Genetic, Physiological, and Industrial Aspects of the Fructophilic Non-Saccharomyces Yeast Species, Starmerella bacillaris
Abstract
:1. Introduction
2. Genetic Aspects
2.1. Starmerella/Wickerhamiella Clade
2.2. S. bacillaris Genome
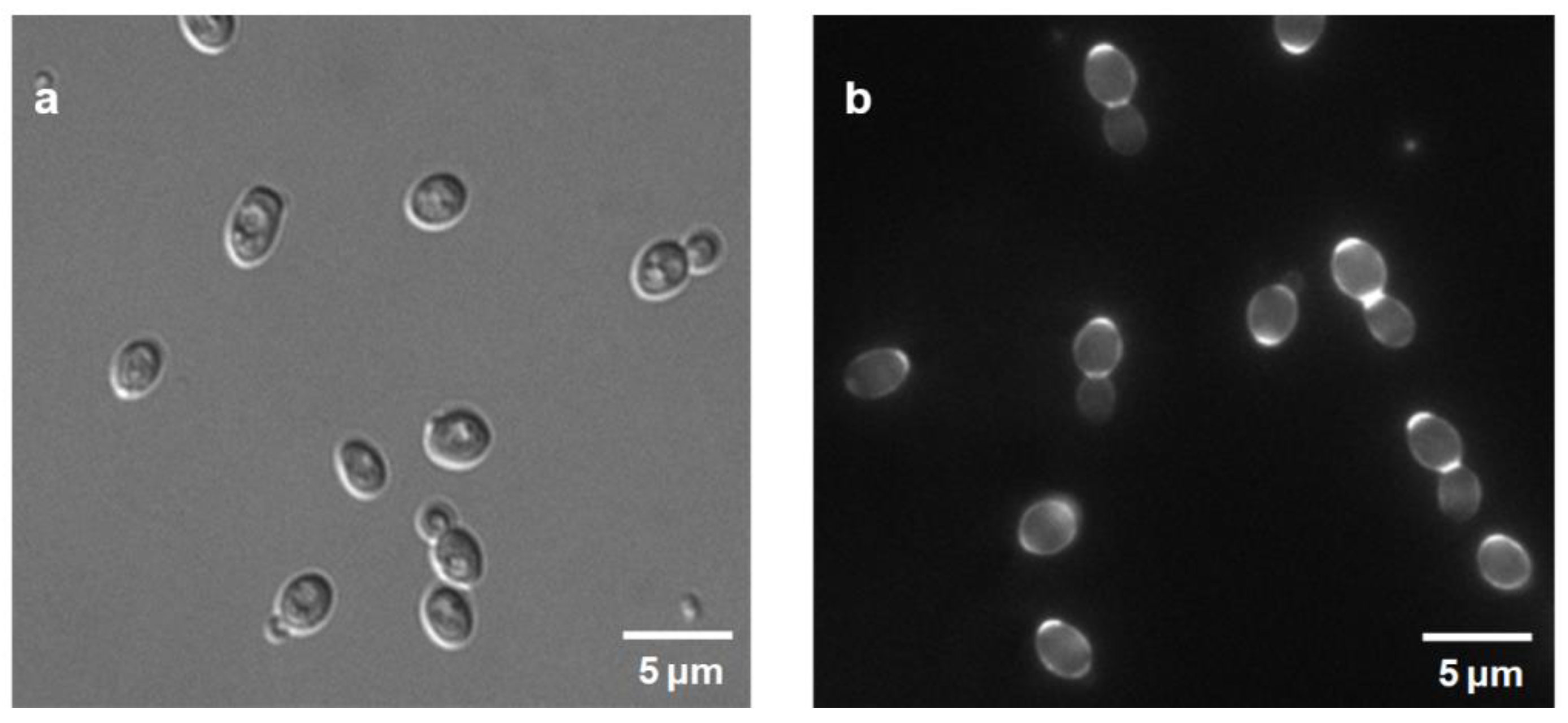
2.3. S. bacillaris Ploidy and Microsatellite Loci
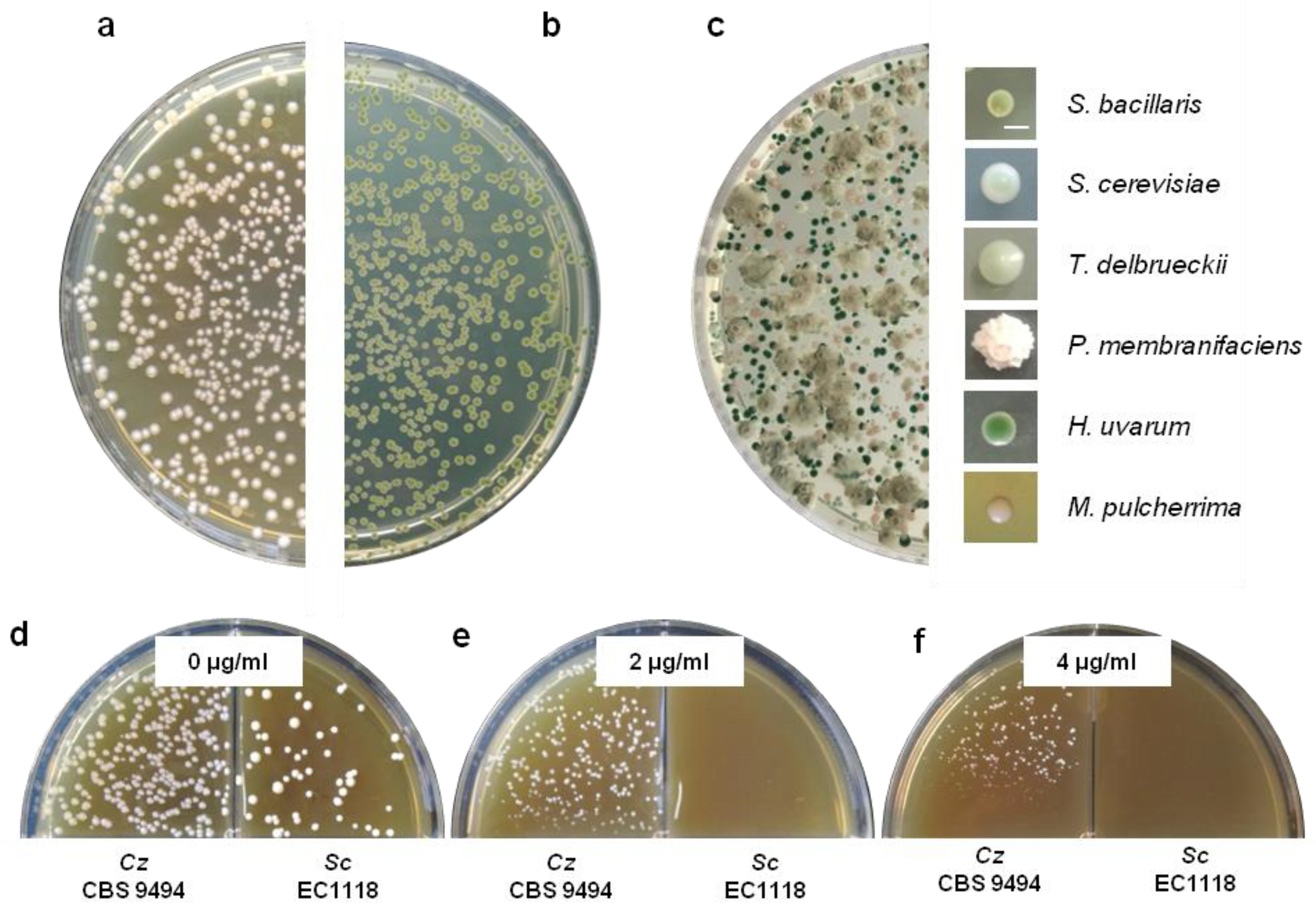
2.4. S. bacillaris Species and Strain Identification
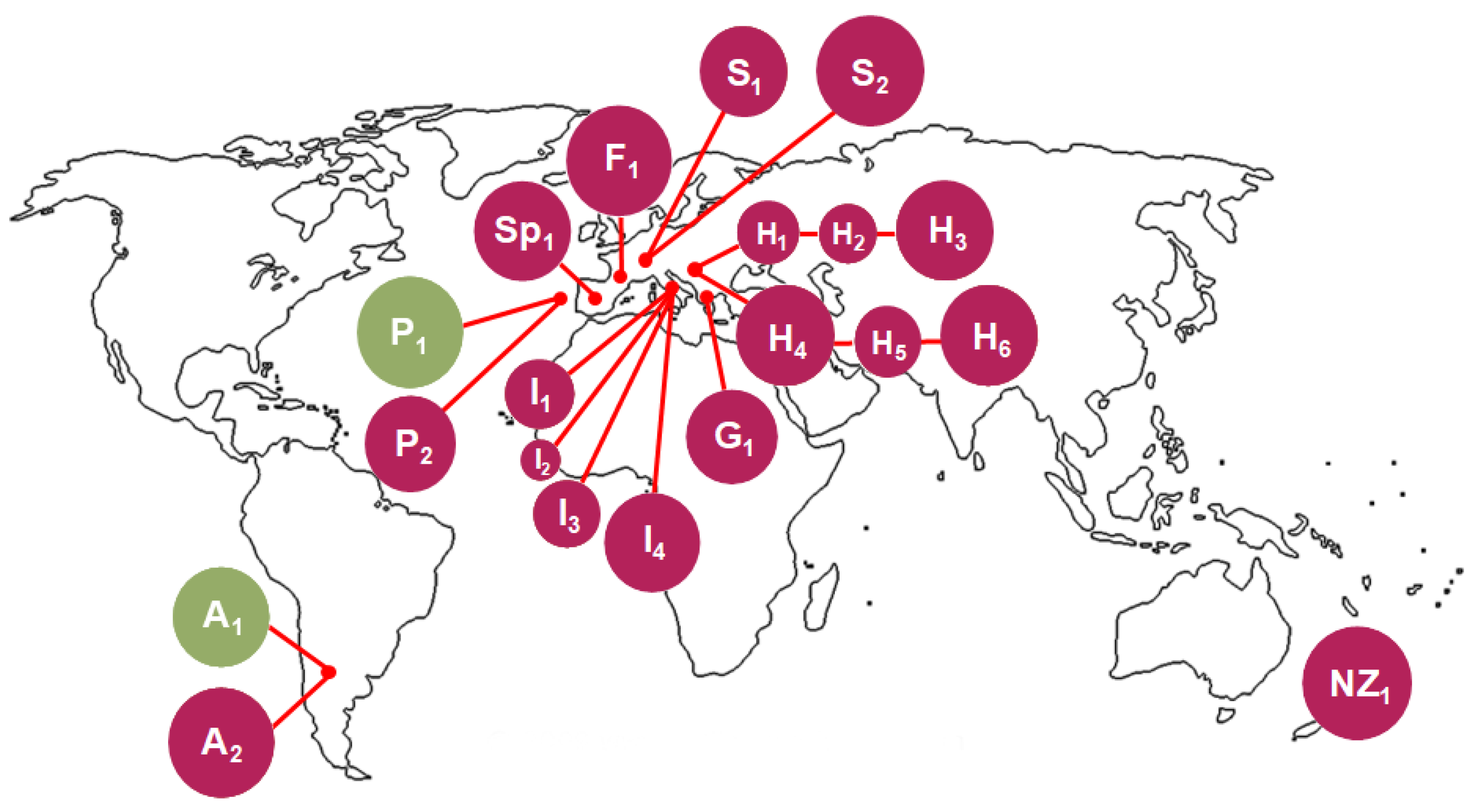
2.5. S. bacillaris Ecology
3. S. bacillaris Physiology
4. Industrial Application of S. bacillaris
4.1. S. bacillaris as a Co-Starter in Grape Must Fermentations
4.2. S. bacillaris and the Reduction in Ethanol Levels in Wines
4.3. Chemical Complexity of S. bacillaris and S. cerevisiae Fermented Beverages
4.4. Biocontrol Potential of S. bacillaris
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duarte, F.L.; Pimentel, N.H.; Teixeira, A.; Fonseca, A. Saccharomyces bacillaris is not a synonym of Candida stellata: Reinstatement as Starmerella bacillaris comb. nov. Antonie Van Leeuwenhoek 2012, 102, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.L.; Miot-Sertier, C.; Salin, F.; Sipiczki, M.; Bely, M. Draft Genome Sequence of the Starmerella bacillaris (syn., Candida zemplinina) Type Strain CBS 9494. Microbiol. Resour. Announc. 2018, 7, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csoma, H.; Sipiczki, M. Taxonomic reclassification of Candida stellata strains reveals frequent occurrence of Candida zemplinina in wine fermentation. FEMS Yeast Res. 2008, 8, 328–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drumonde-Neves, J.; Franco-Duarte, R.; Lima, T.; Schuller, D.; Pais, C. Yeast biodiversity in vineyard environments is increased by human intervention. PLoS ONE 2016, 11, e0160579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drumonde-Neves, J.; Franco-Duarte, R.; Lima, T.; Schuller, D.; Pais, C. Association between grape yeast communities and the vineyard ecosystems. PLoS ONE 2017, 12, e0169883. [Google Scholar] [CrossRef]
- Tofalo, R.; Schirone, M.; Torriani, S.; Rantsiou, K.; Cocolin, L.; Perpetuini, G.; Suzzi, G. Diversity of Candida zemplinina strains from grapes and Italian wines. Food Microbiol. 2012, 29, 18–26. [Google Scholar] [CrossRef]
- Magyar, I.; Tóth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Sipiczki, M. Candida zemplinina sp. nov., an osmotolerant and psychrotolerant yeast that ferments sweet botrytized wines. Int. J. Syst. Evol. Microbiol. 2003, 53, 2079–2083. [Google Scholar] [CrossRef] [Green Version]
- Tofalo, R.; Chaves-López, C.; Di Fabio, F.; Schirone, M.; Felis, G.E.; Torriani, S.; Paparella, A.; Suzzi, G. Molecular identification and osmotolerant profile of wine yeasts that ferment a high sugar grape must. Int. J. Food Microbiol. 2009, 130, 179–187. [Google Scholar] [CrossRef]
- Raymond Eder, M.L.; Reynoso, C.; Lauret, S.C.; Rosa, A.L. Isolation and identification of the indigenous yeast population during spontaneous fermentation of Isabella (Vitis labrusca L.) grape must. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond Eder, M.L.; Conti, F.; Rosa, A.L. Differences between indigenous yeast populations in spontaneously fermenting musts from V. vinifera L. and V. labrusca L. Grapes harvested in the same geographic location. Front. Microbiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gaensly, F.; Agustini, B.C.; da Silva, G.A.; Picheth, G.; Bonfim, T.M.B. Autochthonous yeasts with β-glucosidase activity increase resveratrol concentration during the alcoholic fermentation of Vitis labrusca grape must. J. Funct. Foods 2015, 19, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Stamps, J.A.; Yang, L.H.; Morales, V.M.; Boundy-Mills, K.L. Drosophila regulate yeast density and increase yeast community similarity in a natural substrate. PLoS ONE 2012, 7, e42238. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.S.; Hønholt, S.; Tano-Debrah, K.; Jespersen, L. Yeast populations associated with Ghanaian cocoa fermentations analysed using denaturing gradient gel electrophoresis (DGGE). Yeast 2005, 22, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Pfliegler, W.P.; Horváth, E.; Kállai, Z.; Sipiczki, M. Diversity of Candida zemplinina isolates inferred from RAPD, micro/minisatellite and physiological analysis. Microbiol. Res. 2014, 169, 402–410. [Google Scholar] [CrossRef]
- Binati, R.L.; Innocente, G.; Gatto, V.; Celebrin, A.; Polo, M.; Felis, G.E.; Torriani, S. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res. Int. 2019, 122, 432–442. [Google Scholar] [CrossRef]
- Mills, D.A.; Johannsen, E.A.; Cocolin, L. Yeast diversity and persistence in botrytis-affected wine fermentations. Appl. Environ. Microbiol. 2002, 68, 4884–4893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 1998, 73, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The interaction between Saccharomyces cerevisiae and non-Saccharomyces yeast during alcoholic fermentation is species and strain specific. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: Physiological and molecular characterizations. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Magyar, I.; Nyitrai-Sárdy, D.; Leskó, A.; Pomázi, A.; Kállay, M. Anaerobic organic acid metabolism of Candida zemplinina in comparison with Saccharomyces wine yeasts. Int. J. Food Microbiol. 2014, 178, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biotechnol. 2016, 100, 5515–5526. [Google Scholar] [CrossRef]
- Lemos Junior, W.J.F.; Bovo, B.; Nadai, C.; Crosato, G.; Carlot, M.; Favaron, F.; Giacomini, A.; Corich, V. Biocontrol ability and action mechanism of Starmerella bacillaris (Synonym Candida zemplinina) isolated from wine musts against gray mold disease agent Botrytis cinerea on grape and their effects on alcoholic fermentation. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Junior, W.J.; Binati, R.L.; Felis, G.E.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Volatile organic compounds from Starmerella bacillaris to control gray mold on apples and modulate cider aroma profile. Food Microbiol. 2020, 89. [Google Scholar] [CrossRef]
- Gonçalves, P.; Gonçalves, C.; Brito, P.H.; Sampaio, J.P. The Wickerhamiella/Starmerella clade—A treasure trove for the study of the evolution of yeast metabolism. Yeast 2020, 37, 313–320. [Google Scholar] [CrossRef]
- Lemos Junior, W.J.; da Silva Duarte, V.; Treu, L.; Campanaro, S.; Nadai, C.; Giacomini, A.; Corich, V. Whole genome comparison of two Starmerella bacillaris strains with other wine yeasts uncovers genes involved in modulating important winemaking traits. FEMS Yeast Res. 2018, 18, foy069. [Google Scholar] [CrossRef]
- Gonçalves, C.; Wisecaver, J.H.; Kominek, J.; Salema-Oom, M.; Leandro, M.J.; Shen, X.X.; Opulente, D.A.; Zhou, X.; Peris, D.; Kurtzman, C.P.; et al. Evidence for loss and reacquisition of alcoholic fermentation in a fructophilic yeast lineage. eLife 2018, 7, e33034. [Google Scholar] [CrossRef]
- Gonçalves, C.; Ferreira, C.; Gonçalves, L.G.; Turner, D.L.; Leandro, M.J.; Salema-Oom, M.; Santos, H.; Gonçalves, P. A New Pathway for Mannitol Metabolism in Yeasts Suggests a Link to the Evolution of Alcoholic Fermentation. Front. Microbiol. 2019, 10, 2510. [Google Scholar] [CrossRef]
- Gonçalves, C.; Gonçalves, P. Multilayered horizontal operon transfers from bacteria reconstruct a thiamine salvage pathway in yeasts. Proc. Natl. Acad. Sci. USA 2019, 116, 22219–22228. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Species identification and comparative molecular and physiological analysis of Candida zemplinina and Candida stellata. J. Basic Microbiol. 2004, 44, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Junior, W.J.; Treu, L.; da Silva Duarte, V.; Campanaro, S.; Nadai, C.; Giacomini, A.; Corich, V. Draft genome sequence of the yeast Starmerella bacillaris (syn., Candida zemplinina) FRI751 isolated from fermenting must of dried Raboso grapes. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Lemos Junior, W.J.F.; Treu, L.; da Silva Duarte, V.; Carlot, M.; Nadai, C.; Campanaro, S.; Giacomini, A.; Corich, V. Whole-genome sequence of Starmerella bacillaris PAS13, a nonconventional enological yeast with antifungal activity. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.-J.; Park, H.J.; Lee, S.H.; Jeong, H.; Bae, J.-H.; Sung, B.H.; Choi, I.-G.; Sohn, J.-H. Draft genome sequence of an acid-tolerant yeast, Candida zemplinina NP2, a potential producer of organic acids. Genome Announc. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond Eder, M.L.; Conti, F.; Bely, M.; Masneuf-Pomarède, I.; Albertin, W.; Rosa, A.L. Vitis species, vintage, and alcoholic fermentation do not drive population structure in Starmerella bacillaris (synonym Candida zemplinina) species. Yeast 2019, 36, 411–420. [Google Scholar] [CrossRef]
- Masneuf-Pomarède, I.; Juquin, E.; Miot-Sertier, C.; Renault, P.; Laizet, Y.; Salin, F.; Alexandre, H.; Capozzi, V.; Cocolin, L.; Colonna-Ceccaldi, B.; et al. The yeast Starmerella bacillaris (synonym Candida zemplinina) shows high genetic diversity in winemaking environments. FEMS Yeast Res. 2015, 15, fov045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raymond Eder, M.L.; Rosa, A.L. Non-tandem repeat polymorphisms at microsatellite loci in wine yeast species. Mol. Genet. Genom. 2020, 295, 685–693. [Google Scholar] [CrossRef]
- Legras, J.L.; Ruh, O.; Merdinoglu, D.; Karst, F. Selection of hypervariable microsatellite loci for the characterization of Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2005, 102, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Bely, M.; Masneuf-Pomarede, I.; Jiranek, V.; Albertin, W. The evolution of Lachancea thermotolerans is driven by geographical determination, anthropisation and flux between different ecosystems. PLoS ONE 2017, 12, e0184652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masneuf-Pomarede, I.; Salin, F.; Börlin, M.; Coton, E.; Coton, M.; Le Jeune, C.; Legras, J.L. Microsatellite analysis of Saccharomyces uvarum diversity. FEMS Yeast Res. 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertin, W.; Chasseriaud, L.; Comte, G.; Panfili, A.; Delcamp, A.; Salin, F.; Marullo, P.; Bely, M. Winemaking and bioprocesses strongly shaped the genetic diversity of the ubiquitous yeast Torulaspora delbrueckii. PLoS ONE 2014, 9, e94246. [Google Scholar] [CrossRef]
- Albertin, W.; Panfili, A.; Miot-Sertier, C.; Goulielmakis, A.; Delcamp, A.; Salin, F.; Lonvaud-Funel, A.; Curtin, C.D.; Masneuf-Pomarede, I. Development of microsatellite markers for the rapid and reliable genotyping of Brettanomyces bruxellensis at strain level. Food Microbiol. 2014, 42, 188–195. [Google Scholar] [CrossRef]
- García, M.; Esteve-Zarzoso, B.; Cabellos, J.M.; Arroyo, T. Advances in the study of Candida stellata. Fermentation 2018, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Csoma, H.; Ács-Szabó, L.; Papp, L.A.; Sipiczki, M. Application of different markers and data-analysis tools to the examination of biodiversity can lead to different results: A case study with Starmerella bacillaris (synonym Candida zemplinina) strains. FEMS Yeast Res. 2018. [Google Scholar] [CrossRef]
- Wang, C.; Esteve-Zarzoso, B.; Mas, A. Monitoring of Saccharomyces cerevisiae, Hanseniaspora uvarum, and Starmerella bacillaris (synonym Candida zemplinina) populations during alcoholic fermentation by fluorescence in situ hybridization. Int. J. Food Microbiol. 2014, 191, 1–9. [Google Scholar] [CrossRef]
- Heard, G.M.; Fleet, G.H. Evaluation of selective media for enumeration of yeasts during wine fermentation. J. Appl. Bacteriol. 1986, 60, 477–481. [Google Scholar] [CrossRef]
- Chasseriaud, L.; Coulon, J.; Marullo, P.; Albertin, W.; Bely, M. New oenological practice to promote non-Saccharomyces species of interest: Saturating grape juice with carbon dioxide. Appl. Microbiol. Biotechnol. 2018, 102, 3779–3791. [Google Scholar] [CrossRef]
- Mestre Furlani, M.V.; Maturano, Y.P.; Combina, M.; Mercado, L.A.; Toro, M.E.; Vazquez, F. Selection of non-Saccharomyces yeasts to be used in grape musts with high alcoholic potential: A strategy to obtain wines with reduced ethanol content. FEMS Yeast Res. 2017, 17, fox010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos Junior, W.J.F.; Nadai, C.; Crepalde, L.T.; de Oliveira, V.S.; Dupas de Matos, A.; Giacomini, A.; Corich, V. Potential use of Starmerella bacillaris as fermentation starter for the production of low-alcohol beverages obtained from unripe grapes. Int. J. Food Microbiol. 2019, 303, 1–8. [Google Scholar] [CrossRef]
- Gonçalves, C.; Coelho, M.A.; Salema-Oom, M.; Gonçalves, P. Stepwise functional evolution in a fungal sugar transporter family. Mol. Biol. Evol. 2016, 33, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.J.; Cabral, S.; Prista, C.; Loureiro-Dias, M.C.; Sychrová, H. The high-capacity specific fructose facilitator ZrFfz1 is essential for the fructophilic behavior of Zygosaccharomyces rouxii CBS 732T. Eukaryot. Cell 2014, 13, 1371–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flikweert, M.T.; Van Der Zanden, L.; Janssen, W.M.; Steensma, H.Y.; Van Dijken, J.P.; Pronk, J.T. Pyruvate decarboxylase: An indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 1996, 12, 247–257. [Google Scholar] [CrossRef]
- Flikweert, M.T.; de Swaaf, M.; van Dijken, J.P.; Pronk, J.T. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1999, 174, 73–79. [Google Scholar] [CrossRef]
- Barnett, J.A. Beginnings of microbiology and biochemistry: The contribution of yeast research. Microbiology 2003, 149, 557–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bely, M.; Sicard, D.; Bely, M.; Renault, P.; Silva, T.; Masneuf-Pomarède, I.; Albertin, W. Non-conventional yeasts and alcohol level reduction. BIO Web Conf. 2013, 33. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Giacosa, S.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profiles and chromatic characteristics of red wines produced with Starmerella bacillaris and Saccharomyces cerevisiae. Food Res. Int. 2018, 109, 298–309. [Google Scholar] [CrossRef]
- Englezos, V.; Pollon, M.; Rantsiou, K.; Ortiz-Julien, A.; Botto, R.; Río Segade, S.; Giacosa, S.; Rolle, L.; Cocolin, L. Saccharomyces cerevisiae-Starmerella bacillaris strains interaction modulates chemical and volatile profile in red wine mixed fermentations. Food Res. Int. 2019, 122, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Nisiotou, A.; Sgouros, G.; Mallouchos, A.; Nisiotis, C.S.; Michaelidis, C.; Tassou, C.; Banilas, G. The use of indigenous Saccharomyces cerevisiae and Starmerella bacillaris strains as a tool to create chemical complexity in local wines. Food Res. Int. 2018, 111, 498–508. [Google Scholar] [CrossRef]
- Suzzi, G.; Schirone, M.; Sergi, M.; Marianella, R.M.; Fasoli, G.; Aguzzi, I.; Tofalo, R. Multistarter from organic viticulture for red wine Montepulciano d’Abruzzo production. Front. Microbiol. 2012, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Englezos, V.; Cocolin, L.; Rantsiou, K.; Ortiz-Julien, A.; Bloem, A.; Dequin, S.; Camarasa, C. Specific phenotypic traits of Starmerella bacillaris related to nitrogen source consumption and central carbon metabolite production during wine fermentation. Appl. Environ. Microbiol. 2018, 84, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Tofalo, R.; Patrignani, F.; Lanciotti, R.; Perpetuini, G.; Schirone, M.; Di Gianvito, P.; Pizzoni, D.; Arfelli, G.; Suzzi, G. Aroma profile of montepulciano d’abruzzo wine fermented by single and co-culture starters of autochthonous Saccharomyces and non-Saccharomyces yeasts. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.; Boido, E.; Ramey, D. Yeasts for Low Input Winemaking: Microbial Terroir and Flavor Differentiation, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 111. [Google Scholar]
- Carrau, F.; Gaggero, C.; Aguilar, P.S. Yeast diversity and native vigor for flavor phenotypes. Trends Biotechnol. 2015, 33, 148–154. [Google Scholar] [CrossRef]
- Tempère, S.; Marchal, A.; Barbe, J.C.; Bely, M.; Masneuf-Pomarede, I.; Marullo, P.; Albertin, W. The complexity of wine: Clarifying the role of microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 3995–4007. [Google Scholar] [CrossRef]
- Albertin, W.; Zimmer, A.; Miot-Sertier, C.; Bernard, M.; Coulon, J.; Moine, V.; Colonna-Ceccaldi, B.; Bely, M.; Marullo, P.; Masneuf-Pomarede, I. Combined effect of the Saccharomyces cerevisiae lag phase and the non-Saccharomyces consortium to enhance wine fruitiness and complexity. Appl. Microbiol. Biotechnol. 2017, 101, 7603–7620. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.B.; Jeon, J.Y.; Park, H.D. Development of air-blast dried non-Saccharomyces yeast starter for improving quality of Korean persimmon wine and apple cider. Int. J. Food Microbiol. 2019, 290, 193–204. [Google Scholar] [CrossRef]
- Bagheri, B.; Zambelli, P.; Vigentini, I.; Bauer, F.F.; Setati, M.E. Investigating the effect of selected non-Saccharomyces species on wine ecosystem function and major volatiles. Front. Bioeng. Biotechnol. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- De Filippis, F.; Aponte, M.; Piombino, P.; Lisanti, M.T.; Moio, L.; Ercolini, D.; Blaiotta, G. Influence of microbial communities on the chemical and sensory features of Falanghina sweet passito wines. Food Res. Int. 2019, 120, 740–747. [Google Scholar] [CrossRef]
- Englezos, V.; Cachón, D.C.; Rantsiou, K.; Blanco, P.; Petrozziello, M.; Pollon, M.; Giacosa, S.; Río Segade, S.; Rolle, L.; Cocolin, L. Effect of mixed species alcoholic fermentation on growth and malolactic activity of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 7687–7702. [Google Scholar] [CrossRef]
- Horváth, B.O.; Sárdy, D.N.; Kellner, N.; Magyar, I. Effects of high sugar content on fermentation dynamics and some metabolites of wine-related yeast species Saccharomyces cerevisiae, S. uvarum and Starmerella bacillaris. Food Technol. Biotechnol. 2020, 58, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, N.; Araque, I.; Ortís, A.; Thornes, G.; Bautista-Gallego, J.; Bordons, A.; Reguant, C. Evaluating the effect of using non-Saccharomyces on Oenococcus oeni and wine malolactic fermentation. Food Res. Int. 2020, 138, 109779. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Giacosa, S.; Río Segade, S.; Rolle, L.; Cocolin, L. Cell-to-cell contact mechanism modulates Starmerella bacillaris death in mixed culture fermentations with Saccharomyces cerevisiae. Int. J. Food Microbiol. 2019, 289, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Valera, M.J.; Morcillo-Parra, M.Á.; Zagórska, I.; Mas, A.; Beltran, G.; Torija, M.J. Effects of melatonin and tryptophol addition on fermentations carried out by Saccharomyces cerevisiae and non-Saccharomyces yeast species under different nitrogen conditions. Int. J. Food Microbiol. 2019, 289, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Cravero, F.; Torchio, F.; Rantsiou, K.; Ortiz-Julien, A.; Lambri, M.; Gerbi, V.; Rolle, L.; Cocolin, L. Oxygen availability and strain combination modulate yeast growth dynamics in mixed culture fermentations of grape must with Starmerella bacillaris and Saccharomyces cerevisiae. Food Microbiol. 2018, 69, 179–188. [Google Scholar] [CrossRef]
- Caballero, A.; Segura, A. The quest for lower alcoholic wines. Microb. Biotechnol. 2017, 10, 238–241. [Google Scholar] [CrossRef]
- Andorrà, I.; Berradre, M.; Rozès, N.; Mas, A.; Guillamón, J.M.; Esteve-Zarzoso, B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Fracassetti, D.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Tufariello, M.; Renna, R.; Tristezza, M.; Taurino, M.; Palombi, L.; Capozzi, V.; Rizzello, C.G.; Grieco, F. New insights into the oenological significance of Candida zemplinina: Impact of selected autochthonous strains on the volatile profile of Apulian wines. Microorganisms 2020, 8, 628. [Google Scholar] [CrossRef]
- Pinto, L.; Malfeito-Ferreira, M.; Quintieri, L.; Silva, A.C.; Baruzzi, F. Growth and metabolite production of a grape sour rot yeast-bacterium consortium on different carbon sources. Int. J. Food Microbiol. 2019, 296, 65–74. [Google Scholar] [CrossRef]
- Prendes, L.P.; Merín, M.G.; Fontana, A.R.; Bottini, R.A.; Ramirez, M.L.; Morata de Ambrosini, V.I. Isolation, identification and selection of antagonistic yeast against Alternaria alternata infection and tenuazonic acid production in wine grapes from Argentina. Int. J. Food Microbiol. 2018, 266, 14–20. [Google Scholar] [CrossRef] [PubMed]
| Restriction Enzyme | Fragment Size (bp) | ||
|---|---|---|---|
| S. bacillaris | C. stellata | S. bombicola | |
| Uncut | 460 | 468 | 467 |
| CfoI | 56 + 103 + 105 + 196 | 56 + 200 + 212 | 39 + 56 + 172 + 200 |
| HaeIII | 460 | 468 | 54 + 87 + 138 + 326 |
| HinfI | 225 + 235 | 229 + 239 | 8 + 227 + 232 |
| DraI | 309 + 115 + 36 | 119 + 349 | 143 + 324 |
| MboI | 22 + 145 + 293 | 22 + 135 + 149 + 162 | 22 + 137 + 138 + 170 |
| Fermentation | S. cerevisiae | S. bacillaris | Inoculation Protocol | Metabolites (Ʃ) | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | Fatty Esters | Fatty Acids | Terpenes and C13 Norisoprenoids | lactones | Acetate Esters | Other | |||||
| C. sauvignon 1 | Uvaferm BC | FC54 | S-24 | ≈ | ↓ | ↓ | ≈ | - | ↓ | ≈ | [58] |
| S-48 | ≈ | ↓ | ↓ | ≈ | - | ↓ | ≈ | ||||
| Merlot 1 | S-24 | ≈ | ↓ | ↓ | ≈ | - | ↓ | ≈ | |||
| S-48 | ≈ | ↓ | ↓ | ≈ | - | ↓ | ↓ | ||||
| Pinot noir 1 | S-24 | ≈ | ↓ | ↓ | ≈ | - | ↓ | ≈ | |||
| S-48 | ↓ | ↓ | ↓ | ≈ | - | ↓ | ↓ | ||||
| Shiraz 1 | S-24 | ≈ | ↓ | ↓ | ↑ | - | ↓ | ≈ | |||
| S-48 | ≈ | ↓ | ↓ | ↑ | - | ↓ | ≈ | ||||
| Chardonnay 1 | S-48 | ≈ | ↓ | ↓ | ↑ | - | ↓ | ≈ | [83] | ||
| Muscat 1 | S-48 | ≈ | ↓ | ↓ | ↓ | - | ↓ | ≈ | |||
| Riesling 1 | S-48 | ≈ | ≈ | ↓ | ≈ | - | ↓ | ≈ | |||
| Sauvignon blanc 1 | S-48 | ↑ | ↑ | ↓ | ↑ | - | ↓ | ≈ | |||
| Golden delicious 2 | EC1118 | CHIAR4 | S-48 | ↓ | ↓ | ↓ | ↑ | - | ↓ | ↑ | [27] |
| PECO4 | S-48 | ↓ | ↓ | ↓ | ↑ | - | ↓ | ↑ | |||
| Sauvignon blanc 1 | PB2023 | MCR-9 | Co | ≈ | ↓ | ≈ | ↑ | ≈ | ↓ | ↓ | [63] |
| K&M 1,3 | SacPK7 | StbPK9 | Co | ≈ | ≈ | ≈ | ≈ | - | ≈ | ≈ | [60] |
| S-23 | ≈ | ≈ | ≈ | ≈ | - | ≈ | ≈ | ||||
| M 4 | SRS1 | STS12 | Co | ↓ | ≈ | ≈ | ≈ | - | - | ≈ | [65] |
| Macabeo | QA23 | CszB4 | Co | ↑ | ↑ | ↓ | - | - | ≈ | - | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raymond Eder, M.L.; Rosa, A.L. Genetic, Physiological, and Industrial Aspects of the Fructophilic Non-Saccharomyces Yeast Species, Starmerella bacillaris. Fermentation 2021, 7, 87. https://doi.org/10.3390/fermentation7020087
Raymond Eder ML, Rosa AL. Genetic, Physiological, and Industrial Aspects of the Fructophilic Non-Saccharomyces Yeast Species, Starmerella bacillaris. Fermentation. 2021; 7(2):87. https://doi.org/10.3390/fermentation7020087
Chicago/Turabian StyleRaymond Eder, María Laura, and Alberto Luis Rosa. 2021. "Genetic, Physiological, and Industrial Aspects of the Fructophilic Non-Saccharomyces Yeast Species, Starmerella bacillaris" Fermentation 7, no. 2: 87. https://doi.org/10.3390/fermentation7020087
APA StyleRaymond Eder, M. L., & Rosa, A. L. (2021). Genetic, Physiological, and Industrial Aspects of the Fructophilic Non-Saccharomyces Yeast Species, Starmerella bacillaris. Fermentation, 7(2), 87. https://doi.org/10.3390/fermentation7020087






