Optimization of Microbial Hydrolysis Parameters of Poultry By-Products Using Probiotic Microorganisms to Obtain Protein Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Ingredients
2.2. Biotechnological Treatment of the Gizzards
2.3. Optimization of Hydrolysis
2.4. Determination of Physical and Chemical Properties
2.5. Determination of the Dispersed Composition
2.6. Scanning Electron Microscopy (SEM)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Hydrolysis
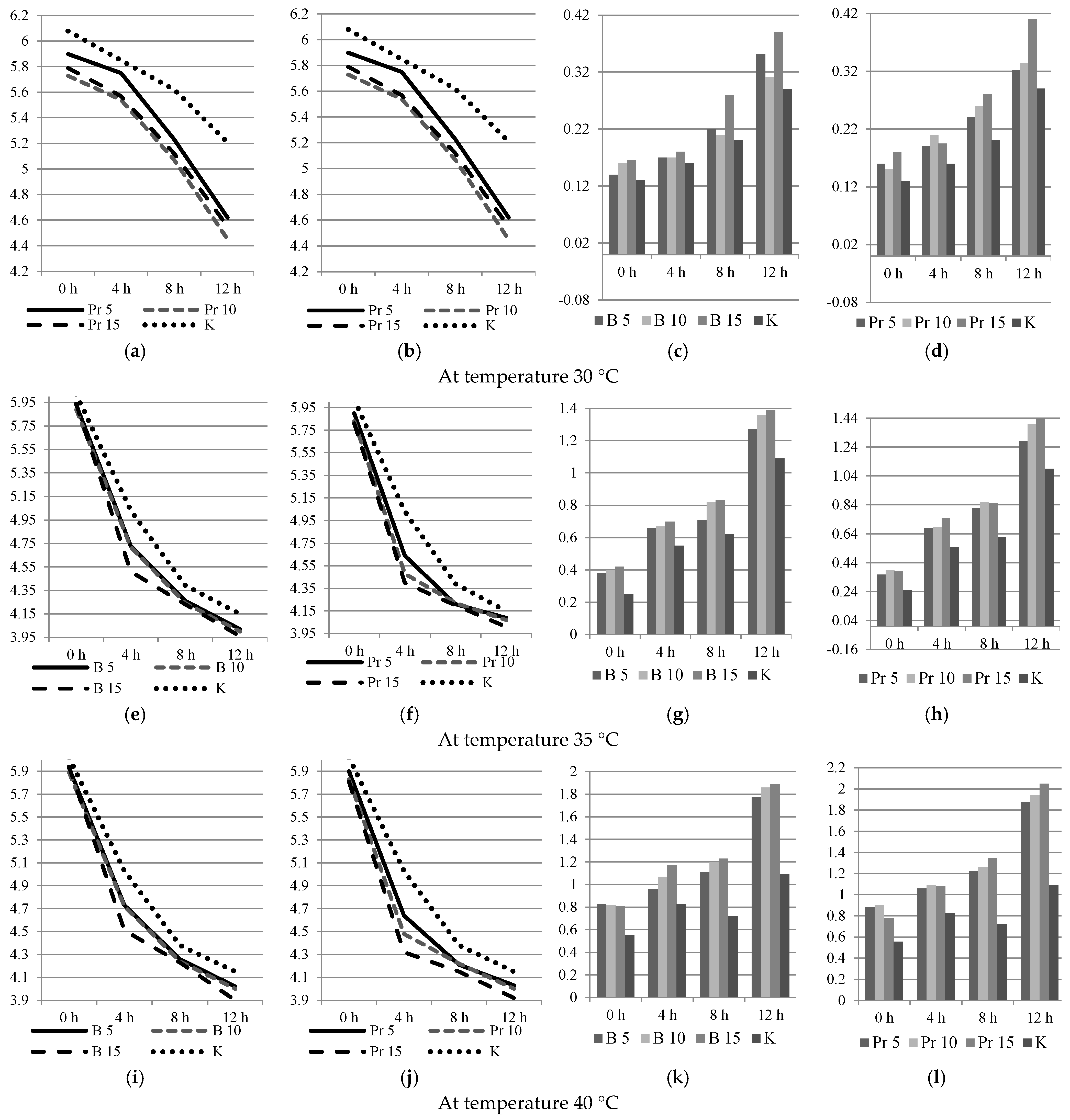
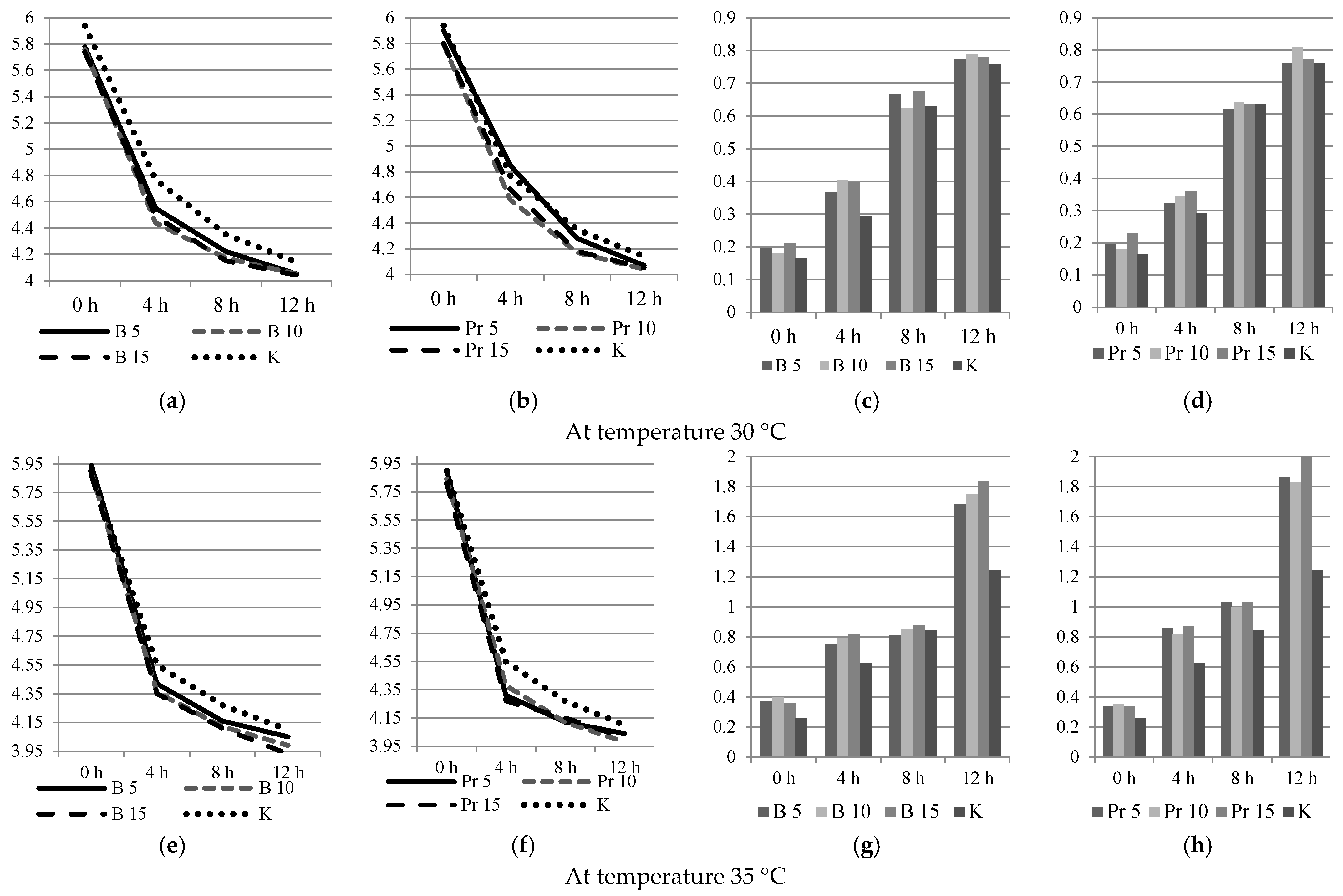
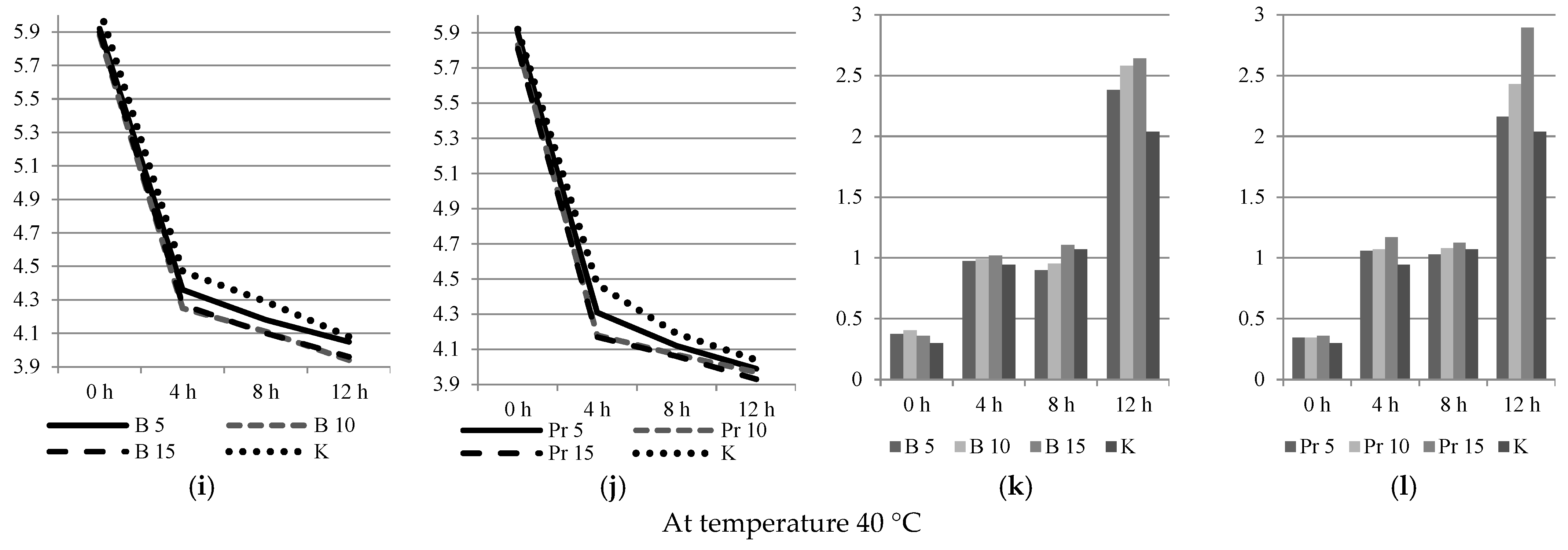
3.2. Physical and Chemical Analyzes
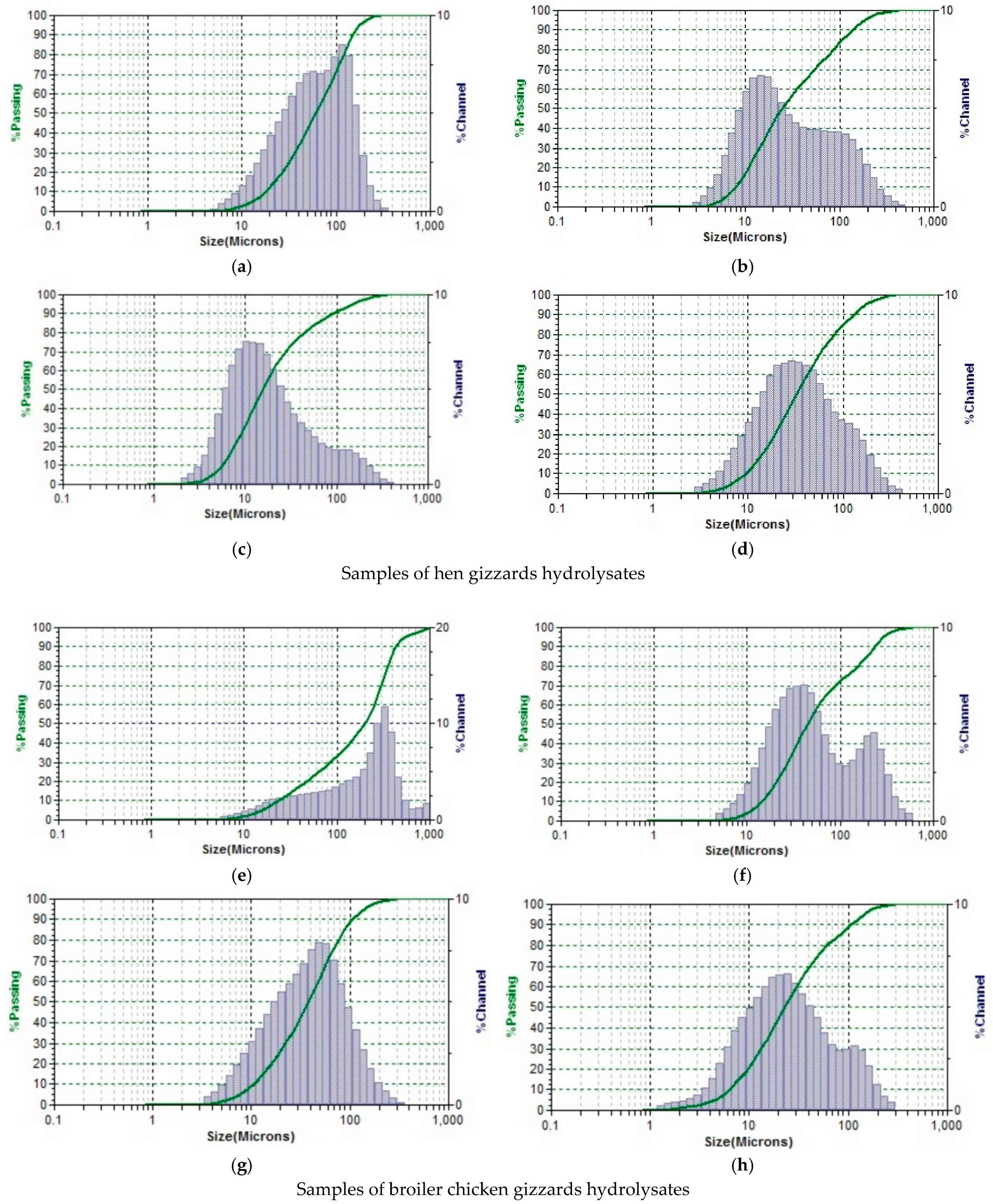
3.3. Dispersed Composition
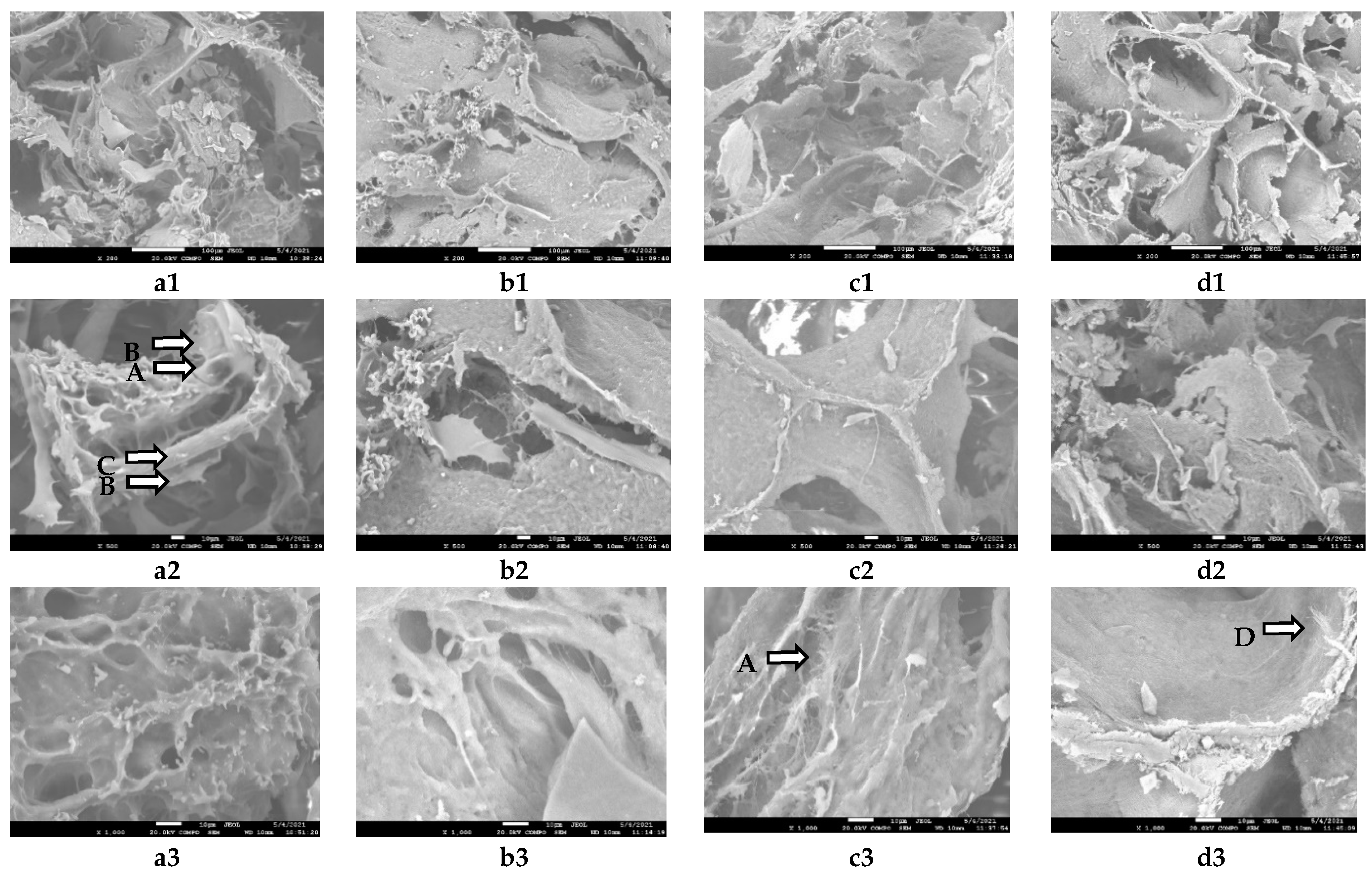
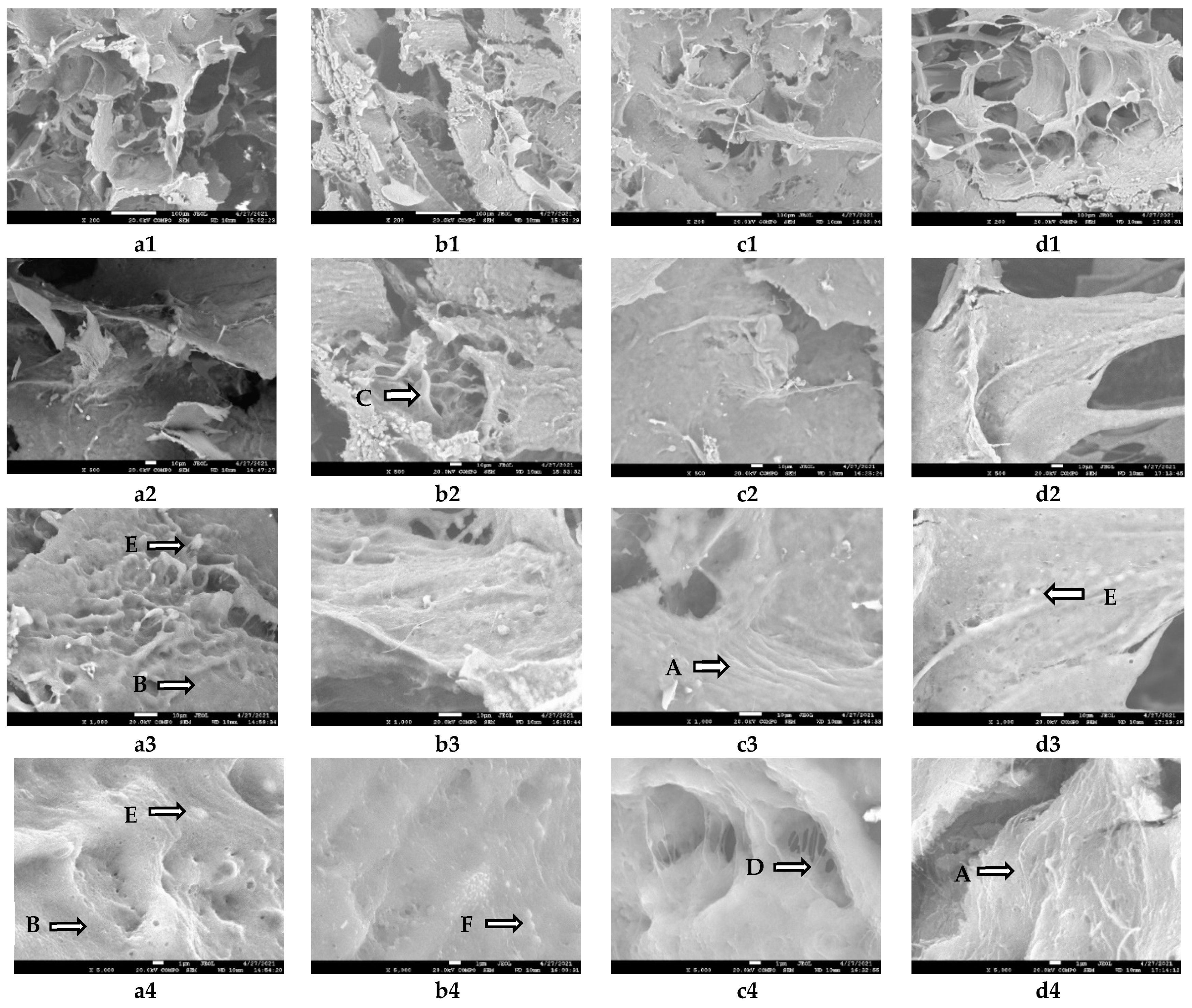
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lasekan, A.; Bakar, F.A.; Hashim, D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013, 33, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Peña-Saldarriaga, L.M.; Fernández-López, J.; Pérez-Alvarez, J.A. Quality of chicken fat by-products: Lipid profile and colour properties. Foods 2020, 9, 1046. [Google Scholar] [CrossRef] [PubMed]
- Roiter, L.M.; Zazykina, L.A.; Eremeeva, N.A. Poultry by-products, reserve for growth of export potential of the industry. IOP Conf. Ser. Earth Environ. Sci. 2019, 341, 012209. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Wan, X.; Fan, Q.; Ma, Y.; Qian, J.; Liu, X.; Shen, Y.; Jiang, J. Hydrolysis technology and kinetics of poultry waste to produce amino acids in subcritical water. J. Anal. Appl. Pyrol. 2010, 88, 187–191. [Google Scholar] [CrossRef]
- Zubair, M.; Wu, J.; Ullah, A. Hybrid Bionanocomposites from Spent Hen Proteins. American Chemical Society. ACS Omega 2019, 4, 3772–3781. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.; Rezende, P.C.; Corrêa, N.M.; Rocha, J.S.; Martins, M.A.; Andrade, T.C.; Fracalossi, D.M.; Vieira, F.N. Protein hydrolysates from poultry by-product and swine liver as an alternative dietary protein source for the Pacific white shrimp. Aquaculture Rep. 2020, 17, 100344. [Google Scholar] [CrossRef]
- Alao, B.O.; Falowo, A.B.; Chulayo, A.; Muchenje, V. The potential of animal by-products in food systems: Production, prospect and challenges. Sustainability 2017, 9, 1089. [Google Scholar] [CrossRef] [Green Version]
- Polaštíková, A.; Gál, R.; Mokrejš, P.; Orsavová, J. Preparation of protein products from collagen-rich poultry tissues. Potravin. Slovak J. Food Sci. 2020, 14, 713–720. [Google Scholar] [CrossRef]
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Rathina Raj, K.; Mahendrakar, N.S. Effect of ensiling and organic solvents treatment on proteolytic enzymes of layer chicken intestine. J. Food Sci. Technol. 2010, 47, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Santos, B.A.S.; Azambuja, S.P.H.; Ávila, P.F.; Pacheco, M.T.B.; Goldbeck, R. n-Butanol production by Saccharomyces cerevisiae from protein-rich agro-industrial by-products. Braz. J. Microbial. 2020, 51, 1655–1664. [Google Scholar] [CrossRef]
- Saadi, S.; Saari, N.; Anwar, F.; Hamid, A.A.; Mohd-Ghazali, H. Recent advances in food biopeptides: Production, biological functionalities and therapeutic applications. Biotechnol. Adv. 2015, 33, 80–116. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Proteinhydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, K.F.; Hui Voo, A.Y.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef] [PubMed]
- García, C.A.; Manrique, I.M. Meat proteins as a potential source of bioactive ingredients for food and pharmaceutical use. In Novel Proteins for Food, Pharmaceuticals and Agriculture: Sources, Applications, and Advances; Hayes, M., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 29–49. [Google Scholar] [CrossRef]
- Smid, E.J.; Lacroix, C. Microbe-microbe interactions in mixed culture food fermentations. Curr. Opin. Biotechnol. 2013, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Assaad, H.; Zhou, L.; Carroll, R.J.; Wu, G. Rapid publication-ready MS-Word tables 597 for one-way ANOVA. SpringerPlus 2014, 3, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei Teshnizi, Z.; Robatjazi, S.M.; Mohammadian Mosaabadi, J. Optimization of the enzymatic hydrolysis of poultry slaughterhouse wastes using alcalase enzyme for the preparation of protein hydrolysates. Appl. Food Biotechnol. 2020, 7, 153–160. [Google Scholar] [CrossRef]
- Kurozawa, L.; Park, K.; Hubinger, M. Optimization of the enzymatic hydrolysis of chicken meat using response surface methodology. J. Food Sci. 2008, 73, 405–512. [Google Scholar] [CrossRef]
- Zhumanova, G.; Rebezov, M.; Assenova, B.; Okuskhanova, E. Prospects of using poultry by-products in the technology of chopped semi-finished products. Inter. J. Eng. Technol. 2018, 7, 495–498. [Google Scholar] [CrossRef]
- Arafa, A.S. Pickled chicken gizzards: 1. Acceptability and proximate analysis. Inter. Poult. Sci. 1977, 56, 1014–1017. [Google Scholar] [CrossRef]
- Abdullah, F.A.A.; Buchtova, H. Comparison of qualitative and quantitative properties of the wings, necks and offal of chicken broilers from organic and conventional production systems. Vet. Med. 2016, 61, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319. [Google Scholar] [CrossRef]
- Sun, F.; Sun, Q.; Zhang, H.; Kong, B.; Xia, X. Purification and biochemical characteristics of the microbial extracellular protease from Lactobacillus curvatus isolated from Harbin dry sausages. Int. J. Biol. Macromol. 2019, 133, 987–997. [Google Scholar] [CrossRef]
- Sun, F.; Tao, R.; Liu, Q.; Wang, H.; Kong, B. Effects of temperature and pH on the structure of a metalloprotease from Lactobacillus fermentum R6 isolated from Harbin dry sausages and molecular docking between protease and meat protein. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Li, X.; Lee, P.R.; Taniasuri, F.; Liu, S.-Q. Biotransformation of pork trimmings into protein hydrolysate using microbial proteases aided by response surface methodology. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Alvarez, R.G.; Vaka, M.R.; Rustad, T.; Sone, I.; Fernandez, E.N. Potential of innovative pre-treatment technologies for the revalorisation of residual materials from the chicken industry through enzymatic hydrolysis Innovative. Food Sci. Emerg. Technol. 2020, 64, 102377. [Google Scholar] [CrossRef]
- Wilkins, D.K.; Grimshaw, S.B.; Receveur, V.; Dobson, C.M.; Jones, J.A.; Smith, L.J. Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR techniques. Biochemistry 1999, 38, 16424–16431. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, D.; Xu, P.; Geng, Z.; Xiong, G.; Zou, Y.; Wang, D.; Xu, W. Structural and antimicrobial properties of Maillard reaction products in chicken liver protein hydrolysate after sonication. Food Chem. 2021, 343, 128417. [Google Scholar] [CrossRef] [PubMed]
- Akram, A.N.; Zhang, C. Extraction of collagen-II with pepsin and ultrasound treatment from chicken sternal cartilage; physicochemical and functional properties. Ultrason. Sonochem. 2020, 64, 105053. [Google Scholar] [CrossRef]
- Aksoy, A.; Karasu, S.; Akcicek, A.; Kayacan, S. Effects of different drying methods on drying kinetics, microstructure, color, and the rehydration ratio of minced meat. Foods 2019, 8, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewska, K.; Mierzwa, D. Influence of ultrasound on the microstructure of plant tissue. Innov. Food Sci. Emerg. Technol. 2017, 43, 117–129. [Google Scholar] [CrossRef]



| Run | X1 | X2 | X3 | DH of Hen Gizzards | DH of Broiler Chicken Gizzards | ||
|---|---|---|---|---|---|---|---|
| Propionix LCSC | BLC | Propionix LCSC | BLC | ||||
| 1 | 30 (−1) | 5 (−1) | 4 (−1) | 10.3 | 15 | 6.1 | 4.5 |
| 2 | 30 (−1) | 15 (+1) | 4 (−1) | 12.9 | 8.5 | 15.4 | 12.4 |
| 3 | 30 (−1) | 5 (−1) | 12 (+1) | 51.3 | 54.8 | 28.5 | 15.2 |
| 4 | 30 (−1) | 15 (+1) | 12 (+1) | 46 | 46 | 52.4 | 29.2 |
| 5 | 40 (+1) | 5 (−1) | 4 (−1) | 18.7 | 23.9 | 16.9 | 16.9 |
| 6 | 40 (+1) | 15 (+1) | 4 (−1) | 15.8 | 20.3 | 27.2 | 21 |
| 7 | 40 (+1) | 5 (−1) | 12 (+1) | 53.7 | 57.9 | 46.7 | 44.1 |
| 8 | 40 (+1) | 15 (+1) | 12 (+1) | 64.6 | 62.1 | 57.8 | 52.2 |
| 9 | 35 (0) | 10 (0) | 8 (0) | 19.7 | 34.6 | 10.6 | 20.3 |
| 10 | 35 (0) | 10 (0) | 8 (0) | 18.1 | 33.5 | 12.5 | 21.9 |
| 11 | 35 (0) | 10 (0) | 8 (0) | 20.8 | 36.2 | 9.8 | 19 |
| 12 | 48.6 (1.2154) | 10 (0) | 8 (0) | 46.7 | 37.5 | 30.7 | 32.3 |
| 13 | 23.5 (−1.2154) | 10 (0) | 8 (0) | 19.5 | 20.3 | 12.3 | 9.8 |
| 14 | 35 (0) | 18.2 (1.2154) | 8 (0) | 29.1 | 35 | 39.2 | 25.8 |
| 15 | 35 (0) | 3.9 (−1.2154) | 8 (0) | 15.3 | 18.5 | 3.4 | 8.5 |
| 16 | 35 (0) | 10 (0) | 14.6 (1.2154) | 55.6 | 57.6 | 52.7 | 34.6 |
| 17 | 35 (0) | 10 (0) | 3.1 (−1.2154) | 15.1 | 17.8 | 9.1 | 6.2 |
| Variables | Hydrolyzing by Propionix LCSC | Hydrolyzing by BLC | ||||
|---|---|---|---|---|---|---|
| Regression Coefficient (b) | t- Student’s Criterion | p-Value | Regression Coefficient | t- Student’s Criterion | p-Value | |
| Y-intersection | 31.6200 | 79.9449 | p ≤ 0.0005 | 33.9867 | 85.9287 | p ≤ 0.0005 |
| Variable X1 | 4.3572 | 8.8432 | p ≤ 0.05 | 4.0536 | 8.2270 | p ≤ 0.05 |
| Variable X2 | 1.4715 | 2.9864 | p ≤ 0.05 | 0.3569 | 0.7244 | p ≥ 0.1 |
| Variable X3 | 13.8082 | 28.0244 | p ≤ 0.0005 | 13.4315 | 27.2598 | p ≤ 0.0005 |
| Variable X1·X2 | 0.7133 | 1.2372 | p ≥ 0.1 | 1.0600 | 1.8384 | p ≥ 0.1 |
| Variable X1·X3 | 0.6467 | 1.1215 | p ≥ 0.1 | -0.1000 | −0.1734 | p ≥ 0.1 |
| Variable X2·X3 | 0.3933 | 0.6821 | p ≥ 0.1 | 0.3667 | 0.6359 | p ≥ 0.1 |
| Variable X1·X2 X3 | 1.4467 | 2.5091 | p ≤ 0.05 | 0.6733 | 1.1678 | p ≥ 0.1 |
| Variable X12 | 1.6472 | 2.1102 | p ≥ 0.1 | 0.1050 | 0.1345 | p ≥ 0.1 |
| Variable X22 | −0.4995 | -0.6399 | p ≥ 0.1 | −0.3184 | −0.4079 | p ≥ 0.1 |
| Variable X32 | 2.0904 | 2.6779 | p ≤ 0.05 | 1.8383 | 2.3548 | p ≤ 0.05 |
| Variables | Hydrolyzing by Propionix LCSC | Hydrolyzing by BLC | ||||
|---|---|---|---|---|---|---|
| Regression Coefficient (b) | t- Student’s Criterion | p-Value | Regression Coefficient (b) | t- Student’s Criterion | p-Value | |
| Y-intersection | 27.2667 | 63.1820 | p ≤ 0.0005 | 22.2000 | 50.8516 | p ≤ 0.0005 |
| Variable X1 | 4.5709 | 8.5012 | p ≤ 0.05 | 6.6831 | 12.2885 | p ≤ 0.001 |
| Variable X2 | 6.5408 | 12.1661 | p ≤ 0.001 | 3.6751 | 6.7575 | p ≤ 0.05 |
| Variable X3 | 11.5194 | 21.4273 | p ≤ 0.0005 | 8.0278 | 14.7611 | p ≤ 0.0005 |
| Variable X1·X2 | −0.7866 | −1.2507 | p ≥ 0.1 | −0.64667 | −1.0161 | p ≥ 0.1 |
| Variable X1·X3 | 0.0666 | 0.1059 | p ≥ 0.1 | 2.0600 | 3.2369 | p ≤ 0.05 |
| Variable X2·X3 | 1.0267 | 1.6319 | p ≥ 0.1 | 0.6733 | 1.0580 | p ≥ 0.1 |
| Variable X1·X2·X3 | −0.9200 | −1.4624 | p ≤ 0.05 | −0.1400 | −0.2199 | p ≥ 0.1 |
| Variable X12 | 1.0551 | 1.2387 | p ≥ 0.1 | 0.9667 | 1.1219 | p ≥ 0.1 |
| Variable X22 | 1.0157 | 1.1925 | p ≥ 0.1 | 0.1985 | 0.2304 | p ≥ 0.1 |
| Variable X32 | 2.9066 | 3.4125 | p ≤ 0.05 | 0.8386 | 0.9733 | p ≥ 0.1 |
| Indicator | Value, % | |
|---|---|---|
| Hen Gizzard | Broiler Chicken Gizzard | |
| Moisture | 75.08 ± 0.14 a | 72.64 ± 0.22 b |
| Protein | 17.88 ± 0.06 a | 20.70 ± 0.08 b |
| Fat | 4.38 ± 0.03 a | 4.62 ± 0.02 a |
| Ash | 1.76 ± 0.01 a | 1.81 ± 0.01 b |
| Indicator | Control Sample in the Start Time | Hydrolyzed Control Sample | Hydrolyzed Test Sample with Propionix LCSC | Hydrolyzed Test Sample with BLC |
|---|---|---|---|---|
| hens’ gizzards | ||||
| MN (MKM) | 11.83 | 6.60 | 4.69 | 2.73 |
| broiler chickens’ gizzards | ||||
| MN (MKM) | 11.29 | 10.63 | 7.22 | 6.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinina, O.; Merenkova, S.; Galimov, D. Optimization of Microbial Hydrolysis Parameters of Poultry By-Products Using Probiotic Microorganisms to Obtain Protein Hydrolysates. Fermentation 2021, 7, 122. https://doi.org/10.3390/fermentation7030122
Zinina O, Merenkova S, Galimov D. Optimization of Microbial Hydrolysis Parameters of Poultry By-Products Using Probiotic Microorganisms to Obtain Protein Hydrolysates. Fermentation. 2021; 7(3):122. https://doi.org/10.3390/fermentation7030122
Chicago/Turabian StyleZinina, Oksana, Svetlana Merenkova, and Damir Galimov. 2021. "Optimization of Microbial Hydrolysis Parameters of Poultry By-Products Using Probiotic Microorganisms to Obtain Protein Hydrolysates" Fermentation 7, no. 3: 122. https://doi.org/10.3390/fermentation7030122
APA StyleZinina, O., Merenkova, S., & Galimov, D. (2021). Optimization of Microbial Hydrolysis Parameters of Poultry By-Products Using Probiotic Microorganisms to Obtain Protein Hydrolysates. Fermentation, 7(3), 122. https://doi.org/10.3390/fermentation7030122






