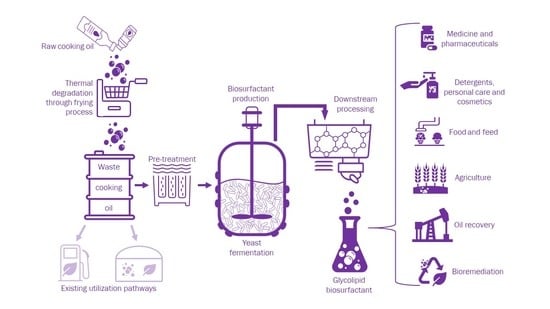
Glycolipid Biosurfactant Production from Waste Cooking Oils by Yeast: Review of Substrates, Producers and Products
Abstract
:1. Introduction
2. Waste Cooking Oils as a Substrate
2.1. Composition of Waste Cooking Oils
| Oil | Fatty Acid Composition, wt% | Property | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Myristic C14:0 | Palmitic C16:0 | Stearic C18:0 | Oleic C18:1 | Linoleic C18:2 | Linolenic C18:3 | Other | Acid Value, mg KOH·g−1 of Oil | Peroxide Value, mEq·kg−1 | ||
| WCO, n/s a | - | 6.70 | 1.60 | 19.30 | 72.40 | - | - | 4.1 | <5 | [22] |
| WCO, n/s | - | 8.50 | 3.10 | 21.20 | 55.20 | 5.90 | 4.20 | 3.6 | 23.1 | [40] |
| WCO, palm | 0.98 | 39.02 | 4.52 | 44.57 | 10.91 | - | - | 2 | 7.1 | [41] |
| WCO, palm olein | 1.93 | 45.68 | 4.25 | 40.19 | 7.95 | - | - | 36.81 | - | [42] |
| WCO, n/s | 0.14 | 11.73 | 2.10 | 27.75 | 53.29 | 0.83 | 2.44 | - | - | [43] |
| WCO, n/s | - | 18.16 | 8.12 | 40.72 | 25.81 | 7.19 | - | - | - | [44] |
| WCO, sunflower | - | 19.12 | 9.43 | 34.33 | 32.22 | - | 4.9 | 8 | 102 | [29] |
| WCO, rapeseed b | 0.14 | 7.17 | 2.83 | 63.62 | 17.39 | 5.81 | - | [45] | ||
| Sunflower | - | 8.92 | 3.36 | 26.27 | 60.24 | 0.38 | - | 0.29 | 0.36 | [46] |
| Sunflower | - | 7 | 5 | 20–25 | 63–68 | 0.2 | - | - | - | [34] |
| Palm | 1.00 | 37–41 | 3–6 | 40–45 | 8–10 | - | - | - | - | [34] |
| Rapeseed | 0.07 | 4.82 | 1.94 | 61.56 | 20.46 | 8.49 | - | 0.08 | 3.62 | [45] |
| Rapeseed | - | 4–5 | 1–2 | 56–64 | 20–26 | 8–10 | 9.1 | - | - | [34] |
2.2. Pretreatment of Waste Cooking Oils
3. Nonconventional Yeasts as Biosurfactant Producers
3.1. Sophorolipids
3.2. Rhamnolipids
3.3. Mannosylerythritol Lipids
3.4. Emerging Glycolipids Produced by Yeasts
3.5. Downstream Processing
4. Properties of WCO-Derived Glycolipid Biosurfactants
4.1. Surface Tension and Interfacial Activity
4.2. Emulsification and De-Emulsification
4.3. Temperature and pH Tolerance
4.4. Biodegradation and Toxicity
5. Applications of WCO-Derived Glycolipid Biosurfactants
5.1. Agriculture and Bioremediation
5.2. Detergents, Personal Care, and Cosmetics
5.3. Oil Recovery
5.4. Medicine and Pharmaceuticals
5.5. Food and Feed
5.6. Other Emerging Applications
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiménez-Peñalver, P.; Rodríguez, A.; Daverey, A.; Font, X.; Gea, T. Use of wastes for sophorolipids production as a transition to circular economy: State of the art and perspectives. Rev. Environ. Sci. Bio Technol. 2019, 18, 413–435. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physico-chemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, S.; Claus, S.; Van Bogaert, I. Yeast glycolipid biosurfactants. FEBS Lett. 2017, 592, 1312–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos-Takaki, G.M.; Sarubbo, L.A.; Albuquerque, C.D.C. Environmentally friendly biosurfactants produced by yeasts. Biosurfactants 2010, 672, 250–260. [Google Scholar] [CrossRef]
- Saharan, B.S.; Sahu, R.K.; Sharma, D. A review on biosurfactants: Fermentation, current developments and perspectives. Genet. Eng. Biotechnol. J. 2012, 2011, 1–14. [Google Scholar]
- Dhanarajan, G.; Sen, R. Cost Analysis of Biosurfactant Production from a Scientist’s Perspective. In Biosurfactants; Kosaric, N., Sukan, F.V., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 164–175. [Google Scholar]
- Market Research Engine. Biosurfactants Market Research Report. 2020. Available online: https://www.marketresearchengine.com/biosurfactants-market (accessed on 16 March 2021).
- Hayes, D.G.; Solaiman, D.K.Y.; Ashby, R.D. Biobased Surfactants Synthesis, Properties and Applications; Academic Press: Cambridge, MA, USA; OCS Press: Boulder Urbana, IL, USA, 2019; Volume 2, ISBN 9780128127056. [Google Scholar]
- Ashby, R.D.; McAloon, A.J.; Solaiman, D.K.Y.; Yee, W.C.; Reed, M. A process model for approximating the production costs of the fermentative synthesis of sophorolipids. J. Surfactants Deterg. 2013, 16, 683–691. [Google Scholar] [CrossRef]
- Baccile, N.; Babonneau, F.; Banat, I.; Ciesielska, K.; Cuvier, A.-S.; Devreese, B.; Everaert, B.; Lydon, H.; Marchant, R.; Mitchell, C.A.; et al. Development of a cradle-to-grave approach for acetylated acidic sophorolipid biosurfactants. ACS Sustain. Chem. Eng. 2016, 5, 1186–1198. [Google Scholar] [CrossRef] [Green Version]
- Makkar, S.C.R. An update on the use of unconventional substrates for biosurfactant production and their new applications. Appl. Microbiol. Biotechnol. 2002, 58, 428–434. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.; Contiero, J. Rhamnolipid surfactants: An update on the general aspects of these remarkable biomolecules. Biotechnol. Prog. 2005, 21, 1593–1600. [Google Scholar] [CrossRef]
- Sobrinho, H.B.; Rufino, R.D.; Luna, J.M.; Salgueiro, A.A.; Campos-Takaki, G.M.; Leite, L.F.; Sarubbo, L.A. Utilization of two agroindustrial by-products for the production of a surfactant by Candida sphaerica UCP0995. Process Biochem. 2008, 43, 912–917. [Google Scholar] [CrossRef]
- Zenati, B.; Chebbi, A.; Badis, A.; Eddouaouda, K.; Boutoumi, H.; El Hattab, M.; Hentati, D.; Chelbi, M.; Sayadi, S.; Chamkha, M.; et al. A non-toxic microbial surfactant from Marinobacter hydrocarbonoclasticus SdK644 for crude oil solubilization enhancement. Ecotoxicol. Environ. Saf. 2018, 154, 100–107. [Google Scholar] [CrossRef]
- Jalkh, R.; El-Rassy, H.; Chehab, G.R.; Abiad, M.G. Assessment of the physico-chemical properties of waste cooking oil and spent coffee grounds oil for potential use as asphalt binder rejuvenators. Waste Biomass Valorization 2017, 9, 2125–2132. [Google Scholar] [CrossRef]
- Kim, J.-H.; Oh, Y.-R.; Hwang, J.; Kang, J.; Kim, H.; Jang, Y.-A.; Lee, S.-S.; Hwang, S.Y.; Park, J.; Eom, G.T. Valorization of waste-cooking oil into sophorolipids and application of their methyl hydroxyl branched fatty acid derivatives to produce engineering bioplastics. Waste Manag. 2021, 124, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Razaviarani, V.; Buchanan, I.D.; Malik, S.; Katalambula, H. Pilot-scale anaerobic co-digestion of municipal wastewater sludge with restaurant grease trap waste. J. Environ. Manag. 2013, 123, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sustain. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Wallace, T.; Gibbons, D.; O’Dwyer, M.; Curran, T.P. International evolution of fat, oil and grease (FOG) waste management—A review. J. Environ. Manag. 2017, 187, 424–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B. Improved synthesis of sophorolipids from waste cooking oil using fed batch approach in the presence of ultrasound. Chem. Eng. J. 2015, 263, 479–487. [Google Scholar] [CrossRef]
- European Biomass Industry Association (EUBIA). Used Cooking Oil. 2021. Available online: https://www.eubia.org/cms/wiki-biomass/biomass-resources/challenges-related-to-biomass/used-cooking-oil-recycling/ (accessed on 5 December 2020).
- European Comission (EC). Regulation (EC) No. 1069/2009 of the European Parliament and of the Council of 21 October 2009—Laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002. Off. J. Eur. Union 2009, 300, 1–33. [Google Scholar]
- European Biomass Industry Association (EUBIA). Transformation of Used Cooking Oil into Biodiesel: From Waste to Resource (UCO to Biodiesel 2030); European Biomass Industry Association: Brussels, Belgium, 2015. [Google Scholar]
- Dahiya, S.; Kumar, A.N.; Sravan, J.S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, J.; Orjuela, A.; Sánchez, D.L.; Narváez, P.C.; Katryniok, B.; Clark, J. Pre-treatment of used cooking oils for the production of green chemicals: A review. J. Clean. Prod. 2021, 289, 125129. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.; Belo, I. Microbial valorization of waste cooking oils for valuable compounds production—A review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 2583–2616. [Google Scholar] [CrossRef]
- Wadekar, S.D.; Kale, S.B.; Lali, A.M.; Bhowmick, D.N.; Pratap, A.P. Microbial synthesis of rhamnolipids by Pseudomonas aeruginosa (ATCC 10145) on waste frying oil as low-cost carbon source. Prep. Biochem. Biotechnol. 2012, 42, 249–266. [Google Scholar] [CrossRef]
- Mannu, A.; Vlahopoulou, G.; Urgeghe, P.; Ferro, M.; Del Caro, A.; Taras, A.; Garroni, S.; Rourke, J.P.; Cabizza, R.; Petretto, G.L.; et al. Variation of the chemical composition of waste cooking oils upon bentonite filtration. Resources 2019, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Armendáriz, B.; Cal-Y-Mayor-Luna, C.; El-Kassis, E.G.; Ortega-Martínez, L.D. Use of waste canola oil as a low-cost substrate for rhamnolipid production using Pseudomonas aeruginosa. AMB Express 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Orjuela, A.; Clark, J. Green chemicals from used cooking oils: Trends, challenges and opportunities. Curr. Opin. Green Sustain. Chem. 2020, 26, 100369. [Google Scholar] [CrossRef]
- Bockisch, M. Oil Purification. In Fats and Oils Handbook; Academic Press: Cambridge, MA, USA; OCS Press: Boulder Urbana, IL, USA, 1998; pp. 613–718. ISBN 9780981893600. [Google Scholar]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Husain, I.; Alkhatib, M.F.; Jammi, M.S.; Mirghani, M.E.S.; Bin Zainudin, Z.; Hoda, A. Problems, control, and treatment of fat, oil and grease (FOG): A review. J. Oleo Sci. 2014, 63, 747–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, Y.; Semwal, A.D. A study on monitoring of frying performance and oxidative stability of virgin coconut oil (VCO) during continuous/prolonged deep fat frying process using chemical and FTIR spectroscopy. J. Food Sci. Technol. 2013, 52, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Rincón, L.A.; Cadavid, J.G.; Orjuela, A. Used cooking oils as potential oleochemical feedstock for urban biorefineries—Study case in Bogota, Colombia. Waste Manag. 2019, 88, 200–210. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Codex Standard for Edible Fats and Oils Not Covered by Individual Standards (CXS 19-1981); Food and Agriculture Organization of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Orthoefer, F.T.; List, G.R. Evaluation of used frying oil. In Deep Frying: Chemistry, Nutrition and Practical Applications; AOCS Press: Boulder Urbana, IL, USA, 2007; pp. 329–342. ISBN 9780128043530. [Google Scholar]
- Wen, Z.; Yu, X.; Tu, S.-T.; Yan, J.; Dahlquist, E. Biodiesel production from waste cooking oil catalyzed by TiO2-MgO mixed oxides. Bioresour. Technol. 2010, 101, 9570–9576. [Google Scholar] [CrossRef]
- Chuah, L.F.; Aziz, A.R.A.; Yusup, S.; Bokhari, A.; Klemeš, J.J.; Abdullah, M.Z. Performance and emission of diesel engine fuelled by waste cooking oil methyl ester derived from palm olein using hydrodynamic cavitation. Clean Technol. Environ. Policy 2015, 17, 2229–2241. [Google Scholar] [CrossRef]
- Abdulkareem-Alsultan, G.; Asikin, N.; Mansir, N.; Lee, H.; Zainal, Z.; Islam, A.; Taufiq-Yap, Y. Pyrolytic deoxygenation of waste cooking oil for green diesel production over Ag2O3-La2O3/AC nano-catalyst. J. Anal. Appl. Pyrolysis 2019, 137, 171–184. [Google Scholar] [CrossRef]
- Shah, V.; Jurjevic, M.; Badia, D. Utilization of restaurant waste oil as a precursor for sophorolipid production. Biotechnol. Prog. 2007, 23, 512–515. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Cai, B.-X.; Xu, W.-J.; Gang, H.-Z.; Liu, J.-F.; Yang, S.-Z.; Mu, B.-Z. The rebirth of waste cooking oil to novel bio-based surfactants. Sci. Rep. 2015, 5, 9971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aniołowska, M.; Zahran, H.; Kita, A. The effect of pan frying on thermooxidative stability of refined rapeseed oil and professional blend. J. Food Sci. Technol. 2015, 53, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadekar, S.; Kale, S.; Lali, A.; Bhowmick, D.; Pratap, A. Sophorolipid production by Starmerella bombicola (ATCC 22214) from virgin and waste frying oils, and the effects of activated earth treatment of the waste oils. J. Am. Oil Chem. Soc. 2011, 89, 1029–1039. [Google Scholar] [CrossRef]
- Rincón, L.A.; Ramírez, J.C.; Orjuela, A. Assessment of degumming and bleaching processes for used cooking oils upgrading into oleochemical feedstocks. J. Environ. Chem. Eng. 2021, 9, 104610. [Google Scholar] [CrossRef]
- Mannu, A.; Garroni, S.; Porras, J.I.; Mele, A. Available technologies and materials for waste cooking oil recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Icyer, N.C.; Durak, M.Z. Ultrasound-assisted bleaching of canola oil: Improve the bleaching process by central composite design. LWT 2018, 97, 640–647. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Generally Recognized as Safe (GRAS). 2019. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 20 January 2021).
- Bourdichon, F.; Casaregola, S.; Farrokh, C.; Frisvad, J.; Gerds, M.L.; Hammes, W.P.; Harnett, J.; Huys, G.; Laulund, S.; Ouwehand, A.; et al. Food fermentations: Microorganisms with technological beneficial use. Int. J. Food Microbiol. 2012, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- De Graeve, M.; De Maeseneire, S.; Roelants, S.L.K.W.; Soetaert, W. Starmerella bombicola, an industrially relevant, yet fundamentally underexplored yeast. FEMS Yeast Res. 2018, 18, foy072. [Google Scholar] [CrossRef]
- Blount, Z.D. The unexhausted potential of E. coli. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, J.; Tümmler, B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Research 2017, 6, 1261. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Baccile, N.; Banat, I.M.; Déziel, E.; Marchant, R.; Roelants, S.; Van Bogaert, I.N.A. Microbial biosurfactant research: Time to improve the rigour in the reporting of synthesis, functional characterization and process development. Microb. Biotechnol. 2020, 14, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Roy, A. A review on the biosurfactants: Properties, types and its applications. J. Fundam. Renew. Energy Appl. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Ratsep, P.; Shah, V. Identification and quantification of sophorolipid analogs using ultra-fast liquid chromatography-mass spectrometry. J. Microbiol. Methods 2009, 78, 354–356. [Google Scholar] [CrossRef]
- Solaiman, D.K.Y.; Ashby, R.D.; Birbir, M.; Caglayan, P. Antibacterial activity of sophorolipids produced by candida bombicola on Gram-positive and Gram-negative bacteria isolated from salted hides. J. Am. Leather Chem. Assoc. 2016, 111, 358–363. [Google Scholar]
- Spencer, J.F.T.; Gorin, P.A.J.; Tulloch, A.P. Torulopsis bombicola sp. n. Antonie Leeuwenhoek 1970, 36, 129–133. [Google Scholar] [CrossRef]
- Ma, X.; Meng, L.; Zhang, H.; Zhou, L.; Yue, J.; Zhu, H.; Yao, R. Sophorolipid biosynthesis and production from diverse hydrophilic and hydrophobic carbon substrates. Appl. Microbiol. Biotechnol. 2019, 104, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Van Bogaert, I.N.A.; Holvoet, K.; Roelants, S.L.K.W.; Li, B.; Lin, Y.-C.; Van de Peer, Y.; Soetaert, W. The biosynthetic gene cluster for sophorolipids: A biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol. Microbiol. 2013, 88, 501–509. [Google Scholar] [CrossRef]
- Saerens, K.M.; Roelants, S.L.; Van Bogaert, I.N.; Soetaert, W. Identification of the UDP-glucosyltransferase gene UGTA1, responsible for the first glucosylation step in the sophorolipid biosynthetic pathway of Candida bombicola ATCC 22214. FEMS Yeast Res. 2010, 11, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielska, K.; Van Bogaert, I.; Chevineau, S.; Li, B.; Groeneboer, S.; Soetaert, W.; Van de Peer, Y.; Devreese, B. Exoproteome analysis of Starmerella bombicola results in the discovery of an esterase required for lactonization of sophorolipids. J. Proteom. 2014, 98, 159–174. [Google Scholar] [CrossRef]
- Casas, J.A.; Garcia-Ochoa, F. Sophorolipid production by Candida bombicola: Medium composition and culture methods. J. Biosci. Bioeng. 1999, 88, 488–494. [Google Scholar] [CrossRef]
- Cavalero, D.A.; Cooper, D.G. The effect of medium composition on the structure and physical state of sophorolipids produced by Candida bombicola ATCC 22214. J. Biotechnol. 2003, 103, 31–41. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.; Zhang, J.; Soetaert, W. Microbial synthesis of sophorolipids. Process Biochem. 2011, 46, 821–833. [Google Scholar] [CrossRef]
- Hommel, R.; Weber, L.; Weiss, A.; Himmelreich, U.; Rilke, O.; Kleber, H.-P. Production of sophorose lipid by Candida (Torulopsis) apicola grown on glucose. J. Biotechnol. 1994, 33, 147–155. [Google Scholar] [CrossRef]
- Takahashi, M.; Morita, T.; Wada, K.; Hirose, N.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of sophorolipid glycolipid biosurfactants from sugarcane molasses using Starmerella bombicola NBRC 10243. J. Oleo Sci. 2011, 60, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Bogaert, I.N.A.; Roelants, S.; Develter, D.; Soetaert, W. Sophorolipid production by Candida bombicola on oils with a special fatty acid composition and their consequences on cell viability. Biotechnol. Lett. 2010, 32, 1509–1514. [Google Scholar] [CrossRef]
- Saerens, K.M.; Van Bogaert, I.N.; Soetaert, W. Characterization of sophorolipid biosynthetic enzymes from Starmerella bombicola. FEMS Yeast Res. 2015, 15, fov075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleurackers, S.J.J. On the use of waste frying oil in the synthesis of sophorolipids. Eur. J. Lipid Sci. Technol. 2006, 108, 5–12. [Google Scholar] [CrossRef]
- Samad, A.; Zhang, J.; Chen, D.; Chen, X.; Tucker, M.; Liang, Y. Sweet sorghum bagasse and corn stover serving as substrates for producing sophorolipids. J. Ind. Microbiol. Biotechnol. 2016, 44, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittgens, A.; Kovacic, F.; Müller, M.M.; Gerlitzki, M.; Santiago-Schübel, B.; Hofmann, D.; Tiso, T.; Blank, L.M.; Henkel, M.; Hausmann, R.; et al. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Appl. Microbiol. Biotechnol. 2016, 101, 2865–2878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiso, T.; Thies, S.; Müller, M.; Tsvetanova, L.; Carraresi, L.; Bröring, S.; Jaeger, K.-E.; Blank, L.M. Rhamnolipids: Production, performance, and application. In Consequences of Microbial Interactions with Hydrocarbons, Oils, and Lipids: Production of Fuels and Chemicals; Sang, Y.L., Ed.; Springer: Berlin, Germany, 2017; pp. 587–622. [Google Scholar]
- Lan, G.; Fan, Q.; Liu, Y.; Chen, C.; Li, G.; Liu, Y.; Yin, X. Rhamnolipid production from waste cooking oil using Pseudomonas SWP-4. Biochem. Eng. J. 2015, 101, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Haba, E.; Espuny, M.J.; Busquets, M.; Manresa, A. Screening and production of rhamnolipids by Pseudomonas aeruginosa 47T2 NCIB 40044 from waste frying oils. J. Appl. Microbiol. 2000, 88, 379–387. [Google Scholar] [CrossRef]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next generation rhamnolipid production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Bahia, F.M.; De Almeida, G.C.; De Andrade, L.P.; Campos, C.G.; Queiroz, L.R.; Da Silva, R.L.V.; Abdelnur, P.V.; Correa, J.; Bettiga, M.; Parachin, N. Rhamnolipids production from sucrose by engineered Saccharomyces cerevisiae. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Wu, J.; Wang, W.; Chen, Q. Production and characterization of a new glycolipid, mannosylerythritol lipid, from waste cooking oil biotransformation by Pseudozyma aphidis ZJUDM34. Food Sci. Nutr. 2019, 7, 937–948. [Google Scholar] [CrossRef] [Green Version]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Production of mannosylerythritol lipids and their application in cosmetics. Appl. Microbiol. Biotechnol. 2013, 97, 4691–4700. [Google Scholar] [CrossRef] [PubMed]
- Saika, A.; Koike, H.; Fukuoka, T.; Yamamoto, S.; Kishimoto, T.; Morita, T. A gene cluster for biosynthesis of mannosylerythritol lipids consisted of 4-O-β-D-mannopyranosyl-(2R,3S)-erythritol as the sugar moiety in a basidiomycetous yeast Pseudozyma tsukubaensis. PLoS ONE 2016, 11, e0157858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewald, S.; Linne, U.; Scherer, M.; Marahiel, M.A.; Kämper, J.; Bölker, M. Identification of a gene cluster for biosynthesis of mannosylerythritol lipids in the basidiomycetous fungus Ustilago maydis. Appl. Environ. Microbiol. 2006, 72, 5469–5477. [Google Scholar] [CrossRef] [Green Version]
- Kitamoto, D.; Nemoto, T.; Yanagishita, H.; Nakane, T.; Kitamoto, H.; Nakahara, T. Fatty-acid Metabolism of mannosylerythritol lipids as biosurfactants produced by Candida antarctica. J. Jpn. Oil Chem. Soc. 1993, 42, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Kitamoto, D.; Yanagishita, H.; Haraya, K.; Kitamoto, H.K. Contribution of a chain-shortening pathway to the biosynthesis of the fatty acids of mannosylerythritol lipid (biosurfactant) in the yeast Candida antarctica: Effect of β-oxidation inhibitors on biosurfactant synthesis. Biotechnol. Lett. 1998, 20, 813–818. [Google Scholar] [CrossRef]
- Kitamoto, D.; Haneishi, K.; Nakahara, T.; Tabuchi, T. Production of mannosylerythritol lipids by Candida antarctica from vegetable oils. Agric. Biol. Chem. 1990, 54, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumari, P.; Mani, K. Structural characterization of a novel xylolipid biosurfactant from Lactococcus lactis and analysis of antibacterial activity against multi-drug resistant pathogens. Bioresour. Technol. 2010, 101, 8851–8854. [Google Scholar] [CrossRef]
- Joshi-Navare, K.; Singh, P.K.; Prabhune, A.A. New yeast isolate Pichia caribbica synthesizes xylolipid biosurfactant with enhanced functionality. Eur. J. Lipid Sci. Technol. 2014, 116, 1070–1079. [Google Scholar] [CrossRef]
- Cheng, Y.; Bélanger, R.R. Protoplast preparation and regeneration from spores of the biocontrol fungus Pseudozyma flocculosa. FEMS Microbiol. Lett. 2000, 190, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Oraby, A.; Werner, N.; Sungur, Z.; Zibek, S. Factors affecting the synthesis of cellobiose lipids by Sporisorium scitamineum. Front. Bioeng. Biotechnol. 2020, 8, 1280. [Google Scholar] [CrossRef]
- Chen, C.; Li, D.; Li, R.; Shen, F.; Xiao, G.; Zhou, J. Enhanced biosurfactant production in a continuous fermentation coupled with in situ foam separation. Chem. Eng. Process. Process. Intensif. 2021, 159, 108206. [Google Scholar] [CrossRef]
- Jimoh, A.A.; Lin, J. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotoxicol. Environ. Saf. 2019, 184, 109607. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Thavasi, R. Microbial Biosurfactants and Their Environmental and Industrial Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781315271767. [Google Scholar]
- Dolman, B.M.; Kaisermann, C.; Martin, P.; Winterburn, J.B. Integrated sophorolipid production and gravity separation. Process Biochem. 2017, 54, 162–171. [Google Scholar] [CrossRef]
- Biniarz, P.; Henkel, M.; Hausmann, R.; Łukaszewicz, M. Development of a bioprocess for the production of cyclic lipopeptides pseudofactins with efficient purification from collected foam. Front. Bioeng. Biotechnol. 2020, 8, 1340. [Google Scholar] [CrossRef] [PubMed]
- Blesken, C.C.; Strümpfler, T.; Tiso, T.; Blank, L.M. Uncoupling foam fractionation and foam adsorption for enhanced biosurfactant synthesis and recovery. Microorganisms 2020, 8, 2029. [Google Scholar] [CrossRef]
- Zhou, C.; Sha, R.; Long, X.; Meng, Q. Extraction separation of rhamnolipids by n-hexane via forming reverse micelles. J. Surfactants Deterg. 2020, 23, 883–889. [Google Scholar] [CrossRef]
- Shen, L.; Zhu, J.; Lu, J.; Gong, Q.; Jin, M.; Long, X. Isolation and purification of biosurfactant mannosylerythritol lipids from fermentation broth with methanol/water/n-hexane. Sep. Purif. Technol. 2019, 219, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, D.; Sun, W.; Yang, X.; Zhang, C.; Zhao, F.; Lu, W. Semicontinuous sophorolipid fermentation using a novel bioreactor with dual ventilation pipes and dual sieve-plates coupled with a novel separation system. Microb. Biotechnol. 2017, 11, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.; de Andrade, L.M.; Rocco, S.; Sforça, M.; Pastore, G.M.; Jauregi, P. A novel approach for the production and purification of mannosylerythritol lipids (MEL) by Pseudozyma tsukubaensis using cassava wastewater as substrate. Sep. Purif. Technol. 2017, 180, 157–167. [Google Scholar] [CrossRef]
- Zulkifli, W.N.; Razak, N.N.A.; Yatim, A.R.M.; Hayes, D.G. Acid precipitation versus solvent extraction: Two techniques leading to different lactone/acidic sophorolipid ratios. J. Surfactants Deterg. 2018, 22, 365–371. [Google Scholar] [CrossRef]
- Hisham, N.H.M.B.; Ibrahim, M.F.; Ramli, N.; Abd-Aziz, S. Production of Biosurfactant produced from used cooking oil by Bacillus sp. HIP3 for heavy metals removal. Molecules 2019, 24, 2617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.U.H.; Sivapragasam, M.; Moniruzzaman, M.; Talukder, M.R.; Yusup, S.B.; Goto, M. Production of sophorolipids by Starmerella bombicola yeast using new hydrophobic substrates. Biochem. Eng. J. 2017, 127, 60–67. [Google Scholar] [CrossRef]
- George, S.; Jayachandran, K. Production and characterization of rhamnolipid biosurfactant from waste frying coconut oil using a novel Pseudomonas aeruginosa D. J. Appl. Microbiol. 2012, 114, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Peñalver, P.; Gea, T.; Sánchez, A.; Font, X. Production of sophorolipids from winterization oil cake by solid-state fermentation: Optimization, monitoring and effect of mixing. Biochem. Eng. J. 2016, 115, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Konishi, M.; Morita, T.; Fukuoka, T.; Imura, T.; Uemura, S.; Iwabuchi, H.; Kitamoto, D. Selective production of acid-form sophorolipids from glycerol by Candida floricola. J. Oleo Sci. 2017, 66, 1365–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Datta, P.; Kumar, B.; Tiwari, P.; Pandey, L.M. Production of novel rhamnolipids via biodegradation of waste cooking oil using Pseudomonas aeruginosa MTCC7815. Biodegradation 2019, 30, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Najmi, Z.; Ebrahimipour, G.; Franzetti, A.; Banat, I.M. In situ downstream strategies for cost-effective bio/surfactant recovery. Biotechnol. Appl. Biochem. 2018, 65, 523–532. [Google Scholar] [CrossRef]
- Dolman, B.M.; Wang, F.; Winterburn, J.B. Integrated production and separation of biosurfactants. Process Biochem. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Drakontis, C.E.; Amin, S. Biosurfactants: Formulations, properties, and applications. Curr. Opin. Colloid Interface Sci. 2020, 48, 77–90. [Google Scholar] [CrossRef]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Borsanyiova, M.; Patil, A.; Mukherji, R.; Prabhune, A.; Bopegamage, S. Biological activity of sophorolipids and their possible use as antiviral agents. Folia Microbiol. 2015, 61, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Satpute, S.K.; Banpurkar, A.G.; Dhakephalkar, P.K.; Banat, I.; Chopade, B.A. Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Pinzon-Gamez, N.M. Rhamnolipid Biosurfactant Production from Glycerol: New Methods of Analysis and Improved Denitrifying Fermentation. Ph.D. Thesis, The University of Akron, Akron, OH, USA, 2009. [Google Scholar]
- Amaral, P.; Coelho, M.A.Z.; Marrucho, I.; Coutinho, J.A.P. Biosurfactants from yeasts: Characteristics, production and application. Biosurfactants 2010, 672, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; da Silva, R.S.; Brasileiro, P.P.F.; de Luna, J.M.; Da Silva, M.D.G.; Rufino, R.; Costa, A.F.S.; Santos, V.A.; Sarubbo, L.A. Application of a biosurfactant from Candida tropicalis UCP 0996 produced in low-cost substrates for hydrophobic contaminants removal. Chem. Eng. Trans. 2018, 64, 541–546. [Google Scholar] [CrossRef]
- Lira, I.R.A.D.S.; Santos, E.D.M.S.; Filho, A.A.S.; Farias, C.B.B.; Guerra, J.M.C.; Sarubbo, L.A.; De Luna, J.M. Bio-surfactant production from Candida guilliermondii and evaluation of its toxicity. Chem. Eng. Trans. 2020, 79, 457–462. [Google Scholar] [CrossRef]
- Ahuekwe, E.F.; Okoli, B.E.; Stanley, H.O.; Kinigoma, B. Evaluation of hydrocarbon emulsification and heavy metal detoxification potentials of sophorolipid biosurfactants produced from waste substrates using yeast and mushroom. In Proceedings of the 2017 SPE African Health, Safety, Security, Environment, and Social Responsibility Conference and Exhibition, Accra, Ghana, 4–6 October 2016. [Google Scholar]
- Bognolo, G. Biosurfactants as emulsifying agents for hydrocarbons. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 41–52. [Google Scholar] [CrossRef]
- Li, Z.; Dai, L.; Wang, D.; Mao, L.; Gao, Y. Stabilization and rheology of concentrated emulsions using the natural emulsifiers Quillaja saponins and rhamnolipids. J. Agric. Food Chem. 2018, 66, 3922–3929. [Google Scholar] [CrossRef]
- Coimbra, C.D.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Studies of the cell surface properties of Candida species and relation to the production of biosurfactants for environmental applications. Curr. Microbiol. 2008, 58, 245–251. [Google Scholar] [CrossRef]
- Hillion, G.; Marchal, R.; Stoltz, C.; Borzeix, F. Use of a Sophorolipid to Provide Free Radical Formation Inhibiting Activity or Elastase Inhibiting Activity. U.S. Patent 5,756,471, 26 May 1998. [Google Scholar]
- Paulino, B.N.; Pessôa, M.G.; Mano, M.C.R.; Molina, G.; Numa, I.A.N.; Pastore, G.M. Current status in biotechnological production and applications of glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. [Google Scholar] [CrossRef]
- Mohan, P.K.; Nakhla, G.; Yanful, E.K. Biokinetics of biodegradation of surfactants under aerobic, anoxic and anaerobic conditions. Water Res. 2006, 40, 533–540. [Google Scholar] [CrossRef]
- Lima, T.M.S.; Procópio, L.C.; Brandão, F.D.; Carvalho, A.M.X.; Tótola, M.; Borges, A.C. Biodegradability of bacterial surfactants. Biodegradation 2010, 22, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.M.; Filho, A.S.S.; Rufino, R.D.; Sarubbo, L.A. Production of biosurfactant from Candida bombicola URM 3718 for environmental applications. Chem. Eng. Trans. 2016, 49, 583–588. [Google Scholar] [CrossRef]
- Vatsa, P.; Sanchez, L.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 2010, 11, 5095–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Moldes, A.; Vecino, X.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J. Biosurfactants: The Use of Biomolecules in Cosmetics and Detergents. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rodrigues, A.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–185. [Google Scholar]
- Van Bogaert, I.N.A.; Saerens, K.; De Muynck, C.; Develter, D.; Soetaert, W.; Vandamme, E.J. Microbial production and application of sophorolipids. Appl. Microbiol. Biotechnol. 2007, 76, 23–34. [Google Scholar] [CrossRef]
- Varvaresou, A.; Iakovou, K. Biosurfactants in cosmetics and biopharmaceuticals. Lett. Appl. Microbiol. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Morita, T.; Fukuoka, T.; Imura, T.; Kitamoto, D. Mannosylerythritol lipids: Production and applications. J. Oleo Sci. 2015, 64, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Toyobo—Cosmetics Ingredients Department. SurfMellow Pseudozyma tsukubaensis. 2021. Available online: http://toyobo.cn/seihin/cosme/surfmellow.htm (accessed on 27 July 2021).
- De Almeida, D.G.; Silva, R.D.C.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, L.; Banat, I.; Teixeira, J.; Oliveira, R. Biosurfactants: Potential applications in medicine. J. Antimicrob. Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, P.; Nawale, L.; Sarkar, D.; Nisal, A.; Prabhune, A. Sophorolipid assisted tunable and rapid gelation of silk fibroin to form porous biomedical scaffolds. RSC Adv. 2015, 5, 33955–33962. [Google Scholar] [CrossRef]
- Allingham, R.P. Sophoroside Esters in Prepared Food Products. U.S. Patent 3,622,344, 23 November 1971. [Google Scholar]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the glycolipid biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb. Cell Factories 2021, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, K.; Yamamoto, M.; Shibuki, M.; Nomura, Y.; Sengoku, K.; Higashine, S. Method of Improving Quality of Wheat Flour. U.S. Patent 4,524,080, 18 June 1985. [Google Scholar]
- Kasture, M.B.; Patel, P.; Prabhune, A.A.; Ramana, C.V.; Kulkarni, A.A.; Prasad, B.L.V. Synthesis of silver nanoparticles by sophorolipids: Effect of temperature and sophorolipid structure on the size of particles. J. Chem. Sci. 2008, 120, 515–520. [Google Scholar] [CrossRef]
- Saerens, K.; Van Bogaert, I.; Soetaert, W.; Vandamme, E. Production of glucolipids and specialty fatty acids from sophorolipids by Penicillium decumbens naringinase: Optimization and kinetics. Biotechnol. J. 2009, 4, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Hipólito, A.; Caretta, T.D.O.; Silveira, V.A.I.; Bersaneti, G.T.; Mali, S.; Celligoi, M.A.P.C. Active biodegradable cassava starch films containing sophorolipids produced by Starmerella bombicola ATCC® 22214™. J. Polym. Environ. 2021, 1–11. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; Buyst, D.; Martins, J.C.; Roelants, S.L.K.W.; Soetaert, W.K. Synthesis of bolaform biosurfactants by an engineered Starmerella bombicola yeast. Biotechnol. Bioeng. 2016, 113, 2644–2651. [Google Scholar] [CrossRef]




| Substrate | Hydrophobic and Hydrophilic Substrate Ratio | Nitrogen Source | Titer, g·L−1 | Yield, g·g−1 | Volumetric Productivity, g·L−1·h−1 | Cultivation Method | Reference | |
|---|---|---|---|---|---|---|---|---|
| Hydrophobic | Hydrophilic | |||||||
| Restaurant oil waste | - | - | Yeast extract, urea | 34.0 | 0.23 | 0.14 | Batch in flask | [43] |
| WCO | Glucose | 0.27 | Yeast extract, urea | 50.0 | 0.24 | 0.25 | Fed-batch in a bioreactor | [71] |
| WCO | Glucose | 1.00 | Yeast extract, urea | 55.6 | 0.40 | 0.23 | Fed-batch with ultrasound in a fermenter | [22] |
| Raw sunflower oil | Glucose | 1.00 | Yeast extract, urea | 12.31 | 0.06 | 0.06 | Batch in flask | [46] |
| Waste frying (sunflower) oil | Glucose | 1.00 | Yeast extract, urea | 4.26 | 0.02 | 0.02 | Batch in flask | [46] |
| Yellow grease (soybean oil) | Sorghum bagasse hydrolysate | 0.18 a | - | 35.9 | 0.56 | 0.11 | Batch in flask | [72] |
| Yellow grease (soybean oil) | Corn stover hydrolysate | 2.13 a | - | 52.1 | 0.34 | 0.31 | Batch in a fermenter | [72] |
| WCO | Glucose | 1.00 | Yeast extract, peptone | 84.8 | 0.42 | 0.59 | Batch in flask | [18] |
| WCO | Glucose | 1.00 | Yeast extract, peptone | 315.6 | - | - | Fed-batch in a fermenter | [18] |
| Raw cooking oil | Glucose | 1.00 | Yeast extract, peptone | 365.8 | - | - | Fed-batch in a fermenter | [18] |
| Fatty Acid a, % | Caprylic C8:0 | Capric C10:0 | Decanoic C10:1 | Lauric C12:0 | Palmitic C16:0 | Stearic C18:0 | Oleic C18:1 | Linoleic C18:2 |
|---|---|---|---|---|---|---|---|---|
| Raw soybean oil | - | - | - | - | 13.08 | 4.36 | 33.6 | 48.11 |
| WCO | - | - | - | - | 18.59 | 5.13 | 43.57 | 30.63 |
| MELs from raw soybean oil | 23.19 | 47.49 | 0.7 | 5.94 | 2.13 | 1.6 | 7.37 | 9.57 |
| MELs from WCO | 1.5 | 6.66 | 3.22 | 0.31 | 7.92 | 3.79 | 59.78 | 13.78 |
| BS Extraction Method | BS Type | Purity, % | Recovery Rate, % | Method Used to Quantify BS | Reference |
|---|---|---|---|---|---|
| Foam fractionation + foam adsorption | Rhamnolipids | No data | 40 | HPLC + charged aerosol detector | [96] |
| Acid precipitation + solvent extraction (with n-hexane) | Rhamnolipids | 90 | 99 | Anthrone-sulfuric acid assay a | [97] |
| Integrated gravity separation + solvent extraction (with hexane and ethyl acetate) | Sophorolipids | No data | 86 | Gravimetry | [94] |
| Filtration + solvent extraction (with methanol/water (pH 2)/n-hexane) | MELs | 100 | 90 | Orcinol method a | [98] |
| Multi-step integrated gravity separation | Sophorolipids | 74 | No data | Anthrone method a (residual glucose deduced from total sugar content to get sophorolipid content) | [99] |
| Foam fractionation + ultrafiltration | MELs | No data | 80 | Reverse phase HPLC | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liepins, J.; Balina, K.; Soloha, R.; Berzina, I.; Lukasa, L.K.; Dace, E. Glycolipid Biosurfactant Production from Waste Cooking Oils by Yeast: Review of Substrates, Producers and Products. Fermentation 2021, 7, 136. https://doi.org/10.3390/fermentation7030136
Liepins J, Balina K, Soloha R, Berzina I, Lukasa LK, Dace E. Glycolipid Biosurfactant Production from Waste Cooking Oils by Yeast: Review of Substrates, Producers and Products. Fermentation. 2021; 7(3):136. https://doi.org/10.3390/fermentation7030136
Chicago/Turabian StyleLiepins, Janis, Karina Balina, Raimonda Soloha, Ieva Berzina, Liva Kristiana Lukasa, and Elina Dace. 2021. "Glycolipid Biosurfactant Production from Waste Cooking Oils by Yeast: Review of Substrates, Producers and Products" Fermentation 7, no. 3: 136. https://doi.org/10.3390/fermentation7030136
APA StyleLiepins, J., Balina, K., Soloha, R., Berzina, I., Lukasa, L. K., & Dace, E. (2021). Glycolipid Biosurfactant Production from Waste Cooking Oils by Yeast: Review of Substrates, Producers and Products. Fermentation, 7(3), 136. https://doi.org/10.3390/fermentation7030136