The Antioxidant, Anti-Diabetic, and Anti-Adipogenesis Potential and Probiotic Properties of Lactic Acid Bacteria Isolated from Human and Fermented Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Probiotic Candidates and Culture Conditions
2.3. Cell-Free Extract Preparation of the Isolates
2.4. Antioxidant Activity
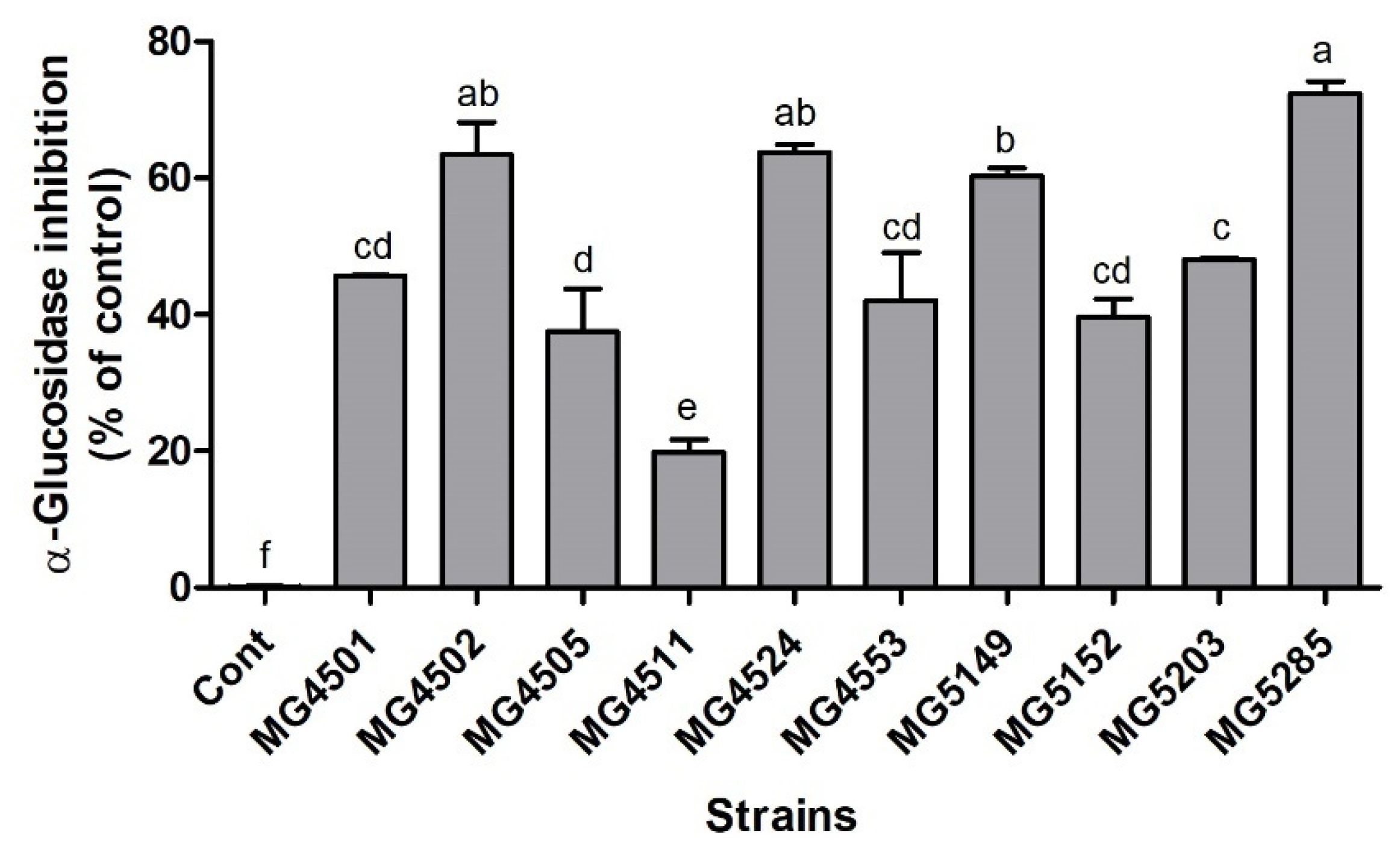
2.5. α-Glucosidase Inhibitory Activity
2.6. Adipocyte Culture and Differentiation
2.7. Anti-Adipogenesis Activity
2.8. Strain Survival under Simulated Human Gastrointestinal Tract Conditions
2.9. Adhesion to HT-29 Cells
2.10. Enzyme Activity and Biochemical Profile Characterization
2.11. Antibiotic Susceptibility
2.12. Hemolytic Activity
2.13. Statistical Analysis
3. Results & Discussion
3.1. Antioxidant Activities of LAB
3.2. α-Glucosidase Inhibition Activity of LAB
3.3. LAB Inhibit Adipogenesis in 3T3-L1 Cells
3.4. Survival under Simulated Human Gastrointestinal Tract Conditions
3.5. Adherence Ability of Probiotics
3.6. Assessment of Safety
3.7. Carbohydrate Utilization and Enzyme Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 April 2020).
- Alford, S.; Patel, D.; Perakakis, N.; Mantzoros, C. Obesity as a risk factor for Alzheimer’s disease: Weighing the evidence. Obes. Rev. 2018, 19, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a disease. Med. Clin. N. Am. 2018, 102, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Nowicka, G. Obesity, DNA damage, and development of obesity-related diseases. Int. J. Mol. Sci. 2019, 20, 1146. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin. Drug Saf. 2012, 11, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Medical treatment of obesity: The past, the present and the future. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 665–684. [Google Scholar] [CrossRef]
- Schippa, S.; Conte, M.P. Dysbiotic events in gut microbiota: Impact on human health. Nutrients 2014, 6, 5786–5805. [Google Scholar] [CrossRef]
- Gordo, R.; López-Andrés, N.; Fernández-Celis, A.; Gutiérrrez-Miranda, B.; Nieto, M.L.; Alarcon, T.; Alba, C.; Cachofeiro, V. The Interaction between Mitochondrial Oxidative Stress and Gut Microbiota in the Cardiometabolic Consequences in Diet-Induced Obese Rats. Antioxidants 2020, 9, 640. [Google Scholar]
- WHO. Report on Joint FAO/WHO Guidelines for the Evaluation of Probiotics in Food. Available online: https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (accessed on 30 April 2002).
- Ku, S.; Park, M.S.; Ji, G.E.; You, H.J. Review on Bifidobacterium bifidum BGN4: Functionality and nutraceutical applications as a probiotic microorganism. Int. J. Mol. Sci. 2016, 17, 1544. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Zhang, Q.; Dang, H.; Liu, X.; Tian, F.; Zhao, J.; Chen, Y.; Zhang, H.; Chen, W. Screening for potential new probiotic based on probiotic properties and α-glucosidase inhibitory activity. Food Control 2014, 35, 65–72. [Google Scholar] [CrossRef]
- Koh, W.Y.; Utra, U.; Ahmad, R.; Rather, I.A.; Park, Y.-H. Evaluation of probiotic potential and anti-hyperglycemic properties of a novel Lactobacillus strain isolated from water kefir grains. Food Sci. Biotechnol. 2018, 27, 1369–1376. [Google Scholar] [CrossRef]
- Burgos-Morón, E.; Abad-Jiménez, Z.; Martinez de Maranon, A.; Iannantuoni, F.; Escribano-López, I.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: The battle continues. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Chang, C.E.; Sylvia, I.P.; Lin, T.; Eun-Ki, K.; Seung, C.K.; Hyun, S.Y.; Jae-Seong, S. Molecular identification of vaginal Lactobacillus spp. isolated from Korean women. J. Microbiol. Biotechnol. 2002, 12, 312–317. [Google Scholar]
- Kim, H.; Kim, J.-S.; Kim, Y.; Jeong, Y.; Kim, J.-E.; Paek, N.-S.; Kang, C.-H. Antioxidant and Probiotic Properties of Lactobacilli and Bifidobacteria of Human Origins. Biotechnol. Bioprocess. Eng. 2020, 25, 421–430. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Lee, J.; Kim, A.-R.; Lee, J.-J. Ramie leaf extracts suppresses adipogenic differentiation in 3T3-L1 cells and pig preadipocytes. Asian Australas. J. Anim. Sci. 2016, 29, 1338. [Google Scholar] [CrossRef] [Green Version]
- Rizzatti, V.; Boschi, F.; Pedrotti, M.; Zoico, E.; Sbarbati, A.; Zamboni, M. Lipid droplets characterization in adipocyte differentiated 3T3-L1 cells: Size and optical density distribution. Eur. J. Histochem. 2013, 57, e24. [Google Scholar] [CrossRef] [Green Version]
- Maragkoudakis, P.A.; Zoumpopoulou, G.; Miaris, C.; Kalantzopoulos, G.; Pot, B.; Tsakalidou, E. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 2006, 16, 189–199. [Google Scholar] [CrossRef]
- Kim, S.; Choi, S.-I.; Jang, M.; Jeong, Y.; Kang, C.-H. Anti-adipogenic effect of Lactobacillus fermentum MG4231 and MG4244 through AMPK pathway in 3T3-L1 preadipocytes. Food Sci. Biotechnol. 2020, 29, 1541–1551. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products; Substances Used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, e1801944. [Google Scholar] [CrossRef]
- Ji, Y.-J.; Lee, E.Y.; Lee, J.Y.; Lee, Y.J.; Lee, S.E.; Seo, K.H.; Kim, H.D. Antioxidant and Anti-Diabetic Effects of Agastache rugosa Extract. J. East. Asian Soc. Diet. Life 2020, 30, 297–305. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.-Y.; Seo, H.T.; Seong, H.-A.; Ji, Y.-J.; Lee, S.E.; Seo, K.H.; Kim, H.D. Samnamul (Shoots of Aruncus dioicus) Inhibit Adipogenesis by Downregulating Adipocyte-Specific Transcription Factors in 3T3-L1 Adipocytes. Processes 2020, 8, 1576. [Google Scholar] [CrossRef]
- Marella, S.; Hema, K.; Shameer, S.; Prasad, T. Nano-ellagic acid: Inhibitory actions on aldose reductase and α-glucosidase in secondary complications of diabetes, strengthened by in silico docking studies. 3 Biotech 2020, 10, 439. [Google Scholar] [CrossRef]
- Tabuchi, M.; Ozaki, M.; Tamura, A.; Yamada, N.; Ishida, T.; Hosoda, M.; Hosono, A. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2003, 67, 1421–1424. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, J.; Zuo, F.; Zhang, Y.; Ma, H.; Chen, S. Screening for potential novel probiotic Lactobacillus strains based on high dipeptidyl peptidase IV and α-glucosidase inhibitory activity. J. Funct. Foods 2016, 20, 486–495. [Google Scholar] [CrossRef]
- Ejtahed, H.-S.; Angoorani, P.; Soroush, A.-R.; Atlasi, R.; Hasani-Ranjbar, S.; Mortazavian, A.M.; Larijani, B. Probiotics supplementation for the obesity management; A systematic review of animal studies and clinical trials. J. Funct. Foods 2019, 52, 228–242. [Google Scholar] [CrossRef]
- Jung, S.-P.; Lee, K.-M.; Kang, J.-H.; Yun, S.-I.; Park, H.-O.; Moon, Y.; Kim, J.-Y. Effect of Lactobacillus gasseri BNR17 on overweight and obese adults: A randomized, double-blind clinical trial. Korean J. Fam. Med. 2013, 34, 80. [Google Scholar] [CrossRef] [Green Version]
- Park, D.-Y.; Ahn, Y.-T.; Huh, C.-S.; Jeon, S.-M.; Choi, M.-S. The inhibitory effect of Lactobacillus plantarum KY1032 cell extract on the adipogenesis of 3T3-L1 Cells. J. Med. Food. 2011, 14, 670–675. [Google Scholar] [CrossRef]
- Kim, S.; Huang, E.; Park, S.; Holzapfel, W.; Lim, S.-D. Physiological characteristics and anti-obesity effect of Lactobacillus plantarum K10. Korean J. Food Sci. Anim. Resour. 2018, 38, 554. [Google Scholar]
- Morelli, L. In vitro assessment of probiotic bacteria: From survival to functionality. Int. Dairy J. 2007, 17, 1278–1283. [Google Scholar] [CrossRef]
- Patel, A.; Lindström, C.; Patel, A.; Prajapati, J.; Holst, O. Probiotic properties of exopolysaccharide producing lactic acid bacteria isolated from vegetables and traditional Indian fermented foods. Int. J. Fermented Foods 2012, 1, 87–101. [Google Scholar]
- Kang, C.-H.; Kim, Y.G.; Han, S.H.; Jeong, Y.; Paek, N.-S. Antibacterial activity and probiotic properties of lactic acid bacteria from Korean Intestine Origin. KSBB J. 2017, 32, 153–159. [Google Scholar] [CrossRef]
- Srisesharam, S.; Park, H.S.; Soundharrajan, I.; Kuppusamy, P.; Kim, D.H.; Jayraaj, I.A.; Lee, K.D.; Choi, K.C. Evaluation of probiotic Lactobacillus plantarum against foodborne pathogens and its fermentation potential in improving Lolium multiflorum silage quality. 3 Biotech 2018, 8, 443. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-S.; Yeu, J.-E.; Hong, S.-P. Safety evaluation of oral care probiotics Weissella cibaria CMU and CMS1 by phenotypic and genotypic analysis. Int. J. Mol. Sci. 2019, 20, 2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunyakti, A.; Asan-Ozusaglam, M. Lactobacillus gasseri from human milk with probiotic potential and some technological properties. LWT 2019, 109, 261–269. [Google Scholar] [CrossRef]
- Shi, Y.; Cui, X.; Gu, S.; Yan, X.; Li, R.; Xia, S.; Chen, H.; Ge, J. Antioxidative and probiotic activities of lactic acid bacteria isolated from traditional artisanal milk cheese from Northeast China. Probiot. Antimicrob. Proteins 2019, 11, 1086–1099. [Google Scholar] [CrossRef]
- Yasmin, I.; Saeed, M.; Khan, W.A.; Khaliq, A.; Chughtai, M.F.J.; Iqbal, R.; Tehseen, S.; Naz, S.; Liaqat, A.; Mehmood, T. In Vitro Probiotic Potential and Safety Evaluation (Hemolytic, Cytotoxic Activity) of Bifidobacterium Strains Isolated from Raw Camel Milk. Microorganisms 2020, 8, 354. [Google Scholar] [CrossRef] [Green Version]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1675–1683. [Google Scholar] [CrossRef]
- Yang, S.-J.; Lee, J.-E.; Lim, S.-M.; Kim, Y.-J.; Lee, N.-K.; Paik, H.-D. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci. Biotechnol. 2019, 28, 491–499. [Google Scholar] [CrossRef]



| Factors a | DPPH | ABTS | α-Glucosidase | Adipogenesis |
|---|---|---|---|---|
| DPPH | 1.000 | 0.685 ** | 0.362 * | 0.380 * |
| ABTS | 1.000 | 0.248 | 0.533 ** | |
| α-Glucosidase | 1.000 | 0.337 * | ||
| Adipogenesis | 1.000 |
| Strains | Initial | Viable Count | |||
|---|---|---|---|---|---|
| Simulated Gastric Fluid a | Simulated Intestinal Fluid b | ||||
| pH 3 | pH 4 | pH 7 | pH 8 | ||
| MG4502 | 8.6 ± 0.1 | 8.6 ± 0.0 | 8.6 ± 0.2 | 8.7 ± 0.1 | 8.6 ± 0.0 |
| MG4524 | 8.3 ± 0.0 | 7.6 ± 0.1 | 7.5 ± 0.1 | 8.9 ± 0.1 | 8.8 ± 0.0 |
| MG5149 | 8.8 ± 0.0 | 8.7 ± 0.0 | 8.7 ± 0.1 | 8.8 ± 0.0 | 8.8 ± 0.0 |
| MG5285 | 7.8 ± 0.1 | 7.3 ± 0.1 | 7.7 ± 0.1 | 7.8 ± 0.0 | 7.9 ± 0.1 |
| Antibiotics | In This Study | |||
|---|---|---|---|---|
| MG4502 | MG4524 | MG5149 | MG5285 | |
| Ampicillin | 0.75 | 0.125 | 0.5 | 0.25 |
| Gentamicin | 4 | 2 | 2 | 2 |
| Kanamycin | 64 | 32 | 48 | 16 |
| Streptomycin | 8 | 4 | 12 | 24 |
| Tetracycline | 0.5 | 1 | 8 | 1 |
| Chloramphenicol | 4 | 3 | 4 | 4 |
| Erythromycin | 0.064 | 0.016 | 0.023 | 0.064 |
| Vancomycin | n.r. | 0.75 | n.r. | n.r. |
| Clindamycin | 0.19 | 0.19 | 0.016 | 0.047 |
| Substrate | MG4524 | MG5149 | MG4502 | MG5285 |
|---|---|---|---|---|
| d-arabinose | − | − | + | − |
| l-arabinose | − | + | − | + |
| d-ribose | − | + | − | + |
| d-xylose | − | − | − | + |
| d-fructose | + | − | + | + |
| d-mannose | + | − | + | + |
| Dulcitol | − | − | + | − |
| Inositol | − | − | + | − |
| d-mannitol | − | − | + | + |
| d-sorbitol | − | − | + | + |
| N-acetyl-glucosamine | + | − | + | + |
| Amygdalin | + | − | + | + |
| Arbutin | + | − | + | + |
| Salicin | + | − | + | + |
| d-cellobiose | + | − | + | + |
| d-maltose | + | + | − | + |
| d-lactose | + | + | − | − |
| d-melibiose | − | − | − | + |
| d-sucrose | + | + | − | + |
| d-trehalose | + | − | + | + |
| d-melezitose | − | − | + | − |
| Starch | + | − | − | − |
| Gentiobiose | + | − | + | + |
| d-turanose | + | − | − | + |
| d-tagatose | + | − | + | − |
| l-fucose | − | − | + | − |
| Gluconate | − | + | + | + |
| 2-keto-gluconate | − | − | − | + |
| Enzyme Assayed for | MG4524 | MG5149 | MG4502 | MG5285 |
|---|---|---|---|---|
| Alkaline phosphatase | 0 | 0 | 2 | 0 |
| Esterase (C4) | 1 | 1 | 3 | 0 |
| Esterase Lipase (C8) | 0 | 1 | 3 | 0 |
| Lipase (C14) | 0 | 0 | 2 | 0 |
| Leucine arylamidase | 4 | 1 | 5 | 0 |
| Valine arylamidase | 1 | 3 | 5 | 0 |
| Crystine arylamidase | 1 | 0 | 3 | 0 |
| α-Chymotrypsin | 0 | 0 | 1 | 0 |
| Acid phosphatase | 1 | 2 | 3 | 4 |
| Naphtol-AS-BI-Phosphohydrolase | 1 | 2 | 5 | 1 |
| α-Galactosidase | 3 | 0 | 2 | 0 |
| β-Galactosidase | 1 | 4 | 3 | 0 |
| α-Glucosidase | 1 | 1 | 2 | 0 |
| β-Glucosidase | 5 | 0 | 5 | 0 |
| N-Acetyl-β-glucosaminidase | 3 | 0 | 0 | 0 |
| α-Fucosidase | 0 | 0 | 3 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Kim, H.; Lee, J.Y.; Won, G.; Choi, S.-I.; Kim, G.-H.; Kang, C.-H. The Antioxidant, Anti-Diabetic, and Anti-Adipogenesis Potential and Probiotic Properties of Lactic Acid Bacteria Isolated from Human and Fermented Foods. Fermentation 2021, 7, 123. https://doi.org/10.3390/fermentation7030123
Jeong Y, Kim H, Lee JY, Won G, Choi S-I, Kim G-H, Kang C-H. The Antioxidant, Anti-Diabetic, and Anti-Adipogenesis Potential and Probiotic Properties of Lactic Acid Bacteria Isolated from Human and Fermented Foods. Fermentation. 2021; 7(3):123. https://doi.org/10.3390/fermentation7030123
Chicago/Turabian StyleJeong, Yulah, Hyemin Kim, Ji Yeon Lee, GaYeong Won, Soo-Im Choi, Gun-Hee Kim, and Chang-Ho Kang. 2021. "The Antioxidant, Anti-Diabetic, and Anti-Adipogenesis Potential and Probiotic Properties of Lactic Acid Bacteria Isolated from Human and Fermented Foods" Fermentation 7, no. 3: 123. https://doi.org/10.3390/fermentation7030123
APA StyleJeong, Y., Kim, H., Lee, J. Y., Won, G., Choi, S.-I., Kim, G.-H., & Kang, C.-H. (2021). The Antioxidant, Anti-Diabetic, and Anti-Adipogenesis Potential and Probiotic Properties of Lactic Acid Bacteria Isolated from Human and Fermented Foods. Fermentation, 7(3), 123. https://doi.org/10.3390/fermentation7030123






