Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience
Abstract
:1. Microbial Resources and Food Fermentations: The ‘Oldest Biotechnologies’
2. Food Systems and Negative Externalities
3. Microbial Biotechnologies to Reduce Negative Externalities in Agri-Food Systems
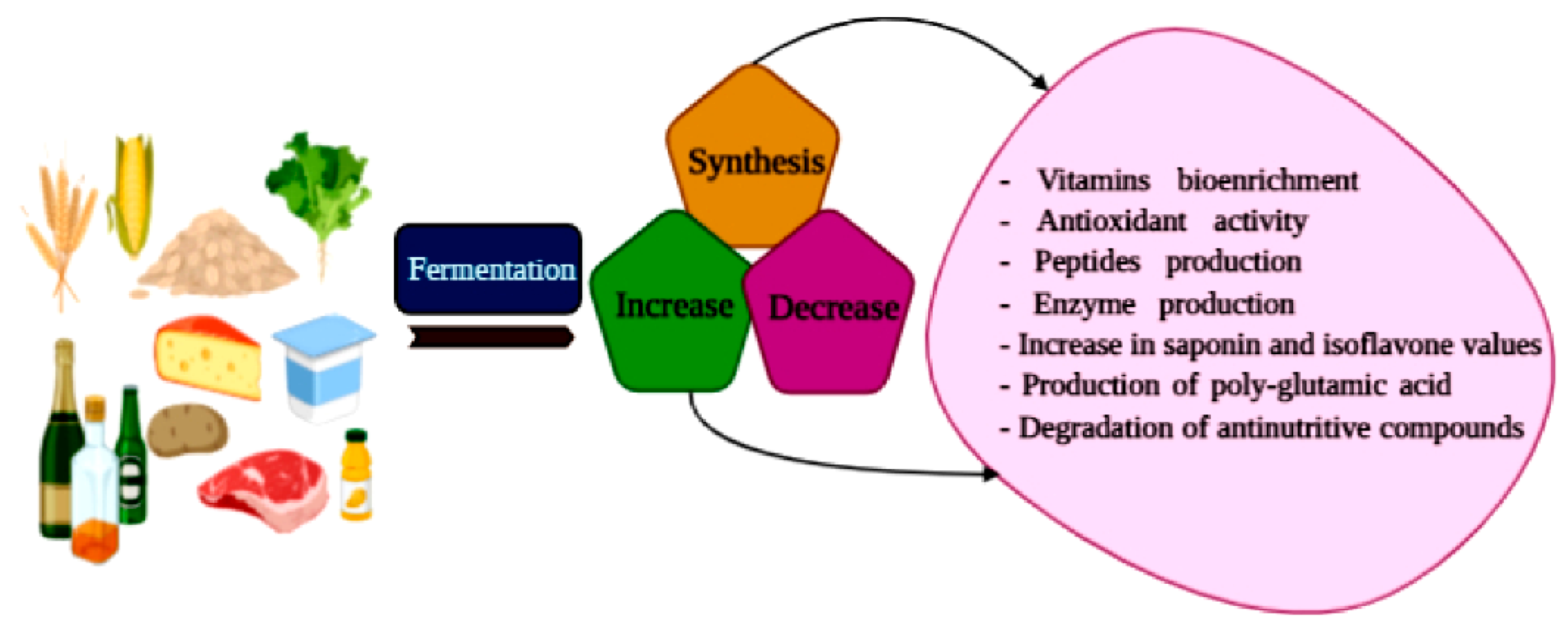
4. Tailored Food Fermentative Processes to Reduce Negative Externalities in Food Systems
5. Microbes as Mitigating Agents: A Common Denominator of R&D Activities in the Field
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Timmis, K.; de Vos, W.M.; Ramos, J.L.; Vlaeminck, S.E.; Prieto, A.; Danchin, A.; Verstraete, W.; de Lorenzo, V.; Lee, S.Y.; Brüssow, H.; et al. The Contribution of Microbial Biotechnology to Sustainable Development Goals. Microb. Biotechnol. 2017, 10, 984–987. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Espinosa, R.M. Microorganisms and Their Metabolic Capabilities in the Context of the Biogeochemical Nitrogen Cycle at Extreme Environments. Int. J. Mol. Sci. 2020, 21, 4228. [Google Scholar] [CrossRef]
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial Diversity in Extreme Marine Habitats and Their Biomolecules. Microorganisms 2017, 5, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented Foods: Definitions and Characteristics, Impact on the Gut Microbiota and Effects on Gastrointestinal Health and Disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef] [Green Version]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, R.F.; Hammes, W.P.; Habermeyer, M.; Engel, K.-H.; Knorr, D.; Eisenbrand, G. Microbial Food Cultures—Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG). Mol. Nutr. Food Res. 2011, 55, 654–662. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- De Simone, N.; Pace, B.; Grieco, F.; Chimienti, M.; Tyibilika, V.; Santoro, V.; Capozzi, V.; Colelli, G.; Spano, G.; Russo, P. Botrytis cinerea and Table Grapes: A Review of the Main Physical, Chemical, and Bio-Based Control Treatments in Post-Harvest. Foods 2020, 9, 1138. [Google Scholar] [CrossRef]
- Castellano, P.; Pérez Ibarreche, M.; Blanco Massani, M.; Fontana, C.; Vignolo, G.M. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Ruben, R.; Verhagen, J.; Plaisier, C. The Challenge of Food Systems Research: What Difference Does It Make? Sustainability 2019, 11, 171. [Google Scholar] [CrossRef] [Green Version]
- OECD Glossary of Statistical Terms—Externalities—OECD Definition. Available online: https://stats.oecd.org/glossary/detail.asp?ID=3215 (accessed on 31 December 2020).
- Ziolo, M.; Filipiak, B.Z.; Bąk, I.; Cheba, K.; Tîrca, D.M.; Novo-Corti, I. Finance, Sustainability and Negative Externalities. An Overview of the European Context. Sustainability 2019, 11, 4249. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Buzby, J.C.; Hyman, J. Total and per Capita Value of Food Loss in the United States. Food Policy 2012, 37, 561–570. [Google Scholar] [CrossRef]
- Shafiee-Jood, M.; Cai, X. Reducing Food Loss and Waste to Enhance Food Security and Environmental Sustainability. Environ. Sci. Technol. 2016, 50, 8432–8443. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, W.; Vrieze, J.D. Microbial Technology with Major Potentials for the Urgent Environmental Needs of the next Decades. Microb. Biotechnol. 2017, 10, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, P.; Berbegal, C.; De Ceglie, C.; Grieco, F.; Spano, G.; Capozzi, V. Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations. Fermentation 2019, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Matkovski, B.; Đokić, D.; Zekić, S.; Jurjević, Ž. Determining Food Security in Crisis Conditions: A Comparative Analysis of the Western Balkans and the EU. Sustainability 2020, 12, 9924. [Google Scholar] [CrossRef]
- de Lorenzo, V. Seven Microbial Bio-Processes to Help the Planet. Microb. Biotechnol. 2017, 10, 995–998. [Google Scholar] [CrossRef]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting Biological Nitrogen Fixation: A Route Towards a Sustainable Agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef]
- Alaswad, A.A.; Oehrle, N.W.; Krishnan, H.B. Classical Soybean (Glycine Max (L.) Merr) Symbionts, Sinorhizobium fredii USDA191 and Bradyrhizobium diazoefficiens USDA110, Reveal Contrasting Symbiotic Phenotype on Pigeon Pea (Cajanus Cajan (L.) Millsp). Int. J. Mol. Sci. 2019, 20, 1091. [Google Scholar] [CrossRef] [Green Version]
- Tagliavia, M.; Nicosia, A. Advanced Strategies for Food-Grade Protein Production: A New E. Coli/Lactic Acid Bacteria Shuttle Vector for Improved Cloning and Food-Grade Expression. Microorganisms 2019, 7, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajiboye, T.O.; Kuvarega, A.T.; Onwudiwe, D.C. Recent Strategies for Environmental Remediation of Organochlorine Pesticides. Appl. Sci. 2020, 10, 6286. [Google Scholar] [CrossRef]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Chaudhary, H.J. Microbial Biotechnology as an Emerging Industrial Wastewater Treatment Process for Arsenic Mitigation: A Critical Review. J. Clean. Prod. 2017, 151, 427–438. [Google Scholar] [CrossRef]
- Wang, R.; Wu, B.; Zheng, J.; Chen, H.; Rao, P.; Yan, L.; Chai, F. Biodegradation of Total Petroleum Hydrocarbons in Soil: Isolation and Characterization of Bacterial Strains from Oil Contaminated Soil. Appl. Sci. 2020, 10, 4173. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Malandrino, M.; Fabbri, D. Bioremediation Methods for the Recovery of Lead-Contaminated Soils: A Review. Appl. Sci. 2020, 10, 3528. [Google Scholar] [CrossRef]
- Fowler, S.J.; Smets, B.F. Microbial Biotechnologies for Potable Water Production. Microb. Biotechnol. 2017, 10, 1094–1097. [Google Scholar] [CrossRef] [Green Version]
- Byrne, J.M.; Kappler, A. Current and Future Microbiological Strategies to Remove as and Cd from Drinking Water. Microb. Biotechnol. 2017, 10, 1098–1101. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, P.; Schenk, P.M.; Wallenstein, M.D.; Singh, B.K. Tiny Microbes, Big Yields: Enhancing Food Crop Production with Biological Solutions. Microb. Biotechnol. 2017, 10, 999–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of Phage-Based Inhibition of Listeria monocytogenes in Food Products and Food Processing Environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef] [PubMed]
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowakiewicz, A.; Zięba, P.; Gnat, S.; Matuszewski, Ł. Last Call for Replacement of Antimicrobials in Animal Production: Modern Challenges, Opportunities, and Potential Solutions. Antibiotics 2020, 9, 883. [Google Scholar] [CrossRef]
- Goicoechea, N.; Antolín, M.C. Increased Nutritional Value in Food Crops. Microb. Biotechnol. 2017, 10, 1004–1007. [Google Scholar] [CrossRef] [Green Version]
- Pedone-Bonfim, M.V.L.; da Silva, F.S.B.; Maia, L.C. Production of Secondary Metabolites by Mycorrhizal Plants with Medicinal or Nutritional Potential. Acta Physiol. Plant. 2015, 37, 27. [Google Scholar] [CrossRef]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, Old Sustainable Food and Fashion Nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae Characterization for Consolidated and New Application in Human Food, Animal Feed and Nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Immerseel, F.V.; Eeckhaut, V.; Moore, R.J.; Choct, M.; Ducatelle, R. Beneficial Microbial Signals from Alternative Feed Ingredients: A Way to Improve Sustainability of Broiler Production? Microb. Biotechnol. 2017, 10, 1008–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbegal, C.; Spano, G.; Fragasso, M.; Grieco, F.; Russo, P.; Capozzi, V. Starter Cultures as Biocontrol Strategy to Prevent Brettanomyces bruxellensis Proliferation in Wine. Appl. Microbiol. Biotechnol. 2018, 102, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbegal, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of Autochthonous Yeasts and Bacteria in Order to Control Brettanomyces bruxellensis in Wine. Fermentation 2017, 3, 65. [Google Scholar] [CrossRef] [Green Version]
- Odedina, G.F.; Vongkamjan, K.; Voravuthikunchai, S.P. Potential Bio-Control Agent from Rhodomyrtus tomentosa against Listeria monocytogenes. Nutrients 2015, 7, 7451–7468. [Google Scholar] [CrossRef] [Green Version]
- Russo, P.; Fares, C.; Longo, A.; Spano, G.; Capozzi, V. Lactobacillus plantarum with Broad Antifungal Activity as a Protective Starter Culture for Bread Production. Foods 2017, 6, 110. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. Exploration of the Microbial Biodiversity Associated with North Apulian Sourdoughs and the Effect of the Increasing Number of Inoculated Lactic Acid Bacteria Strains on the Biocontrol against Fungal Spoilage. Fermentation 2019, 5, 97. [Google Scholar] [CrossRef] [Green Version]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-Based Biocontrol Solutions for Fruits and Vegetables: Recent Insight, Patents, and Innovative Trends. Recent Pat. Food Nutr. Agric. 2021. [Google Scholar] [CrossRef]
- Fang, Q.; Du, M.; Chen, J.; Liu, T.; Zheng, Y.; Liao, Z.; Zhong, Q.; Wang, L.; Fang, X.; Wang, J. Degradation and Detoxification of Aflatoxin B1 by Tea-Derived Aspergillus niger RAF106. Toxins 2020, 12, 777. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on Biological Degradation of Mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Spano, G.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Metabolites of Microbial Origin with an Impact on Health: Ochratoxin A and Biogenic Amines. Front. Microbiol. 2016, 7, 482. [Google Scholar] [CrossRef]
- Nardi, T. Microbial Resources as a Tool for Enhancing Sustainability in Winemaking. Microorganisms 2020, 8, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Zara, G.; Comitini, F.; Budroni, M.; Ciani, M. Yeast Killer Toxins: From Ecological Significance to Application. Crit. Rev. Biotechnol. 2019, 39, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Kim, Y.-T.; Ryu, S.; Lee, J.-H. Biocontrol and Rapid Detection of Food-Borne Pathogens Using Bacteriophages and Endolysins. Front. Microbiol. 2016, 7, 474. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V.; Spano, G. Functional Starters for Functional Yogurt. Foods 2015, 4, 15–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yépez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In Situ Riboflavin Fortification of Different Kefir-like Cereal-Based Beverages Using Selected Andean LAB Strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Fragasso, M.; de Vita, P.; Fiocco, D.; Spano, G. Biotechnology and Pasta-Making: Lactic Acid Bacteria as a New Driver of Innovation. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef] [Green Version]
- Petrova, P.; Petrov, K. Lactic Acid Fermentation of Cereals and Pseudocereals: Ancient Nutritional Biotechnologies with Modern Applications. Nutrients 2020, 12, 1118. [Google Scholar] [CrossRef] [Green Version]
- Krausova, G.; Kana, A.; Hyrslova, I.; Mrvikova, I.; Kavkova, M. Development of Selenized Lactic Acid Bacteria and Their Selenium Bioaccummulation Capacity. Fermentation 2020, 6, 91. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.P.; Russo, P.; Capozzi, V.; Rascón, A.; Felis, G.E.; Spano, G.; Fiocco, D. Combinations of Cereal β-Glucans and Probiotics Can Enhance the Anti-Inflammatory Activity on Host Cells by a Synergistic Effect. J. Funct. Foods 2016, 23, 12–23. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, A.; Mohedano, M.L.; López, P.; Spano, G.; Fiocco, D.; Russo, P.; Capozzi, V. In Situ β-Glucan Fortification of Cereal-Based Matrices by Pediococcus parvulus 2.6: Technological Aspects and Prebiotic Potential. Int. J. Mol. Sci. 2017, 18, 1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, P.W.; Paoli, M. The Contribution of Microbial Biotechnology to Sustainable Development Goals: Microbiome Therapies. Microb. Biotechnol. 2017, 10, 1066–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satokari, R. Modulation of Gut Microbiota for Health by Current and Next-Generation Probiotics. Nutrients 2019, 11, 1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, T.; Hansbro, P.M.; Sohal, S.S.; Dingle, P.; Eri, R.; Stanley, R. Microbiota Modulating Nutritional Approaches to Countering the Effects of Viral Respiratory Infections Including SARS-CoV-2 through Promoting Metabolic and Immune Fitness with Probiotics and Plant Bioactives. Microorganisms 2020, 8, 921. [Google Scholar] [CrossRef]
- Barathikannan, K.; Chelliah, R.; Rubab, M.; Daliri, E.B.-M.; Elahi, F.; Kim, D.-H.; Agastian, P.; Oh, S.-Y.; Oh, D.H. Gut Microbiome Modulation Based on Probiotic Application for Anti-Obesity: A Review on Efficacy and Validation. Microorganisms 2019, 7, 456. [Google Scholar] [CrossRef] [Green Version]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries—Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Mora-Villalobos, J.A.; Montero-Zamora, J.; Barboza, N.; Rojas-Garbanzo, C.; Usaga, J.; Redondo-Solano, M.; Schroedter, L.; Olszewska-Widdrat, A.; López-Gómez, J.P. Multi-Product Lactic Acid Bacteria Fermentations: A Review. Fermentation 2020, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.; Summa, D.; Semeraro, B.; Zappaterra, F.; Rugiero, I.; Tamburini, E. Fermentation as a Strategy for Bio-Transforming Waste into Resources: Lactic Acid Production from Agri-Food Residues. Fermentation 2021, 7, 3. [Google Scholar] [CrossRef]
- Krull, S.; Brock, S.; Prüße, U.; Kuenz, A. Hydrolyzed Agricultural Residues—Low-Cost Nutrient Sources for l-Lactic Acid Production. Fermentation 2020, 6, 97. [Google Scholar] [CrossRef]
- Alexandri, M.; López-Gómez, J.P.; Olszewska-Widdrat, A.; Venus, J. Valorising Agro-Industrial Wastes within the Circular Bioeconomy Concept: The Case of Defatted Rice Bran with Emphasis on Bioconversion Strategies. Fermentation 2020, 6, 42. [Google Scholar] [CrossRef]
- Kurniawan, A.; Kwon, S.Y.; Shin, J.-H.; Hur, J.; Cho, J. Acid Fermentation Process Combined with Post Denitrification for the Treatment of Primary Sludge and Wastewater with High Strength Nitrate. Water 2016, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Souza Filho, P.F.; Brancoli, P.; Bolton, K.; Zamani, A.; Taherzadeh, M.J. Techno-Economic and Life Cycle Assessment of Wastewater Management from Potato Starch Production: Present Status and Alternative Biotreatments. Fermentation 2017, 3, 56. [Google Scholar] [CrossRef] [Green Version]
- Harb, M.; Hong, P.-Y. Anaerobic Membrane Bioreactor Effluent Reuse: A Review of Microbial Safety Concerns. Fermentation 2017, 3, 39. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, V.; Fragasso, M.; Russo, P. Microbiological Safety and the Management of Microbial Resources in Artisanal Foods and Beverages: The Need for a Transdisciplinary Assessment to Conciliate Actual Trends and Risks Avoidance. Microorganisms 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capozzi, V.; Russo, P.; Spano, G. Microbial Information Regimen in EU Geographical Indications. World Pat. Inf. 2012, 34, 229–231. [Google Scholar] [CrossRef]
- Spano, G.; Capozzi, V. Food Microbial Biodiversity and “Microbes of Protected Origin”. Front. Microbiol. 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial Protein: Future Sustainable Food Supply Route with Low Environmental Footprint. Microb. Biotechnol. 2016, 9, 568–575. [Google Scholar] [CrossRef]
- Kårlund, A.; Gómez-Gallego, C.; Korhonen, J.; Palo-oja, O.-M.; El-Nezami, H.; Kolehmainen, M. Harnessing Microbes for Sustainable Development: Food Fermentation as a Tool for Improving the Nutritional Quality of Alternative Protein Sources. Nutrients 2020, 12, 1020. [Google Scholar] [CrossRef] [Green Version]
- Berbegal, C.; Borruso, L.; Fragasso, M.; Tufariello, M.; Russo, P.; Brusetti, L.; Spano, G.; Capozzi, V. A Metagenomic-Based Approach for the Characterization of Bacterial Diversity Associated with Spontaneous Malolactic Fermentations in Wine. Int. J. Mol. Sci. 2019, 20, 3980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, P.; Spano, G.; Capozzi, V. Safety evaluation of starter cultures. In Starter Cultures in Food Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 101–128. ISBN 978-1-118-93379-4. [Google Scholar]
- Stackebrandt, E.; Schüngel, M.; Martin, D.; Smith, D. The Microbial Resource Research Infrastructure MIRRI: Strength through Coordination. Microorganisms 2015, 3, 890–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vero, L.; Boniotti, M.B.; Budroni, M.; Buzzini, P.; Cassanelli, S.; Comunian, R.; Gullo, M.; Logrieco, A.F.; Mannazzu, I.; Musumeci, R.; et al. Preservation, Characterization and Exploitation of Microbial Biodiversity: The Perspective of the Italian Network of Culture Collections. Microorganisms 2019, 7, 685. [Google Scholar] [CrossRef] [Green Version]
- de Bono, E. Lateral Thinking; Creativity Step by Step; Penguin: London, UK, 1970. [Google Scholar]
- Topleva, S.A.; Prokopov, T.V. Integrated Business Model for Sustainability of Small and Medium-Sized Enterprises in the Food Industry: Creating Value Added through Ecodesign. Br. Food J. 2020, 122, 1463–1483. [Google Scholar] [CrossRef]
- Toussaint, M.; Cabanelas, P.; González-Alvarado, T.E. What about the Consumer Choice? The Influence of Social Sustainability on Consumer’s Purchasing Behavior in the Food Value Chain. Eur. Res. Manag. Bus. Econ. 2021, 27, 100134. [Google Scholar] [CrossRef]
- Demain, A.L. Microbial Biotechnology. Trends Biotechnol. 2000, 18, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Pakseresht, A.; McFadden, B.R.; Lagerkvist, C.J. Consumer Acceptance of Food Biotechnology Based on Policy Context and Upstream Acceptance: Evidence from an Artefactual Field Experiment. Eur. Rev. Agric. Econ. 2017, 44, 757–780. [Google Scholar] [CrossRef] [Green Version]
- McFadden, J.R.; Huffman, W.E. Consumer Valuation of Information about Food Safety Achieved Using Biotechnology: Evidence from New Potato Products. Food Policy 2017, 69, 82–96. [Google Scholar] [CrossRef] [Green Version]
- Roman, M.; Varga, H.; Cvijanovic, V.; Reid, A. Quadruple Helix Models for Sustainable Regional Innovation: Engaging and Facilitating Civil Society Participation. Economies 2020, 8, 48. [Google Scholar] [CrossRef]
- Leydesdorff, L. The Triple Helix, Quadruple Helix, …, and an N-Tuple of Helices: Explanatory Models for Analyzing the Knowledge-Based Economy? J. Knowl. Econ. 2012, 3, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Tobi, R.C.A.; Harris, F.; Rana, R.; Brown, K.A.; Quaife, M.; Green, R. Sustainable Diet Dimensions. Comparing Consumer Preference for Nutrition, Environmental and Social Responsibility Food Labelling: A Systematic Review. Sustainability 2019, 11, 6575. [Google Scholar] [CrossRef] [Green Version]
- Timmis, K.; de Lorenzo, V.; Verstraete, W.; Ramos, J.L.; Danchin, A.; Brüssow, H.; Singh, B.K.; Timmis, J.K. The Contribution of Microbial Biotechnology to Economic Growth and Employment Creation. Microb. Biotechnol. 2017, 10, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Steenson, L.R.; Meng, J. Bacteria in Food and Beverage Production. In The Prokaryotes: Applied Bacteriology and Biotechnology; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 241–256. ISBN 978-3-642-31331-8. [Google Scholar]
- Timmis, K.; Cavicchioli, R.; Garcia, J.L.; Nogales, B.; Chavarría, M.; Stein, L.; McGenity, T.J.; Webster, N.; Singh, B.K.; Handelsman, J.; et al. The Urgent Need for Microbiology Literacy in Society. Environ. Microbiol. 2019, 21, 1513–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Eilam, E.; Prasad, V.; Widdop Quinton, H. Climate Change Education: Mapping the Nature of Climate Change, the Content Knowledge and Examination of Enactment in Upper Secondary Victorian Curriculum. Sustainability 2020, 12, 591. [Google Scholar] [CrossRef] [Green Version]
- Capozzi, V.; Spano, G.; Fiocco, D. Transdisciplinarity and Microbiology Education. J. Microbiol. Biol. Educ. JMBE 2012, 13, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Organisation | Initiatives | Website |
|---|---|---|
| United Nations General Assembly | 2030 Agenda, Sustainable Development Goals (SDGs) | https://www.un.org/sustainabledevelopment/, accessed on 13 December 2020 |
| Food and Agriculture Organization (FAO) | Food and agriculture in the 2030 Agenda for Sustainable Development | http://www.fao.org/sustainable-development-goals/en/, accessed on 10 January 2021 |
| European Commission | Food 2030 | https://ec.europa.eu/info/research-and-innovation/research-area/food-systems/food-2030_en, accessed on 14 December 2020 |
| United States Environmental Protection Agency | Sustainable Management of Food | https://www.epa.gov/sustainable-management-food, accessed on 21 December 2020 |
| United Kingdom Government | Food Industry Sustainability Strategy (FISS) | https://www.gov.uk/government/publications/food-industry-sustainability-strategy-fiss, accessed on 20 November 2020 |
| Microbial Biotechnologies to Counteract/Prevent Negative Externalities | Ref. |
|---|---|
| Biological fixation of nitrogen | [22,23,24] |
| Alternative nitrogen sources to be used as feed or food | [19] |
| Microbial protein production | [19,25] |
| Microbial biotechnology for CO2 capture | [19,22] |
| Microbial biotechnology to limit diffuse methane emissions | [19] |
| Microbial-based bioconversion of pollutants in water | [19,26,27] |
| Microbial-based bioremediation of soil | [28,29] |
| Microbial biotechnologies for potable water production | [30,31] |
| Biodegradation of endocrine disruptors from trophic chains | [22] |
| Optimisation of microbial biofertilizers/biostimulants | [32,33] |
| Optimisation of microbial biopesticides | [32,34] |
| Bioprotection and alternatives to antibiotics | [35,36,37] |
| Rhizospheric microorganisms for improving the nutrient quality of crops | [38,39] |
| Beneficial plant-microbe interactions to breed ‘microbe-optimized plants’ | [32] |
| Microalgae and new application in food, feed, and nutraceuticals chains | [40,41] |
| Microbial-based tailored solutions for sustainable feeding regimen | [42] |
| Fermentative Processes to Counteract/Prevent Negative Externalities | Ref. |
|---|---|
| Microbial-based biocontrol of microbial pathogens and spoilers | [43,44,45,46,47,48] |
| Microbial-based degradation of chemical contaminants | [49,50,51] |
| Bioprotection and alternatives to chemical preservatives | [52,53,54] |
| Microbial production of nutrients | [55,56,57] |
| Microbes to improve nutrient bioavailability | [10,58,59,60] |
| Synbiotic approaches to improve human health and well-being | [61,62,63,64] |
| Microbial biotechnology and microbiome therapies | [65,66,67,68] |
| Microbial resources and strategies to save energy during fermentation | [52,69] |
| Fermentative valorization of foods by-products | [52,70,71] |
| Fermentative valorization of foods wastes | [72,73,74] |
| Microbial-based valorization of wastewater associated with food systems | [75,76,77] |
| Strategies to preserve microbial diversity associated with food fermentation | [78,79,80] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, V.; Fragasso, M.; Bimbo, F. Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience. Fermentation 2021, 7, 54. https://doi.org/10.3390/fermentation7020054
Capozzi V, Fragasso M, Bimbo F. Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience. Fermentation. 2021; 7(2):54. https://doi.org/10.3390/fermentation7020054
Chicago/Turabian StyleCapozzi, Vittorio, Mariagiovanna Fragasso, and Francesco Bimbo. 2021. "Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience" Fermentation 7, no. 2: 54. https://doi.org/10.3390/fermentation7020054
APA StyleCapozzi, V., Fragasso, M., & Bimbo, F. (2021). Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience. Fermentation, 7(2), 54. https://doi.org/10.3390/fermentation7020054







