64 |
| Arachidic | 0.79 | n.d. |
| Eicosenoic | 0.79 | n.m. |
| Eicosadienoic | 0.26 | n.m. |
| Heneicosanoic | 0.06 | n.m. |
| Arachidonic | n.d. | 0.79 |
| Behenic | 1.12 | n.m. |
| Docosenoic | 0.16 | 0.38 |
| Erucic | 0.38 | n.m. |
| Docosadienoic | 0.12 | n.m. |
| Tricosanoic | 0.19 | n.m. |
| Docosatetraenoic | 0.61 | n.m. |
| Lignoceric | 0.82 | n.m. |
| Docosapentaenoic DPA | 0.09 | n.m. |
| Docosahexaenoic DHA | 1.16 | 1.16 |
| Nervonic | 0.70 | n.m. |
| Obtusilic | n.m. | 0.14 |
| Vaccenic | n.m. | 1.15 |
| Barley Rootlet Patents | |||
|---|---|---|---|
| Google Patent Number | Title | Area of Usage | Summary |
| US20070148317A1 [126] | Functional component-enriched barley malt rootlets and process for producing same | Food/cosmetic/medicinal ingredient | Process for the extraction of functional components from rootlets of barley which can be utilised as a raw material in food, cosmetic and medicinal formulations |
| US9326542B2 [127] | Process for producing food and beverage products from malt sprouts | Food and beverage ingredient | Technology for utilising malt sprouts of a specific particle size as a raw material in food or beverages |
| US5034325A [128] | 5’Phosphodiesterase enzyme preparation and method for its production | Enzyme preparation | An extraction method to obtain 5’phosphodiesterase from barley malt sprouts which is stable in storage |
| US3304238A [129] | Enzymatic material and method of preparing same | Enzyme preparation | Preparation of an aqueous enzyme medium from barley (and other grains) rootlets and stems capable of producing mainly 5’nucleotides |
| US3459637A [130] | Enzyme digestion of nucleic acids | Enzymatic production of 5’nucleotides | Method for enzymatically digesting RNA to primarily form 5’nucleotides using the aqueous extract of plant rootlets and stems (including barley) |
| US2925345A [131] | Preparation of an antioxidant from rootlets | Antioxidant extract | Method to limit auto-oxidation in a fatty material which involves the mixing of pulverised rootlets with the fatty material |
| US2694011A [132] | Poultry and swine feeds containing rootlets of germinated barley | Animal feed | Utilisation of barley rootlets within animal feeds for poultry and swine |
| US4613507A [133] | Malt-like flavour from cereal grain root cultures | Food and beverage flavour ingredient | Method of creating malt-like flavour ingredient from roots of grains (including barley) which can be used in food and beverage formulations |
| WO2019238928A1 [134] | Process for preparing a cereal based beverage with malt and malt rootlets | Beverage ingredient | Utilisation of barley rootlets in wort to obtain a malt-based beverage |
| US20200178580A1 [135] | Malt sprouts extracts and their uses | Extract | Use of malt sprouts as raw materials in extract production for various uses |
| WO2018104531A1 [136] | Compositions and methods for stimulating plant growth | Extract | Incorporation of malt sprouts in extract preparation and use as a bio stimulant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neylon, E.; Arendt, E.K.; Lynch, K.M.; Zannini, E.; Bazzoli, P.; Monin, T.; Sahin, A.W. Rootlets, a Malting By-Product with Great Potential. Fermentation 2020, 6, 117. https://doi.org/10.3390/fermentation6040117
Neylon E, Arendt EK, Lynch KM, Zannini E, Bazzoli P, Monin T, Sahin AW. Rootlets, a Malting By-Product with Great Potential. Fermentation. 2020; 6(4):117. https://doi.org/10.3390/fermentation6040117
Chicago/Turabian StyleNeylon, Emma, Elke K. Arendt, Kieran M. Lynch, Emanuele Zannini, Paolo Bazzoli, Thomas Monin, and Aylin W. Sahin. 2020. "Rootlets, a Malting By-Product with Great Potential" Fermentation 6, no. 4: 117. https://doi.org/10.3390/fermentation6040117
APA StyleNeylon, E., Arendt, E. K., Lynch, K. M., Zannini, E., Bazzoli, P., Monin, T., & Sahin, A. W. (2020). Rootlets, a Malting By-Product with Great Potential. Fermentation, 6(4), 117. https://doi.org/10.3390/fermentation6040117