Digital Smoke Taint Detection in Pinot Grigio Wines Using an E-Nose and Machine Learning Algorithms Following Treatment with Activated Carbon and a Cleaving Enzyme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wine Samples and Smoke Taint Amelioration Treatments
2.2. Chemical Measurements
2.3. Electronic Nose
2.4. GC-MS Analysis of Volatile Aroma Compounds
2.5. Statistical Analysis and Machine Learning Modeling
3. Results
3.1. Chemical Measurements
3.2. GC-MS Analysis
3.3. Electronic Nose
3.4. Multivariate Data Analysis
3.5. Artificial Neural Network Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardo-Garcia, A.; Wilkinson, K.; Culbert, J.; Lloyd, N.; Alonso, G.; Salinas, M.R. Accumulation of guaiacol glycoconjugates in fruit, leaves and shoots of vitis vinifera cv. Monastrell following foliar applications of guaiacol or oak extract to grapevines. Food Chem. 2017, 217, 782–789. [Google Scholar] [CrossRef]
- van der Hulst, L.; Munguia, P.; Culbert, J.A.; Ford, C.M.; Burton, R.A.; Wilkinson, K.L. Accumulation of volatile phenol glycoconjugates in grapes following grapevine exposure to smoke and potential mitigation of smoke taint by foliar application of kaolin. Planta 2019, 249, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Kennison, K.; Wilkinson, K.; Pollnitz, A.; Williams, H.; Gibberd, M. Effect of timing and duration of grapevine exposure to smoke on the composition and sensory properties of wine. Aust. J. Grape Wine Res. 2009, 15, 228–237. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Torrico, D.D.; Pang, A.; Fuentes, S. Detection of smoke-derived compounds from bushfires in cabernet-sauvignon grapes, must, and wine using near-infrared spectroscopy and machine learning algorithms. OENO One 2020, 54, 1105–1119. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Baldock, G.; Pardon, K.; Jeffery, D.; Herderich, M. Investigation into the formation of guaiacol conjugates in berries and leaves of grapevine vitis vinifera l. Cv. Cabernet sauvignon using stable isotope tracers combined with hplc-ms and ms/ms analysis. J. Agric. Food Chem. 2010, 58, 2076–2081. [Google Scholar] [CrossRef]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.; Hayasaka, Y.; Fudge, A.; Wilkinson, K. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- Ristic, R.; van der Hulst, L.; Capone, D.; Wilkinson, K. Impact of bottle aging on smoke-tainted wines from different grape cultivars. J. Agric. Food Chem. 2017, 65, 4146–4152. [Google Scholar] [CrossRef]
- Kennison, K.; Gibberd, M.; Pollnitz, A.; Wilkinson, K. Smoke-derived taint in wine: The release of smoke-derived volatile phenols during fermentation of merlot juice following grapevine exposure to smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef]
- Mayr, C.M.; Parker, M.; Baldock, G.A.; Black, C.A.; Pardon, K.H.; Williamson, P.O.; Herderich, M.J.; Francis, I.L. Determination of the importance of in-mouth release of volatile phenol glycoconjugates to the flavor of smoke-tainted wines. J. Agric. Food Chem. 2014, 62, 2327–2336. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Summerson, V.; Gonzalez Viejo, C.; Pang, A.; Torrico, D.D.; Fuentes, S. Review of the effects of grapevine smoke exposure and technologies to assess smoke contamination and taint in grapes and wine. Beverages 2021, 7, 7. [Google Scholar] [CrossRef]
- Szeto, C.; Ristic, R.; Capone, D.; Puglisi, C.; Pagay, V.; Culbert, J.; Jiang, W.; Herderich, M.; Tuke, J.; Wilkinson, K. Uptake and glycosylation of smoke-derived volatile phenols by cabernet sauvignon grapes and their subsequent fate during winemaking. Molecules 2020, 25, 3720. [Google Scholar] [CrossRef]
- Favell, J.W.; Noestheden, M.; Lyons, S.-M.; Zandberg, W.F. Development and evaluation of a vineyard-based strategy to mitigate smoke-taint in wine grapes. J. Agric. Food Chem. 2019, 67. [Google Scholar] [CrossRef]
- Fudge, A.; Ristic, R.; Wollan, D.; Wilkinson, K. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Fudge, A.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- Kelly, D.; Zerihun, A. The effect of phenol composition on the sensory profile of smoke affected wines. Molecules 2015, 20, 9536–9549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, P.; Qian, Y.; He, F.; Li, H.; Qian, M. Comparative characterization of aroma compounds in merlot wine by lichrolut-en-based aroma extract dilution analysis and odor activity value. Chemosens. Percept. 2017, 10, 149–160. [Google Scholar] [CrossRef]
- Fuentes, S.; Summerson, V.; Gonzalez Viejo, C.; Tongson, E.; Lipovetzky, N.; Wilkinson, K.L.; Szeto, C.; Unnithan, R.R. Assessment of smoke contamination in grapevine berries and taint in wines due to bushfires using a low-cost e-nose and an artificial intelligence approach. Sensors 2020, 20, 5108. [Google Scholar] [CrossRef]
- Wilson, A.D. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Chen, Z.; Liu, D.; He, Z.; Wu, J. Constructing an e-nose using metal-ion-induced assembly of graphene oxide for diagnosis of lung cancer via exhaled breath. ACS Appl. Mater. Interfaces 2020, 12, 17713–17724. [Google Scholar] [CrossRef]
- Lekha, S.; Suchetha, M. Recent advancements and future prospects on e-nose sensors technology and machine learning approaches for non-invasive diabetes diagnosis: A review. IEEE Rev. Biomed. Eng. 2020, 14. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; da Silva, A.J.; de Andrade Lima, L.L.; Ferreira, T.A. Wine quality rapid detection using a compact electronic nose system: Application focused on spoilage thresholds by acetic acid. LWT 2019, 108, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Mu, F.; Gu, Y.; Zhang, J.; Zhang, L. Milk source identification and milk quality estimation using an electronic nose and machine learning techniques. Sensors 2020, 20, 4238. [Google Scholar] [CrossRef] [PubMed]
- Nimsuk, N. Improvement of accuracy in beer classification using transient features for electronic nose technology. J. Food Meas. Charact. 2019, 13, 656–662. [Google Scholar] [CrossRef]
- Xu, J.; Liu, K.; Zhang, C. Electronic nose for volatile organic compounds analysis in rice aging. Trends Food Sci. Technol. 2021, 109. [Google Scholar] [CrossRef]
- Zarezadeh, M.R.; Aboonajmi, M.; Varnamkhasti, M.G.; Azarikia, F. Olive oil classification and fraud detection using e-nose and ultrasonic system. Food Anal. Methods 2021, 1–12. [Google Scholar] [CrossRef]
- Antolini, A.; Forniti, R.; Modesti, M.; Bellincontro, A.; Catelli, C.; Mencarelli, F. First application of ozone postharvest fumigation to remove smoke taint from grapes. Ozone Sci. Eng. 2020, 1–9. [Google Scholar] [CrossRef]
- Viejo, C.G.; Fuentes, S.; Godbole, A.; Widdicombe, B.; Unnithan, R.R. Development of a low-cost e-nose to assess aroma profiles: An artificial intelligence application to assess beer quality. Sens. Actuators B Chem. 2020, 308, 127688. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Tongson, E.; Fuentes, S. Integrating a low-cost electronic nose and machine learning modelling to assess coffee aroma profile and intensity. Sensors 2021, 21, 2016. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.D.; Godbole, A.; Dunshea, F.R. Chemical characterization of aromas in beer and their effect on consumers liking. Food Chem. 2019, 293, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Development of artificial neural network models to assess beer acceptability based on sensory properties using a robotic pourer: A comparative model approach to achieve an artificial intelligence system. Beverages 2019, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Issa-Issa, H.; Noguera-Artiaga, L.; Sendra, E.; Pérez-López, A.J.; Burló, F.; Carbonell-Barrachina, Á.A.; López-Lluch, D. Volatile composition, sensory profile, and consumers’ acceptance of fondillón. J. Food Qual. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Ayestarán, B.; Martínez-Lapuente, L.; Guadalupe, Z.; Canals, C.; Adell, E.; Vilanova, M. Effect of the winemaking process on the volatile composition and aromatic profile of tempranillo blanco wines. Food Chem. 2019, 276, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Viejo, C.G.; Fuentes, S. Beer aroma and quality traits assessment using artificial intelligence. Fermentation 2020, 6, 56. [Google Scholar] [CrossRef]
- Filipe-Ribeiro, L.; Milheiro, J.; Matos, C.C.; Cosme, F.; Nunes, F.M. Reduction of 4-ethylphenol and 4-ethylguaiacol in red wine by activated carbons with different physicochemical characteristics: Impact on wine quality. Food Chem. 2017, 229, 242–251. [Google Scholar] [CrossRef]
- Krstic, M.; Johnson, D.; Herderich, M. Review of smoke taint in wine: Smoke-derived volatile phenols and their glycosidic metabolites in grapes and vines as biomarkers for smoke exposure and their role in the sensory perception of smoke taint. Aust. J. Grape Wine Res. 2015, 21, 537–553. [Google Scholar] [CrossRef]
- Mirabelli-Montan, Y.A.; Marangon, M.; Graça, A.; Mayr Marangon, C.M.; Wilkinson, K.L. Techniques for mitigating the effects of smoke taint while maintaining quality in wine production: A review. Molecules 2021, 26, 1672. [Google Scholar] [CrossRef]
- Han, F.; Zhang, D.; Aheto, J.H.; Feng, F.; Duan, T. Integration of a low-cost electronic nose and a voltammetric electronic tongue for red wines identification. Food Sci. Nutr. 2020, 8, 4330–4339. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.; Harbertson, J.F.; Greenwood, D.R.; Villas-Bôas, S.G.; Fiehn, O.; Heymann, H. Reference samples guide variable selection for correlation of wine sensory and volatile profiling data. Food Chem. 2018, 267, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Summerson, V.; Gonzalez Viejo, C.; Szeto, C.; Wilkinson, K.L.; Torrico, D.D.; Pang, A.; Bei, R.D.; Fuentes, S. Classification of smoke contaminated cabernet sauvignon berries and leaves based on chemical fingerprinting and machine learning algorithms. Sensors 2020, 20, 5099. [Google Scholar] [CrossRef] [PubMed]
- Herderich, M.; Krstic, M. Mitigation of Climate Change Impacts on the National Wine Industry by Reduction in Losses from Controlled Burns and Wildfires and Improvement in Public Land Management. Available online: https://www.awri.com.au/research_and_development/2017-2025-rde-plan-projects/project-3-4-3/ (accessed on 5 June 2021).
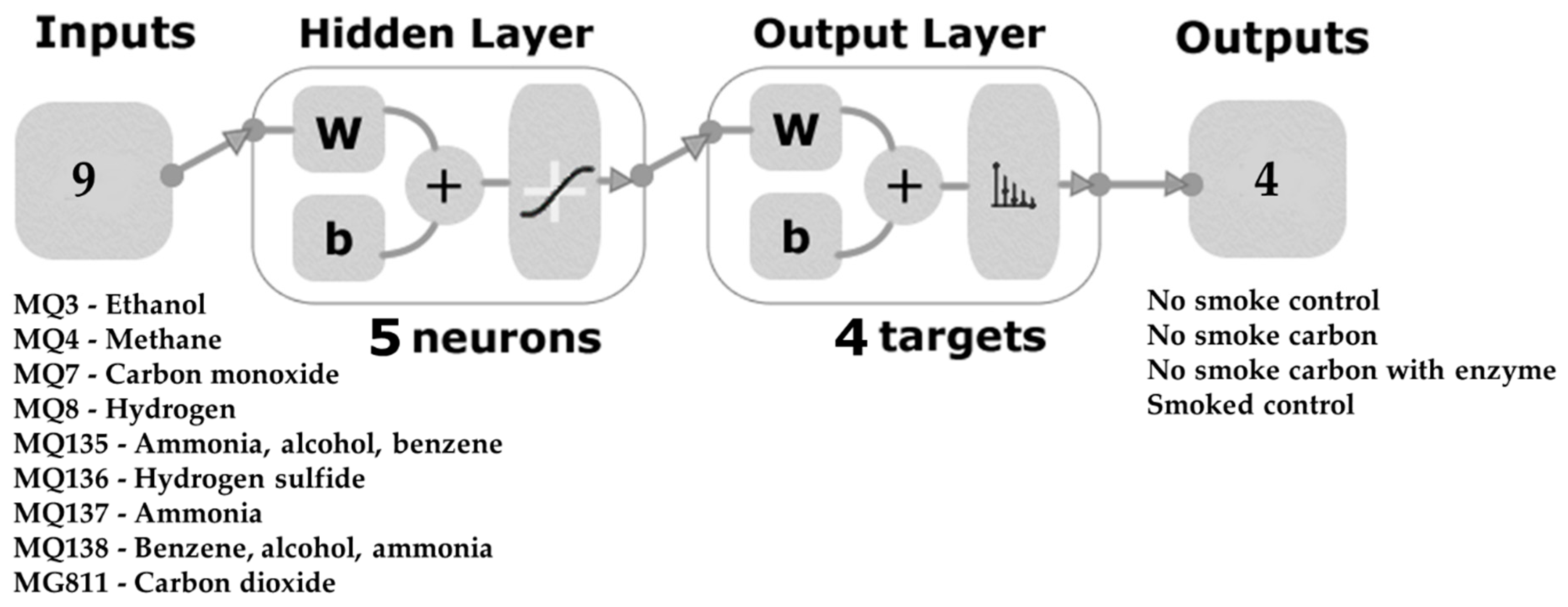
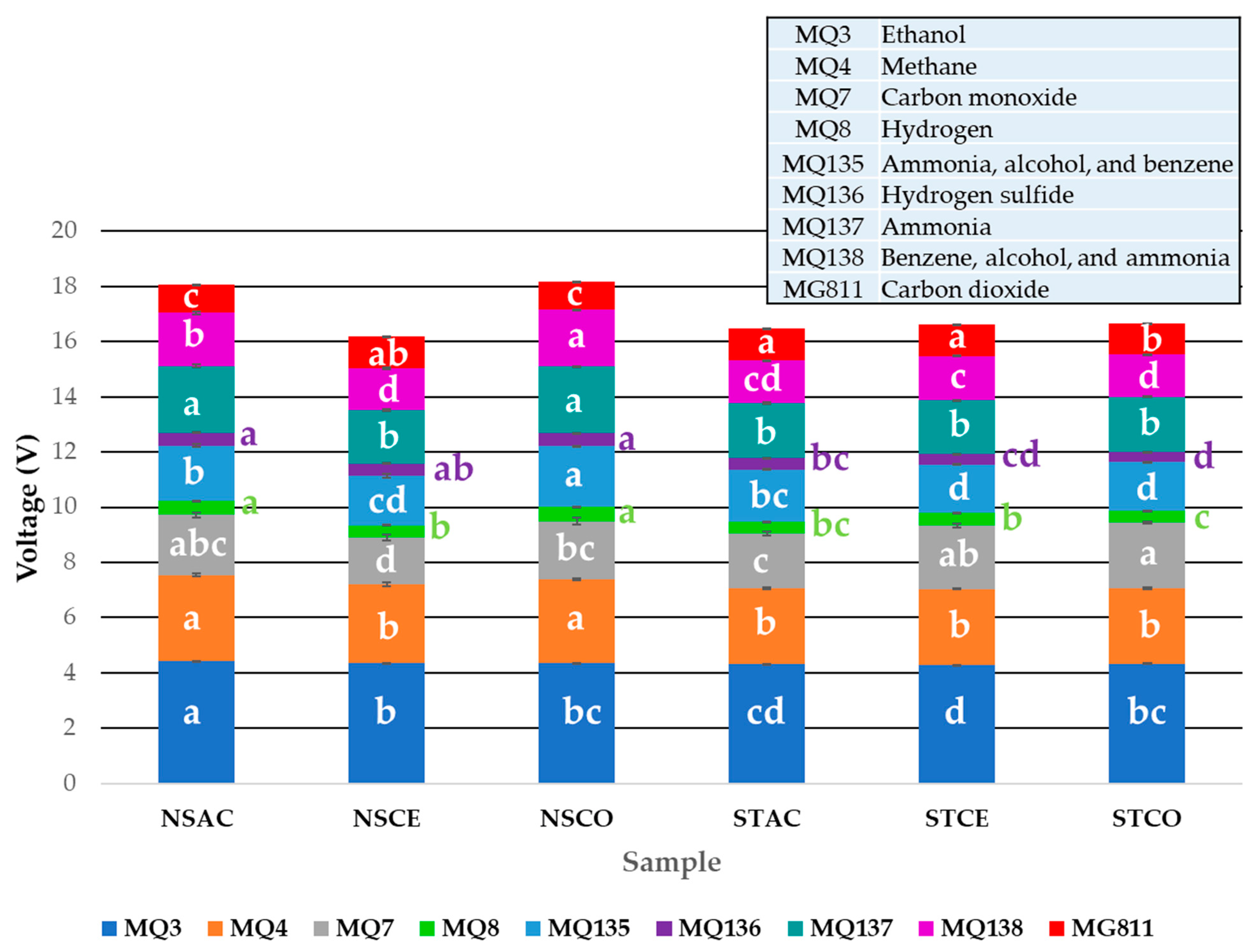
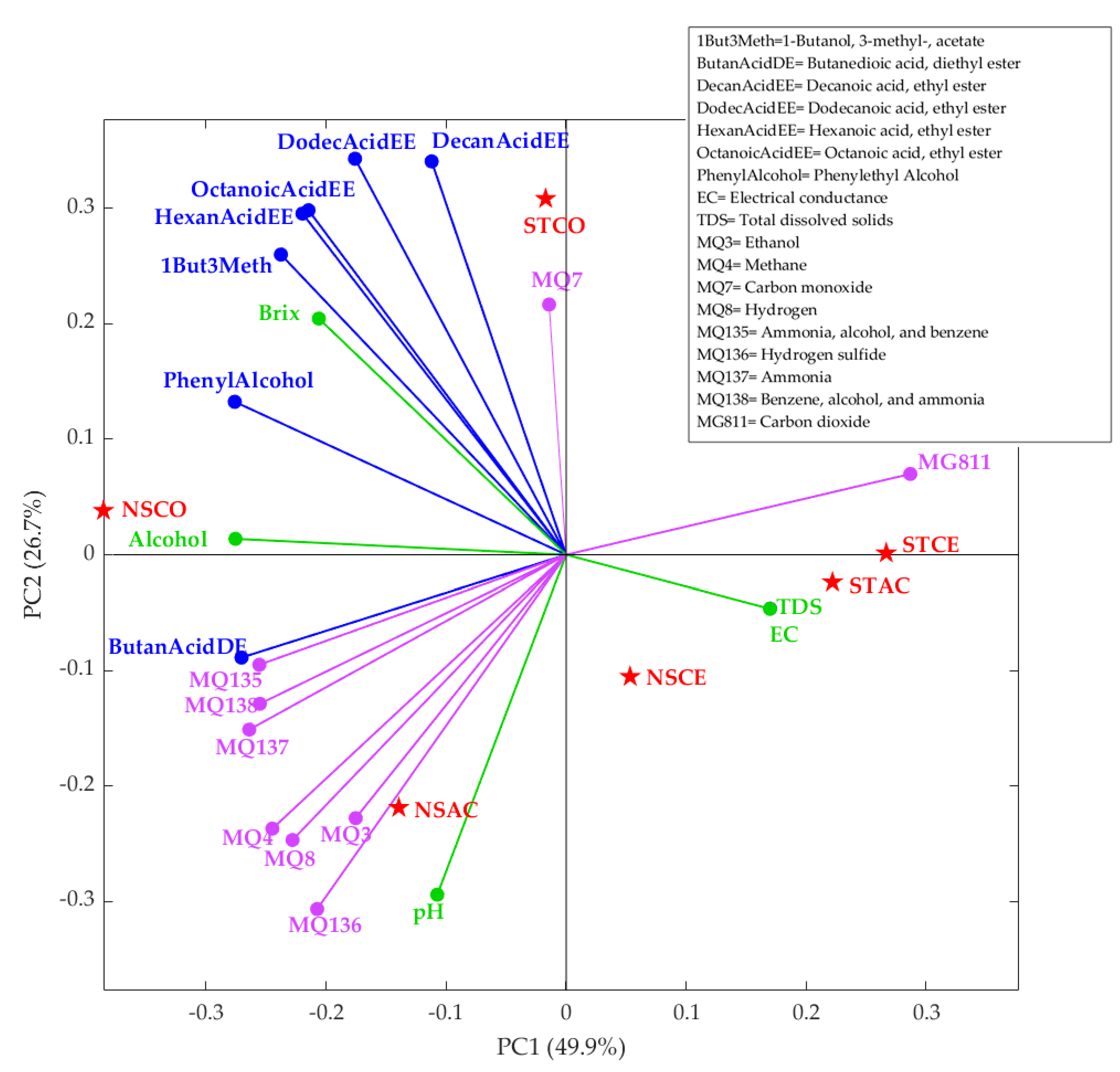
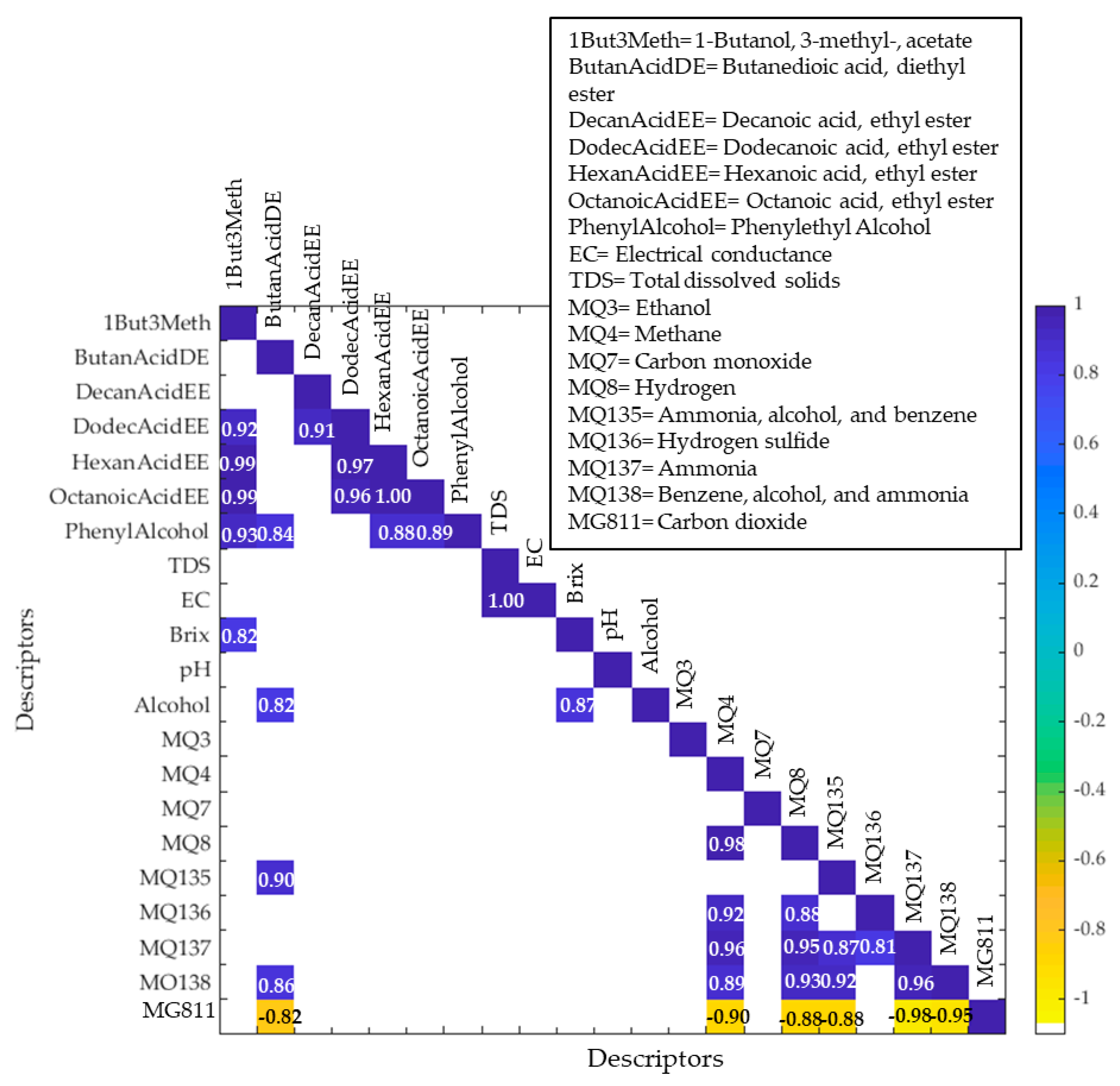
| Sensor * | Gases |
|---|---|
| MQ3 | Ethanol |
| MQ4 | Methane |
| MQ7 | Carbon monoxide |
| MQ8 | Hydrogen |
| MQ135 | Ammonia, alcohol, and benzene |
| MQ136 | Hydrogen sulfide |
| MQ137 | Ammonia |
| MQ138 | Benzene, alcohol, and ammonia |
| MG811 | Carbon dioxide |
| Sample | TDS (ppm) | EC (µs cm−1) | °Brix | pH | Alcohol (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| NSAC | 700.00 bc | ±24.80 | 1489.00 bc | ±52.70 | 5.23 c | ±0.03 | 3.73 b | ±0.03 | 10.70 c | ±0.04 |
| NSCE | 665.70 c | ±16.80 | 1415.70 c | ±35.60 | 5.80 b | ±0.00 | 3.87 a | ±0.07 | 11.34 b | ±0.01 |
| NSCO | 727.00 b | ±8.08 | 1546.30 b | ±17.40 | 6.00 a | ±0.00 | 3.63 b | ±0.03 | 12.18 a | ±0.02 |
| STAC | 802.50 a | ±1.43 | 1706.80 a | ±2.99 | 5.15 cd | ±0.03 | 3.48 c | ±0.03 | 9.64 d | ±0.10 |
| STCE | 832.00 a | ±6.43 | 1769.80 a | ±13.70 | 5.09 d | ±0.05 | 3.48 c | ±0.02 | 9.41 d | ±0.13 |
| STCO | 670.30 c | ±16.50 | 1425.50 c | ±35.20 | 5.90 ab | ±0.03 | 3.43 c | ±0.02 | 10.76 c | ±0.08 |
| Volatile Aromatic Compound | Odor Description | RT (min) | NSAC | NSCE | NSCO | STAC | STCE | STCO |
|---|---|---|---|---|---|---|---|---|
| 1-Butanol, 3-methyl-, acetate | Banana, pear, alcohol | 13.67 | 3.97 c | 4.03 c | 23.88 a | 1.81 d | 2.51 cd | 18.04b |
| ±0.03 | ±0.01 | ±0.20 | ±0.63 | ±0.62 | ±0.42 | |||
| Butanedioic acid, diethyl ester | Fruity, grape, wine [32,33] | 19.18 | 1.71 b | 1.37 b | 4.74 a | 0 | 0.30 b | 0 |
| ±0.09 | ±0.02 | ±2.65 | 0 | ±0.10 | 0 | |||
| Decanoic acid, ethyl ester | Apple, grape, sweet, brandy, waxy [32,33,34,35] | 23.02 | 75.95 b | 10.33 b | 119.21 b | 35.68 b | 1.74 b | 326.15 a |
| ±35.12 | ±2.70 | ±119.21 | ±18.30 | ±0.24 | ±68.10 | |||
| Dodecanoic acid, ethyl ester | floral, waxy, soap [32,35] | 26.33 | 7.61 b | 2.01 b | 49.33 a | 8.36 b | 1.96 b | 63.87 a |
| ±2.71 | ±0.31 | ±12.87 | ±2.56 | ±0.18 | ±6.17 | |||
| Hexanoic acid, ethyl ester | Sweet, fruity, wine [17,32,33,34] | 16.4 | 14.90 c | 12.21 c | 90.43 a | 7.74 d | 7.35 d | 83.60 b |
| ±0.08 | ±0.12 | ±0.78 | ±0.57 | ±1.00 | ±0.81 | |||
| Octanoic acid, ethyl ester | Fruity, banana, sweet, apple, pineapple [17,34,35] | 19.72 | 15.88 b | 9.47 b | 347.84 a | 13.08b | 6.35 b | 361.81 a |
| ±1.87 | ±0.36 | ±32.08 | ±6.11 | ±0.89 | ±26.41 | |||
| Phenylethyl alcohol | Rose, honey, floral [33,35] | 18.93 | 3.11 c | 1.25 cd | 16.27 a | 0.25 d | 0 | 6.21b |
| ±0.53 | ±0.63 | ±1.88 | ±0.25 | 0 | ±0.19 |
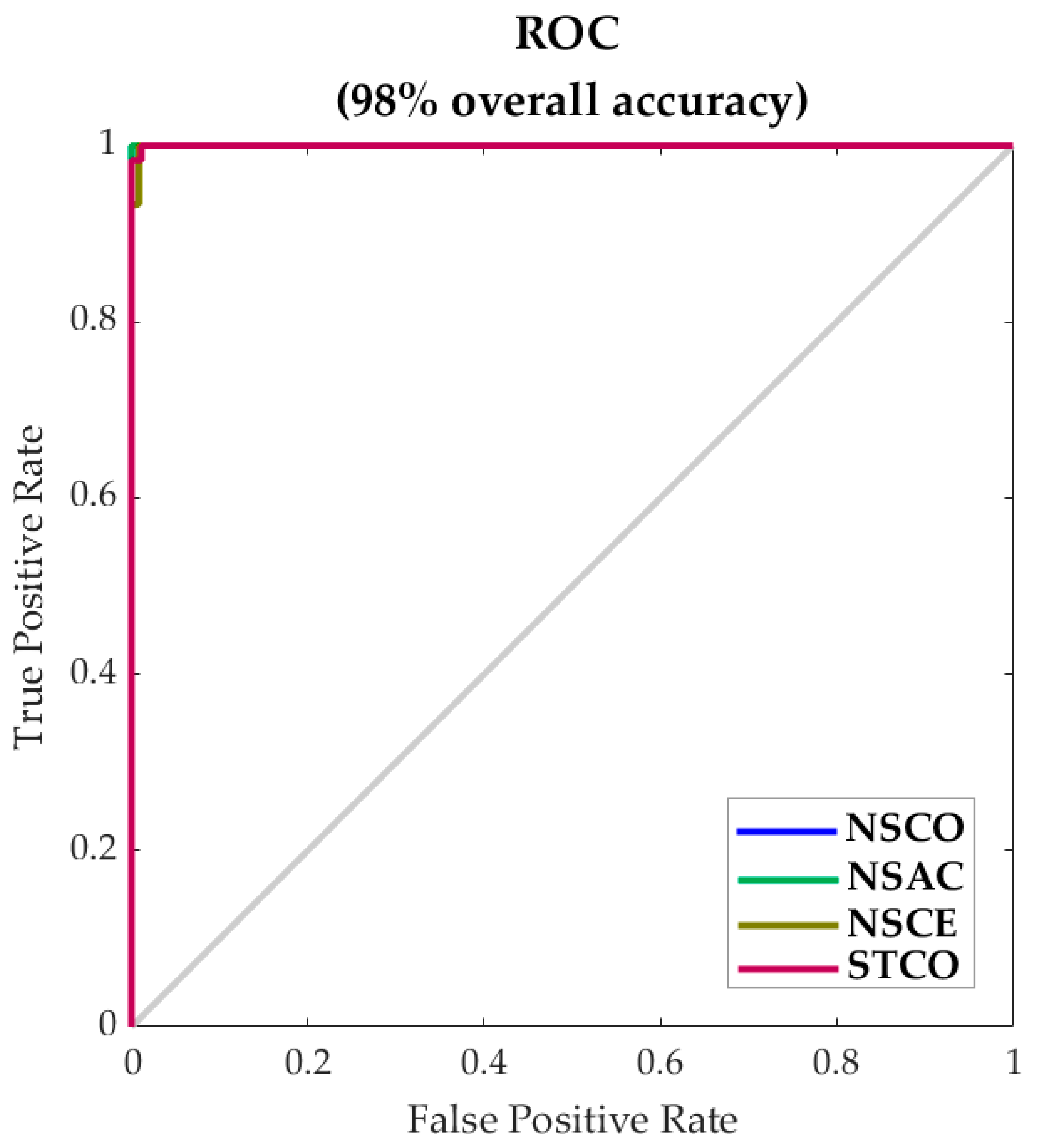
| Stage | Number of Samples | Accuracy (%) | Error (%) | Performance (MSE) |
|---|---|---|---|---|
| Training | 90 | 100 | 0 | <0.01 |
| Testing | 60 | 95 | 5 | 0.02 |
| Overall | 150 | 98 | 2 | - |
| Amelioration Treatments | |||
|---|---|---|---|
| STCE | STAC | ||
| Classification rates | NSCO | 20 | 18 |
| NSAC | 22 | 17 | |
| NSCE | 3 | 1 | |
| STCO | 5 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summerson, V.; Gonzalez Viejo, C.; Torrico, D.D.; Pang, A.; Fuentes, S. Digital Smoke Taint Detection in Pinot Grigio Wines Using an E-Nose and Machine Learning Algorithms Following Treatment with Activated Carbon and a Cleaving Enzyme. Fermentation 2021, 7, 119. https://doi.org/10.3390/fermentation7030119
Summerson V, Gonzalez Viejo C, Torrico DD, Pang A, Fuentes S. Digital Smoke Taint Detection in Pinot Grigio Wines Using an E-Nose and Machine Learning Algorithms Following Treatment with Activated Carbon and a Cleaving Enzyme. Fermentation. 2021; 7(3):119. https://doi.org/10.3390/fermentation7030119
Chicago/Turabian StyleSummerson, Vasiliki, Claudia Gonzalez Viejo, Damir D. Torrico, Alexis Pang, and Sigfredo Fuentes. 2021. "Digital Smoke Taint Detection in Pinot Grigio Wines Using an E-Nose and Machine Learning Algorithms Following Treatment with Activated Carbon and a Cleaving Enzyme" Fermentation 7, no. 3: 119. https://doi.org/10.3390/fermentation7030119
APA StyleSummerson, V., Gonzalez Viejo, C., Torrico, D. D., Pang, A., & Fuentes, S. (2021). Digital Smoke Taint Detection in Pinot Grigio Wines Using an E-Nose and Machine Learning Algorithms Following Treatment with Activated Carbon and a Cleaving Enzyme. Fermentation, 7(3), 119. https://doi.org/10.3390/fermentation7030119









