Conversion of a Thiol Precursor into Aroma Compound 4-mercapto-4-methyl-2-pentanone Using Microbial Cell Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Precursor cys-4MMP
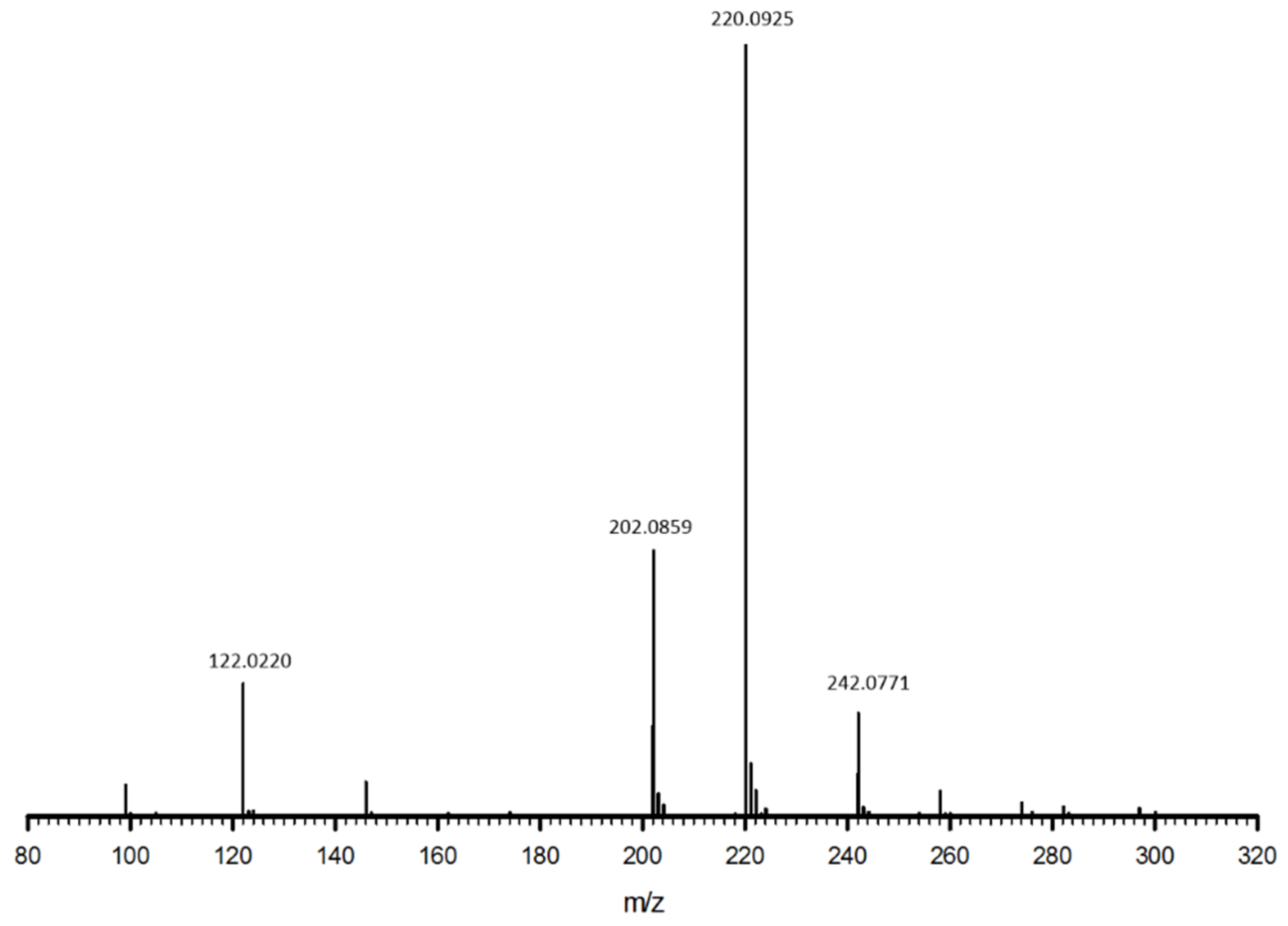
2.2. Liquid Chromatography-Mass Spectrometry
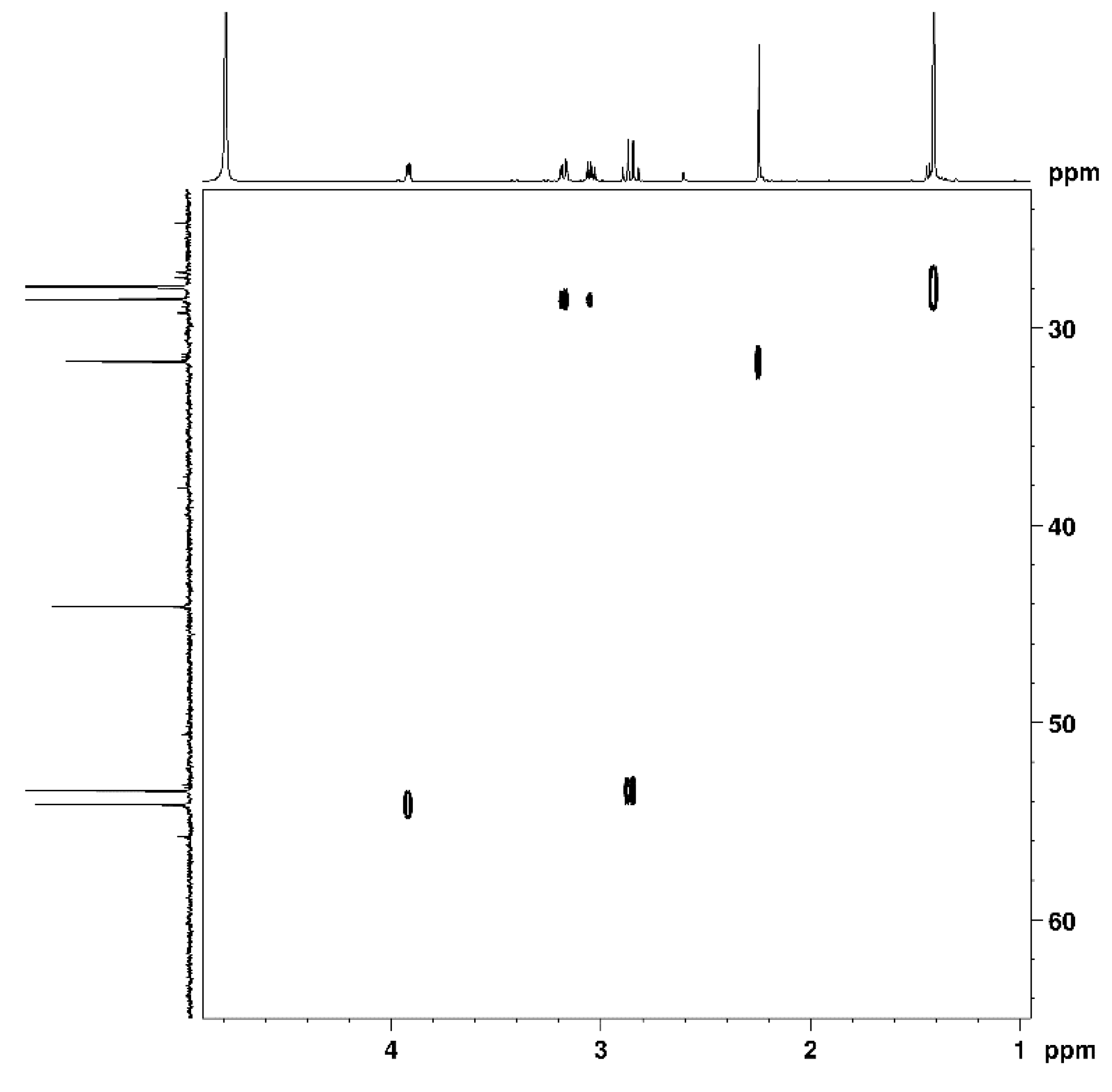
2.3. Nuclear Magnetic Resonance Spectroscopy
2.4. Bacterial and Yeast Strains and Their Cultivation
2.5. Preparation of Bacterial and Yeast Cell Extracts
2.6. β-lyase Activity Assays
2.7. Determination of 4-MMP by Liquid Chromatography-Mass Spectrometry
2.8. Statistical Analysis
3. Results and Discussion
3.1. Cys-4MMP Synthesis
3.2. Screening of Yeast Extracts for β-lyase Activities
3.3. Screening of Bacterial Extracts for β-lyase Activities
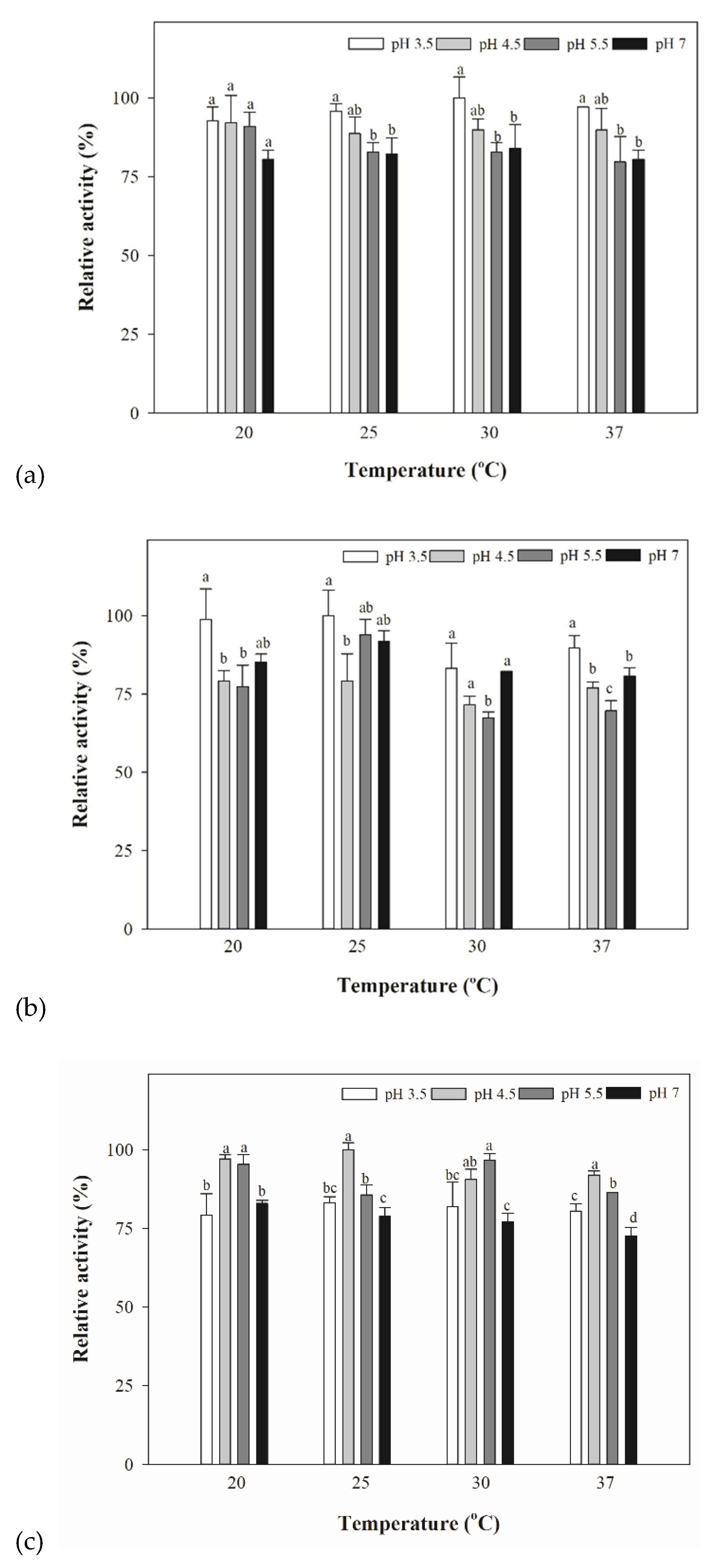
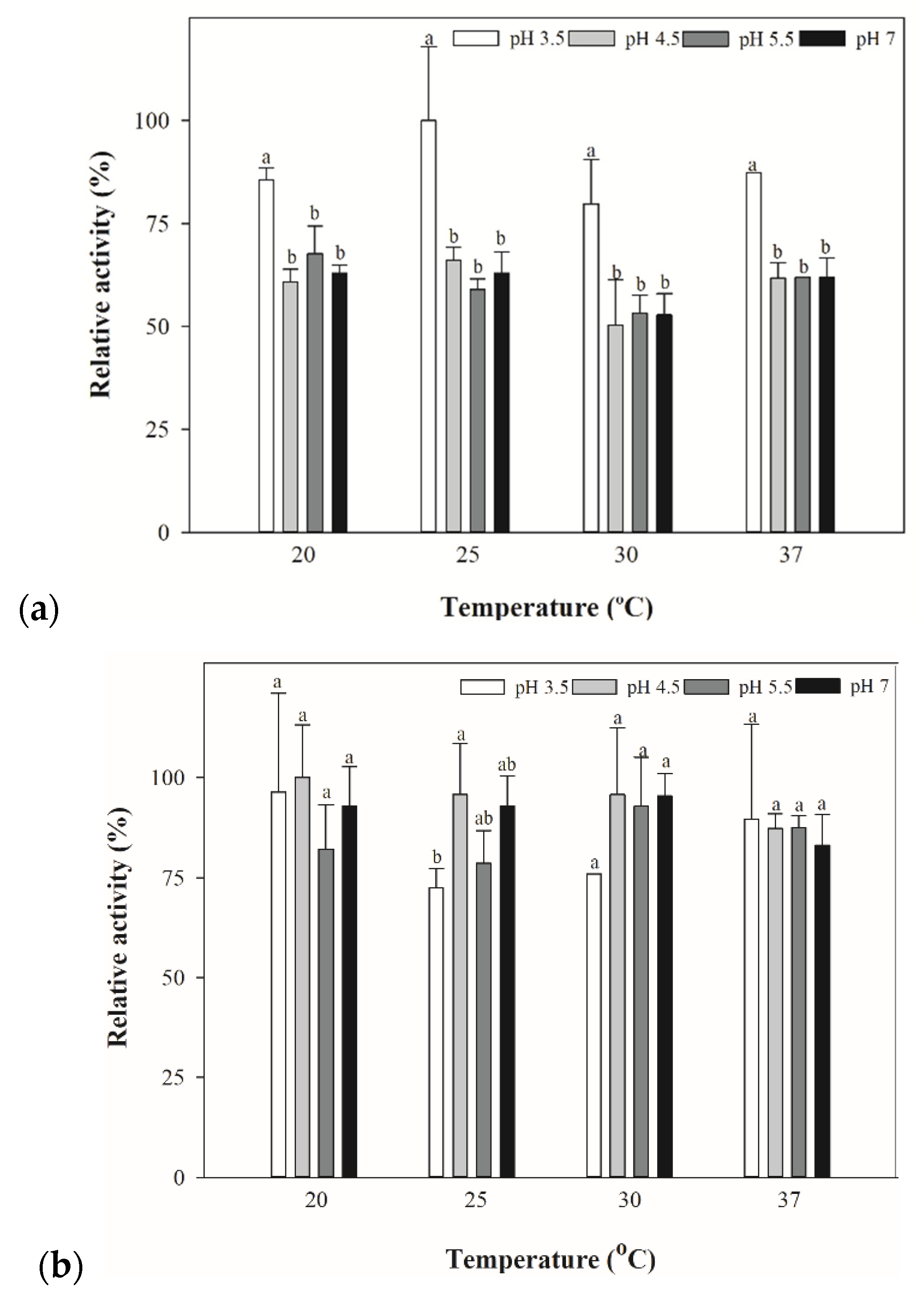
3.4. Optimized Temperature for Yeast and Bacterial Extracts for β-lyase Activities
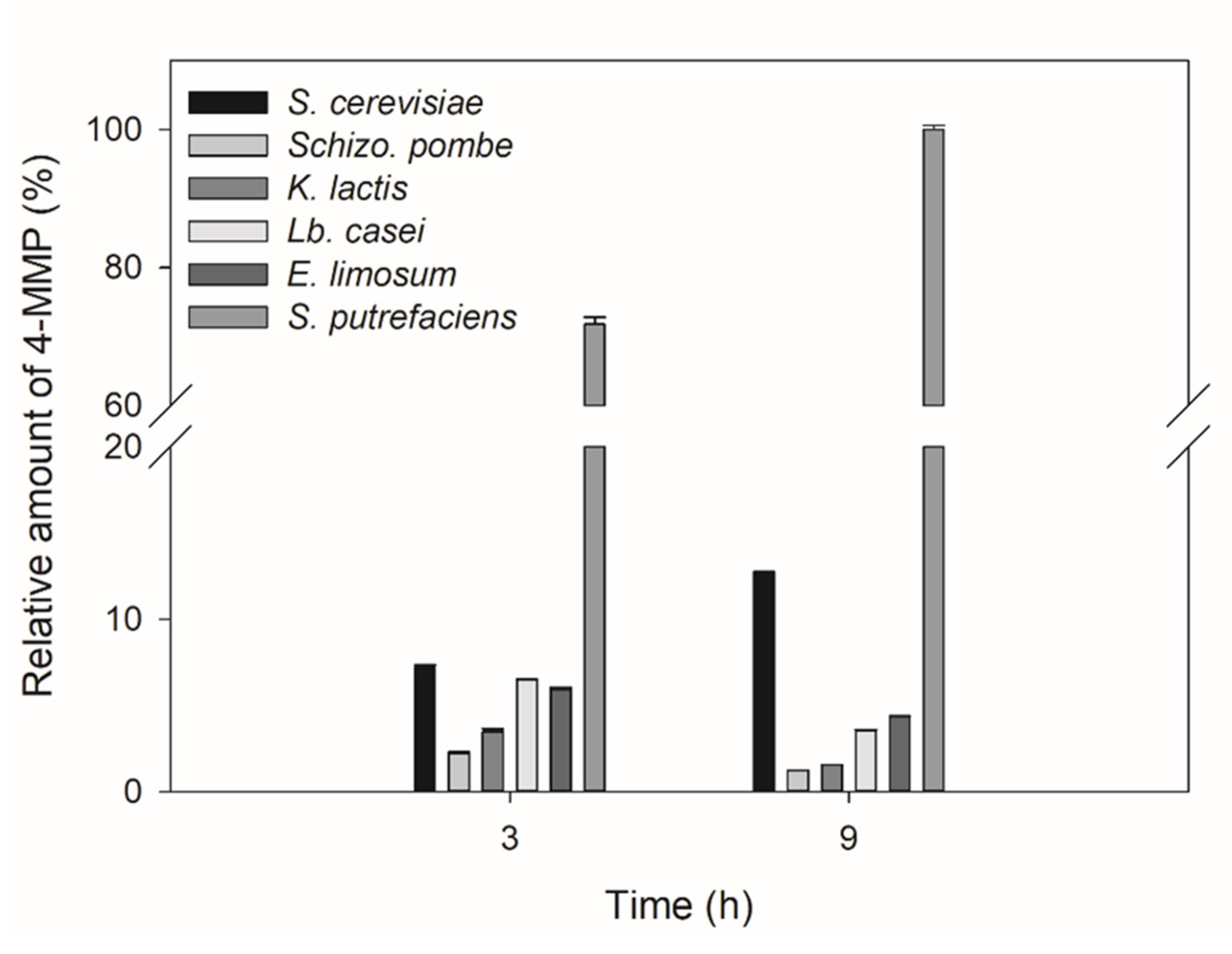
3.5. Conversion of cys-4MMP into Aroma Compounds by Yeasts and Bacteria Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MarketsandMarkets. Food Flavors Market by Type (Chocolate & Browns, Vanilla, Fruit & Nut, Dairy, Spices), Application (Beverages, Dairy, Confectionery, Bakery, Meat, Savory & Snacks), Origin (Natural, Nature identical, Artificial), Form, and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/food-flavors-market-93115891.html?gclid=CjwKCAjwgOGCBhAlEiwA7FUXkqVLaoy5qRdNMauvJdfTe-Lco2tDgZYDUNCq3c7UtmMcus6LdZYbuhoCw40QAvD_BwE (accessed on 23 March 2021).
- Kutyna, D.R.; Borneman, A.R. Heterologous production of flavour and aroma compounds in Saccharomyces cerevisiae. Genes 2018, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Gomez-Torres, N.; Garde, S.; Peiroten, A.; Avila, M. Impact of Clostridium spp. on cheese characteristics: Microbiology, color, formation of volatile compounds and off-flavors. Food Control 2015, 56, 186–194. [Google Scholar] [CrossRef]
- Gallage, N.J.; Moller, B.L. Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol. Plant 2015, 8, 40–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muheim, A.; Mueller, B.; Muench, T.; Wetli, M.G.R.I. Process for the Production of Vanillin. European Patent Office, EP0885968 (A1), 23 December 1998. [Google Scholar]
- Ricci, A.; Levante, A.; Cirlini, M.; Calani, L.; Bernini, V.; Del Rio, D.; Galaverna, G.; Neviani, E.; Lazzi, C. The influence of viable cells and cell-free extracts of Lactobacillus casei on volatile compounds and polyphenolic profile of elderberry juice. Front. Microbiol. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Tominaga, T.; des Gachons, C.P.; Dubourdieu, D. A new type of flavor precursors in Vitis vinifera L cv Sauvignon blanc: S-cysteine conjugates. J. Agric. Food Chem. 1998, 46, 5215–5219. [Google Scholar] [CrossRef]
- Dhake, K.P.; Tambade, P.J.; Qureshi, Z.S.; Singhal, R.S.; Bhanage, B.M. HPMC-PVA film immobilized Rhizopus oryzae lipase as a biocatalyst for transesterification reaction. Acs Catal. 2011, 1, 316–322. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Microbial glyucosidases for wine production. Beverages 2016, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Allegrini, A.; Astegno, A.; La Verde, V.; Dominici, P. Characterization of C-S lyase from Lactobacillus delbrueckii subsp. bulgaricus ATCC BAA-365 and its potential role in food flavour applications. J. Biochem. 2017, 161, 349–360. [Google Scholar] [CrossRef]
- Parker, J.K. Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Pub.: Waltham, MA, USA, 2014; p. 423. [Google Scholar]
- Cooper, A.J.; Krasnikov, B.F.; Niatsetskaya, Z.V.; Pinto, J.T.; Callery, P.S.; Villar, M.T.; Artigues, A.; Bruschi, S.A. Cysteine S-conjugate β-lyases: Important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents. Amino Acids 2011, 41, 7–27. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Banavara, D.S.; Hughes, J.E.; Christiansen, J.K.; Steele, J.L.; Broadbent, J.R.; Rankin, S.A. Role of cystathionine beta-lyase in catabolism of amino acids to sulfur volatiles by genetic variants of Lactobacillus helveticus CNRZ 32. Appl. Environ. Microbiol. 2007, 73, 3034–3039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zha, M.; Yin, S.; Sun, B.; Wang, X.; Wang, C. STR3 and CYS3 contribute to 2-furfurylthiol biosynthesis in Chinese sesame-flavored Baijiu yeast. J. Agric. Food Chem. 2017, 65, 5503–5511. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Sun, B.; Yin, S.; Mehmood, A.; Cheng, L.; Wang, C. Generation of 2-furfurylthiol by carbon-sulfur lyase from the Baijiu Yeast Saccharomyces cerevisiae G20. J. Agric. Food Chem. 2018, 66, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Darriet, P.; Lavigne, V.; Boidron, J.D.D. Identification of a powerful aromatic component of Vitis vinifera L. var. sauvignon wines: 4-mercapto-4-methylpentan-2-one. Flavour Frag J. 1995, 10, 385–392. [Google Scholar] [CrossRef]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of new volatile thiols in the aroma of Vitis vinifera L. var. Sauvignon blanc wines. Flavour Frag. J 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Howell, K.S.; Swiegers, J.H.; Elsey, G.M.; Siebert, T.E.; Bartowsky, E.J.; Fleet, G.H.; Pretorius, I.S.; Pretorius, S.; Lopes, M.A.D. Variation in 4-mercapto-4-methyl-pentan-2-one release by Saccharomyces cerevisiae commercial wine strains. FEMS Microbiol. Lett. 2004, 240, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Murat, M.L.; Masneuf, I.; Darriet, P.; Lavigne, V.; Tominaga, T.; Dubourdieu, D. Effect of Saccharomyces cerevisiae yeast strains on the liberation of volatile thiols in Sauvignon blanc wine. Am. J. Enol. Vitic. 2001, 52, 136–139. [Google Scholar]
- Yoshida, Y.; Negishi, M.; Amano, A.; Oho, T.; Nakano, Y. Differences in the betaC-S lyase activities of viridans group streptococci. Biochem. Biophys. Res. Commun. 2003, 300, 55–60. [Google Scholar] [CrossRef]
- Irmler, S.; Raboud, S.; Beisert, B.; Rauhut, D.; Berthoud, H. Cloning and characterization of two Lactobacillus casei genes encoding a cystathionine lyase. Appl. Environ. Microbiol. 2008, 74, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Capone, D.L.; Ristic, R.; Pardon, K.H.; Jeffery, D.W. Simple quantitative determination of potent thiols at ultratrace levels in wine by derivatization and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) analysis. Anal. Chem. 2015, 87, 1226–1231. [Google Scholar] [CrossRef]
- Starkenmann, C. Analysis of a model reaction system containing cysteine and (E)-2-methyl-2-butenal, (E)-2-hexenal, or mesityl oxide. J. Agric. Food Chem. 2003, 51, 7146–7155. [Google Scholar] [CrossRef]
- Hebditch, K.R.; Nicolau, L.; Brimble, M.A. Synthesis of isotopically labelled thiol volatiles and cysteine conjugates for quantification of Sauvignon Blanc wine. J. Labelled Compd. Radiopharm. 2007, 50, 237–243. [Google Scholar] [CrossRef]
- Holt, S.; Cordente, A.G.; Curtin, C. Saccharomyces cerevisiae STR3 and yeast cystathionine β-lyase enzymes: The potential for engineering increased flavor release. Bioengineered 2012, 3, 178–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, S.; Cordente, A.G.; Williams, S.J.; Capone, D.L.; Jitjaroen, W.; Menz, I.R.; Curtin, C.; Anderson, P.A. Engineering Saccharomyces cerevisiae to release 3-Mercaptohexan-1-ol during fermentation through overexpression of an S. cerevisiae Gene, STR3, for improvement of wine aroma. Appl. Environ. Microbiol. 2011, 77, 3626–3632. [Google Scholar] [CrossRef] [Green Version]
- Alting, A.C.; Engels, W.J.M.; Vanschalkwijk, S.; Exterkate, F.A. Purification and characterization of cystathionine beta-lyase from Lactococcus lactis subsp, cremoris B78 and its possible role in flavor development in cheese. Appl. Environ. Microbiol. 1995, 61, 4037–4042. [Google Scholar] [CrossRef] [Green Version]
- Bruinenberg, P.G.; deRoo, G.; Limsowtin, G.K.Y. Purification and characterization of cystathionine gamma-lyase from Lactococcus lactis subsp cremoris SK11: Possible role in flavor compound formation during cheese maturation. Appl. Environ. Microbiol. 1997, 63, 561–566. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Li, N.; Mao, Y.; Zhou, G.; Gao, H. Endogenous generation of hydrogen sulfide and its regulation in Shewanella oneidensis. Front. Microbiol. 2015, 6, 374. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Kleerebezem, M.; Kuipers, O.P.; Siezen, R.J.; van Kranenburg, R. Regulation of the metC-cysK operon, involved in sulfur metabolism in Lactococcus lactis. J. Bacteriol. 2002, 184, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Fedrizzi, B.; Pardon, K.H.; Sefton, M.A.; Elsey, G.M.; Jeffery, D.W. First identification of 4-S-glutathionyl-4-methylpentan-2-one, a potential precursor of 4-mercapto-4-methylpentan-2-one, in Sauvignon blanc juice. J. Agric. Food Chem. 2009, 57, 991–995. [Google Scholar] [CrossRef]
- Klein, N.; Maillard, M.B.; Thierry, A.; Lortal, S. Conversion of amino acids into aroma compounds by cell-free extracts of Lactobacillus helveticus. J. Appl. Microbiol. 2001, 91, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, W.S.; Queiroz, P.V.; Tavares, G.P.; Santos, F.A.; Soares, F.E.F.; Kasuya, M.C.M.; Queiroz, J.H. Multi-enzyme complex of white rot fungi in saccharification of lignocellulosic material. Braz. J. Microbiol. 2018, 49, 879–884. [Google Scholar] [CrossRef]



| Yeasts | L-Cystathionine | L-Cystine | L-Methionine | |||
|---|---|---|---|---|---|---|
| β-Lyase Activity (U/mL) | Specific Activities (U/mg Protein) | β-Lyase Activity (U/mL) | Specific Activities (U/mg Protein) | β-Lyase Activity (U/mL) | Specific Activities (U/mg Protein) | |
| S. cerevisiae | ||||||
| 21584 | 70.72 ± 7.92 a | 27.63 ± 3.09 a | 22.26 ± 2.41 a | 8.70 ± 0.94 a | 44.87 ± 2.09 b | 17.53 ± 0.82 b |
| 21685 | 56.81 ± 6.96 b | 22.19 ± 2.72 b | 13.57 ± 2.09 cde | 5.30 ± 0.82 b | 40.70 ± 2.09 bc | 15.90 ± 0.81 bc |
| 21686 | 12.75 ± 2.40 c | 6.54 ± 1.19 c | 12.52 ± 0.00 bc | 6.42 ± 0.00 b | 10.43 ± 2.41 e | 5.35 ± 1.24 e |
| K. marxianus | 52.56 ± 5.36 b | 20.53 ± 2.09 b | 15.30 ± 2.41 de | 5.98 ± 0.94 b | 37.57 ± 3.41 c | 14.67 ± 1.33 c |
| K. lactis | 69.57 ± 4.64 a | 27.17 ± 1.81 a | 13.91 ± 4.82 bcd | 5.43 ± 1.88 b | 60.52 ± 7.23 a | 23.64 ± 2.82 a |
| D. hansenii | 17.01 ± 7.08 c | 5.64 ± 2.77 c | 22.96 ± 14.76 e | 8.97 ± 5.76 a | 25.04 ± 7.62 d | 9.78 ± 2.98 d |
| Schizo. pombe | 57.20 ± 2.68 b | 22.34 ± 1.05 b | 23.65 ± 2.41 ab | 9.24 ± 0.94 a | 63.65 ± 6.26 a | 24.86 ± 2.45 a |
| Bacteria | L-Cystathionine | L-Cystine | L-Methionine | |||
|---|---|---|---|---|---|---|
| β-Lyase Activity (U/mL) | Specific Activities (U/mg Protein) | β-Lyase Activity (U/mL) | Specific Activities (U/mg Protein) | β-Lyase Activity (U/mL) | Specific Activities (U/mg Protein) | |
| B. subtilis | 27.99 ± 2.75 c | 8.49 ± 0.84 c | 29.58 ± 4.77 b | 8.08 ± 1.30 b | 21.63 ± 2.75 c | 5.91 ± 0.75 c |
| E. limosum | 13.91 ± 8.03 d | 7.14 ± 4.12 b | 6.96 ± 3.28 c | 3.21 ± 1.51 c | 12.37 ± 5.36 c | 5.71 ± 2.47 b |
| Lb. bulgaricus | 23.95 ± 5.93 c | 7.54 ± 1.86 c | 32.50 ± 2.96 b | 9.20 ± 0.84 b | 13.68 ± 2.96 c | 3.88 ± 0.84 c |
| Lb. casei | 40.19 ± 2.68 b | 11.48 ± 0.77 b | 27.83 ± 4.64 b | 7.16 ± 1.19 b | 32.46 ± 4.64 b | 8.35 ± 1.19 b |
| S. putrefaciens | 305.46 ± 25.66 a | 41.84 ± 3.51 a | 207.62 ± 22.98 a | 25.60 ± 2.83 a | 184.33 ± 10.76 a | 22.73 ± 1.33 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-K.; Chang, C.-F.; Lin, H.-J.; Lin, J.-L.; Lee, Y.-T.; Wu, Y.-H.; Liu, C.-Y.; Lin, T.-C.; Hsu, P.-H.; Lin, H.-T.V. Conversion of a Thiol Precursor into Aroma Compound 4-mercapto-4-methyl-2-pentanone Using Microbial Cell Extracts. Fermentation 2021, 7, 129. https://doi.org/10.3390/fermentation7030129
Li H-K, Chang C-F, Lin H-J, Lin J-L, Lee Y-T, Wu Y-H, Liu C-Y, Lin T-C, Hsu P-H, Lin H-TV. Conversion of a Thiol Precursor into Aroma Compound 4-mercapto-4-methyl-2-pentanone Using Microbial Cell Extracts. Fermentation. 2021; 7(3):129. https://doi.org/10.3390/fermentation7030129
Chicago/Turabian StyleLi, Hao-Kai, Chi-Fon Chang, Hsuan-Ju Lin, Jung-Lee Lin, Yu-Ting Lee, Yu-Hsuan Wu, Chiao-Yen Liu, Tze-Chia Lin, Pang-Hung Hsu, and Hong-Ting Victor Lin. 2021. "Conversion of a Thiol Precursor into Aroma Compound 4-mercapto-4-methyl-2-pentanone Using Microbial Cell Extracts" Fermentation 7, no. 3: 129. https://doi.org/10.3390/fermentation7030129
APA StyleLi, H.-K., Chang, C.-F., Lin, H.-J., Lin, J.-L., Lee, Y.-T., Wu, Y.-H., Liu, C.-Y., Lin, T.-C., Hsu, P.-H., & Lin, H.-T. V. (2021). Conversion of a Thiol Precursor into Aroma Compound 4-mercapto-4-methyl-2-pentanone Using Microbial Cell Extracts. Fermentation, 7(3), 129. https://doi.org/10.3390/fermentation7030129






