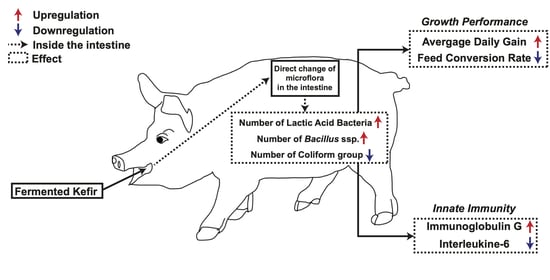
The Effect of Fermented Kefir as Functional Feed Additive in Post-Weaned Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Kefir
2.2. Determination of Vible Colonies in Fermented Kefir
2.3. Experimental Design of Animals
2.4. Analysis of Microorganisms in Fecal Sample of Pigs
2.5. Analysis of Porcine TNF-α, IL-6, IgA and IgG in Serum of Pigs
2.6. Statistical Analysis
3. Results
3.1. The Optimization of Culture Conditions for Kefir Fermentation
3.2. The Effect of Fermented Kefir on Microflora in the Fecal Samples of Post-Weaned Pigs
3.3. The Effect of Fermented Kefir on Innate Immunity of Post-Weaned Pigs
3.4. The Effect of Fermented Kefir on ADG and FCR as Growth Performance Measures in Post-Weaned Pigs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Modesto, M.; D’Aimmo, M.R.; Stefanini, I.; Trevisi, P.; De Filippi, S.; Casini, L.; Mazzoni, M.; Bosi, P.; Biavati, B. A Novel Strategy to Select Bifidobacterium Strains and Prebiotics as Natural Growth Promoters in Newly Weaned Pigs. Livest. Sci. 2009, 122, 248–258. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Yeung, S. Patterns of Antibiotic Use in Global Pig Production: A Systematic Review. Vet. Anim. Sci. 2019, 7, 100058. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial Food Animal Production, Antimicrobial Resistance, and Human Health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Leite, A.M.d.O.; Miguel, M.A.L.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.F. Microbiological, Technological and Therapeutic Properties of Kefir: A Natural Probiotic Beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, W.; Choi, C.-W.; Son, D.-B.; Jeong, B.-C.; Kim, H.-C.; Lee, H.; Suh, J.-W. Effects of Fermented Kefir as a Functional Feed Additive in Litopenaeus Vannamei Farming. Fermentation 2020, 6, 118. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows, version 22.0; IBM Corp.: Armonk, NY, USA, 2020.
- Allan, P.; Bilkei, G. Oregano Improves Reproductive Performance of Sows. Theriogenology 2005, 63, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Stein, H.H.; Kil, D.Y. Reduced Use of Antibiotic Growth Promoters in Diets Fed to Weanling Pigs: Dietary Tools, Part 2. Anim. Biotechnol. 2006, 17, 217–231. [Google Scholar] [CrossRef]
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of Phytogenic Products as Feed Additives for Swine and Poultry. J. Anim. Sci. 2008, 86, E140–E148. [Google Scholar] [CrossRef]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, N.W.; et al. Non-Antibiotic Feed Additives in Diets for Pigs: A Review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Zhang, D.; Liu, H.; Wang, S.; Shan, D.; Wang, Y. Assessment of Probiotic Properties of Lactobacillus Plantarum ZLP001 Isolated from Gastrointestinal Tract of Weaning Pigs. Afr. J. Biotechnol. 2011, 10, 11303–11308. [Google Scholar] [CrossRef] [Green Version]
- Dowarah, R.; Verma, A.K.; Agarwal, N. The Use of Lactobacillus as an Alternative of Antibiotic Growth Promoters in Pigs: A Review. Anim. Nutr. 2017, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bogere, P.; Choi, Y.J.; Heo, J. Probiotics as Alternatives to Antibiotics in Treating Post-Weaning Diarrhoea in Pigs: Review Paper. S. Afr. J. Anim. Sci. 2019, 49, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Hermann-Bank, M.L.; Skovgaard, K.; Stockmarr, A.; Strube, M.L.; Larsen, N.; Kongsted, H.; Ingerslev, H.C.; Mølbak, L.; Boye, M. Characterization of the Bacterial Gut Microbiota of Piglets Suffering from New Neonatal Porcine Diarrhoea. BMC Vet. Res. 2015, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, S.F.; Nyachoti, M. Using Probiotics to Improve Swine Gut Health and Nutrient Utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Gaudino Caputo, L.R.; Tavares Carvalho, J.C.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and Healing Activity of Kefir and Kefiran Extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Wang, S.; Yao, B.; Gao, H.; Zang, J.; Tao, S.; Zhang, S.; Huang, S.; He, B.; Wang, J. Combined Supplementation of Lactobacillus fermentum and Pediococcus acidilactici Promoted Growth Performance, Alleviated Inflammation, and Modulated Intestinal Microbiota in Weaned Pigs. BMC Vet. Res. 2019, 15, 239. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.Q.; Liu, H.Y.; Lai, T.; Ma, J.L.; Wang, J.F.; Zhu, Y.H. Evaluation of Lactobacillus rhamnosus GG Using an Escherichia coli K88 Model of Piglet Diarrhoea: Effects on Diarrhoea Incidence, Faecal Microflora and Immune Responses. Vet. Microbiol. 2010, 141, 142–148. [Google Scholar] [CrossRef]
- Frick, J.S.; Schenk, K.; Quitadamo, M.; Kahl, F.; Köberle, M.; Bohn, E.; Aepfelbacher, M.; Autenrieth, I.B. Lactobacillus fermentum Attenuates the proinflammatory Effect of Yersinia enterocolitica on Human Epithelial Cells. Inflamm. Bowel Dis. 2007, 13, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Candela, M.; Perna, F.; Carnevali, P.; Vitali, B.; Ciati, R.; Gionchetti, P.; Rizzello, F.; Campieri, M.; Brigidi, P. Interaction of Probiotic Lactobacillus and Bifidobacterium Strains with Human Intestinal Epithelial Cells: Adhesion Properties, Competition against Enteropathogens and Modulation of IL-8 Production. Int. J. Food Microbiol. 2008, 125, 286–292. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, F.; Wan, C.; Xiong, Y.; Shah, N.P.; Wei, H.; Tao, X. Evaluation of Probiotic Properties of Lactobacillus plantarum WLPL04 Isolated from Human Breast Milk. J. Dairy Sci. 2016, 99, 1736–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Component | Composition (%, w/v) |
|---|---|
| Glucose | 2 |
| Whey protein | 1 |
| Dipotassium phosphate | 0.02 |
| Yeast extract | 2 |
| Ammonium sulfate | 0.1 |
| MgSO4 | 0.01 |
| MnSO4 | 0.05 |
| Inoculation size | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, W.; Son, D.B.; Hong, J.; Jeong, D.; Kim, H.-C.; Lee, H.; Suh, J.-W. The Effect of Fermented Kefir as Functional Feed Additive in Post-Weaned Pigs. Fermentation 2021, 7, 23. https://doi.org/10.3390/fermentation7010023
Choi W, Son DB, Hong J, Jeong D, Kim H-C, Lee H, Suh J-W. The Effect of Fermented Kefir as Functional Feed Additive in Post-Weaned Pigs. Fermentation. 2021; 7(1):23. https://doi.org/10.3390/fermentation7010023
Chicago/Turabian StyleChoi, Woosik, Dang Bao Son, Jeongpyo Hong, Dabeen Jeong, Hee-Chang Kim, Hanki Lee, and Joo-Won Suh. 2021. "The Effect of Fermented Kefir as Functional Feed Additive in Post-Weaned Pigs" Fermentation 7, no. 1: 23. https://doi.org/10.3390/fermentation7010023
APA StyleChoi, W., Son, D. B., Hong, J., Jeong, D., Kim, H.-C., Lee, H., & Suh, J.-W. (2021). The Effect of Fermented Kefir as Functional Feed Additive in Post-Weaned Pigs. Fermentation, 7(1), 23. https://doi.org/10.3390/fermentation7010023